Abstract
A physiologic role for Notch signaling in hematopoiesis has been clearly defined in lymphoid differentiation, with evidence suggesting a critical role in T-cell versus B-cell fate decisions. Previously, we demonstrated that activation of endogenous Notch receptors by culture of murine lin−Sca-1+c-kit+ (LSK) hematopoietic progenitors with exogenously presented Notch ligand, Delta1ext-IgG, consisting of the extracellular domain of Delta1 fused to the Fc domain of human IgG1, promoted early T-cell differentiation and increased the number of progenitors capable of short-term lymphoid and myeloid reconstitution. Here we show that culture of LSK precursors with Delta1ext-IgG increases the number of progenitors that are able to rapidly repopulate the thymus and accelerate early T-cell reconstitution with a diversified T-cell receptor repertoire. Most of the early T-cell reconstitution originated from cells that expressed lymphoid-associated antigens: B220, Thy1, CD25, and/or IL7Rα, whereas the most efficient thymic repopulation on a per cell basis originated from the smaller number of cultured cells that did not express lymphoid-associated antigens. These findings demonstrate the potential of Delta1ext-IgG-cultured cells for accelerating early immune reconstitution after hematopoietic cell transplantation.
Introduction
Delayed immune reconstitution increases the risks of opportunistic infection after hematopoietic cell transplantation (HCT).1 Recovery of T-cell immunity after HCT proceeds through thymus-dependent, de novo generation of T cells and through thymus-independent peripheral expansion of mature T cells present in the stem cell graft.2,3 Although peripheral expansion of mature T cells is an important source for initial T-cell recovery after HCT, this pathway produces a limited T-cell receptor repertoire and is associated with graft-versus-host disease.2 Full recovery of T-cell immune function relies on generation of sufficient numbers of T cells with a diversified repertoire of T-cell receptors, which occurs only after repopulation by thymus-derived T cells.4,5 Regeneration of CD4+ cells appears to be exclusively thymus-dependent, whereas regeneration of CD8+ cells occurs through thymus-dependent and thymus-independent pathways.6,7 De novo generation of donor stem cell–derived T cells, in particular CD4+ T cells, is an extremely slow process, and prolonged lymphoid deficiency is directly correlated with increased risk of infection.8 Thus, strategies to hasten immune recovery are needed to decrease the mortality and morbidity associated with HCT
The rate of immune reconstitution correlates positively with the number of infused hematopoietic stem cells (HSCs).9-11 Furthermore, results of previous studies have suggested that transplantation of a more differentiated progenitor cell can hasten T-cell reconstitution.12 Lymphoid-committed progenitors regenerate the T-cell compartment faster than noncommitted HSCs, suggesting that transplantation of donor lymphoid-committed progenitors can accelerate the de novo generation of donor T cells after HCT.13-19 Alternatively, seeding of the thymus by primitive lin−Sca-1+c-kit+ (LSK) cells may play a key role.20 A means to increase the number of progenitors that preferentially commit to the T-cell lineage could have profound effects on the rate of T-cell reconstitution.
In efforts to hasten thymic repopulation, ex vivo systems have been used to generate thymic progenitors from hematopoietic precursors, but these efforts have met with only modest success.21-23 Our laboratory and others have demonstrated that coculture of hematopoietic precursor cells with a Notch ligand may provide a means of generating a multilog increase in the number of T-cell precursors.24-28
A role for Notch signaling in T-cell commitment and early thymocyte development is well defined. Notch1 is essential for T-cell lineage commitment, and inhibition of Notch1 blocks differentiation of double negative (DN) thymocytes at the DN1-to-DN2 transition.29 Conversely, overexpression of constitutively active Notch1 in hematopoietic progenitors inhibits B-cell development and promotes T-cell development to the double positive (CD4+CD8+) stage.30 These and other studies31-33 indicate an important role for Notch in directing marrow-derived lymphoid precursors toward a T-cell versus B-cell fate, and in promoting T-cell differentiation.
Our more recent studies using the Notch ligand Delta1ext-IgG in immobilized form demonstrated a profound increase in the number of murine Sca-1+c-kit+ cells with short-term lymphoid and myeloid repopulating ability.34,35 Here we show that culture of LSK precursors with Delta1ext-IgG generates a multilog increase in the number of progenitors that can accelerate and enhance the initial T-cell reconstitution after HCT. Furthermore, our findings suggest the potential usefulness of this augmentation approach in accelerating immune recovery after HCT.
Materials and methods
Generation and immobilization of Delta1ext-IgG
Delta1ext-IgG was prepared as described.36 Briefly, Delta1ext-IgG was purified from conditioned medium generated by NSO myeloma cells electroporated with constructs expressing the ligand. Purified human IgG1 was purchased from Sigma (St Louis, MO). Ligand was immobilized on the plastic surface of the culture wells as described.34 Briefly, culture wells were incubated with control human IgG1 antibody (10 μg/mL) in phosphate-buffered saline (PBS) or in PBS with various concentrations of Delta1ext-IgG (2.5, 5, or 10 μg/mL) for 2 hours at 37°C. The culture wells were then washed extensively with PBS, and blocked for at least 30 minutes with PBS containing 2% bovine serum albumin (BSA).
Immunofluorescence studies
Immunofluorescence analysis was performed using a LSR cytometer (Becton Dickinson, Mountain View, CA) as described.37 Antibodies were purchased from Pharmingen (San Diego, CA) unless noted otherwise. All reagents for flow analysis were diluted in PBS containing 2% fetal bovine serum (PBS/FBS). Briefly, cells were resuspended in PBS/FBS, preincubated with anti–mouse FcγRII block for 10 minutes at 4°C, and stained with one or more of the following antibodies: fluorescein isothiocyanate (FITC)–conjugated monoclonal antibodies against Ly5.2; phycoerythrin (PE)–conjugated monoclonal antibodies against CD3, CD4, CD8, CD19, CD25, CD127, NK, Thy1, Gr1, B220, and F4/80 (Caltag, Burlingame, CA); biotinylated antibodies against Sca-1 secondarily stained with streptavidin-PE-Cy7; and allophycocyanin (APC)–monoclonal antibodies against c-kit. T-cell receptor diversity was screened using FITC-conjugated monoclonal antibodies against Vβ2,3,4,5.1 and 5.2,6,7,8.1 and 8.2,8.3,9,10b,11,12,13,14, and 17a T-cell receptors. Dead cells were gated based on 6′-diamidino-2-phenylindole (DAPI; Sigma) staining.
Cell isolation
Bone marrow (BM) was obtained from C57BL/6 (Ly5.2) mice (8-10 weeks of age) from The Jackson Laboratory (Bar Harbor, ME). LSK cells from BM were enriched by flow cytometric sorting with the use of a Vantage Cell Sorter (Becton Dickinson) as described.37 Cells expressing CD2, CD3, CD8a, CD5, CD11b, B220, GR-1, and TER-119 were depleted, and the remaining population was sorted for cells with high Sca-1 and c-kit expression. For flow cytometric isolation of cultured Sca-1+c-kit+ cells, cells were prepared as described37 and stained with biotinylated antibodies against Sca-1 secondarily stained with streptavidin-PE-Cy7 and APC-monoclonal antibodies against c-kit. Cultured cells were also stained with a mixture of PE-conjugated anti-CD25, anti-B220, anti-Thy, and anti-IL7Rα to isolated Sca-1+c-kit+ cells expressing lymphoid-associated antigens. Sorted cells were at least 95% pure, as determined by postsort analysis. Cells were stained with DAPI to gate for dead cells.
Cell culture
LSK cells (103) were cultured with growth medium and immobilized Delta1ext-IgG or control IgG in each well of a 48-well plate (Falcon non–tissue culture treated; Becton Dickinson, Franklin Lakes, NJ) as described.34 The growth medium (Iscoves modified Dulbecco medium) was supplemented with 20% FBS and 4 growth factors (4GF): murine stem cell factor, human Flt-3 ligand, and human IL-6, each at 100 ng/mL, and human IL-11 at 10 ng/mL (PeproTech, Rocky Hill, NJ). Cell density was maintained at approximately 2.5 × 105 cells/cm2 during the experiment by transferring the cultures to larger vessels every 3 to 5 days during the first 2 weeks. Thereafter, approximately four-fifths of the culture was removed and replaced with fresh medium twice weekly for 2 weeks.
Competitive repopulation assays
Congenic C57BL/6.SJL-Ly5.1-Pep3b (Ly5.1) mice and Rag−/−(Ly5.1) mice bred at the Fred Hutchinson Cancer Research Center (Seattle, WA) were housed in specific pathogen-free conditions and maintained on autoclaved chow ad libitum. Recipients were exposed to γ-irradiation (10.0 Gy) from a linear accelerator at a rate of 20 cGy/min. Cultured Ly5.2 cells (1 × 106) and noncultured Ly5.1 BM cells (1 × 105, 1 × 106, or 1 × 107) were injected via tail vein. Blood was obtained from recipients at various times after transplantation. Red blood cells were removed with ammonium chloride lysis buffer. Nucleated cells were washed in PBS/FBS, preincubated with FcγRII block for 10 minutes at 4°C, and stained with the respective PE-conjugated lineage-specific antibodies as described in “Immunofluorescence studies.” To identify Ly5.2+ donors, cells were stained with FITC-conjugated Ly5.2 (clone 104). All stained cells were washed with PBS/FBS, resuspended in PBS/FBS containing PI (1 μg/mL), and analyzed by FACS.
Quantitation of lymphocytes
Heparinized blood (50 μL) was processed in the Sysmex XT2000i (Sysmex America, Mundelein, IL), a quantitative automated hematology analyzer for in vitro diagnostic use in determining blood cell parameters. Samples were evaluated in the reticulated mode using flow cytometry with a semiconductor laser exploiting the differences in cell size, complexity, and RNA/DNA content. Absolute white blood count was calculated automatically by the XT2000i.
Limiting dilution analysis (LDA)
For LDA, noncultured LSK cells in limiting doses of 10, 50, 100, 500, and 1000 cells were injected into lethally irradiated congenic mice and 500, 1 × 103, or 1 × 104 cells were injected into nonirradiated Rag−/− mice. Similarly, Delta1ext-IgG-cultured cells in limiting doses of 2 × 103, 2 × 104, 2 × 105, and 2 × 106 cells were injected together with 1 × 105 syngeneic Ly5.1 BM cells into lethally irradiated mice, and 1 × 105, 5 × 105, 1 × 106, 1 × 107 Delta1ext-IgG-cultured cells were injected into nonirradiated Rag−/− mice. To determine the percentage of CD3+ cells, FACs analysis was performed on blood samples obtained at 3 weeks after HCT in irradiated congenic mice and at 5 weeks in nonirradiated Rag−/− mice. Successful T-cell reconstitution was defined as the presence of at least 0.5% donor CD3+ T cells. The data from several limiting dilution experiments were pooled and analyzed by applying Poisson statistics to the single-hit model. The frequency of progenitor cells was calculated using L-Calc software (StemCell Technologies, Vancouver, BC, Canada).
Results
Generation of T-cell precursor on Delta1ext-IgG
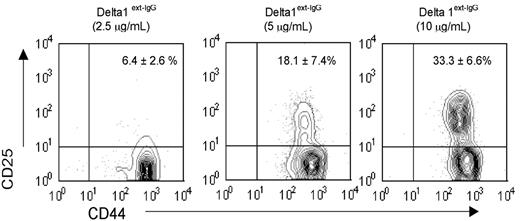
Previously we showed that LSK cells cultured with increasing densities of immobilized Delta1ext-IgG enhanced the generation of Sca-1+c-kit+ cells and Thy1+ CD25+ early T-cell precursors. To address whether higher densities of Delta1ext-IgG generated increased numbers of progenitors with T-cell reconstitution potential, we tested in vivo T-cell reconstitution by the progeny of LSK cells cultured with various densities of Delta1ext-IgG in a competitive repopulation assay. LSK cells (1 × 103) cultured with various concentrations of Delta1ext-IgG (2.5, 5.0, or 10 μg/mL) and 4GF for 28 days generated at least 1 × 1013 cells, with no significant difference in the number of cells generated on different concentrations of Delta1ext-IgG (data not shown). Cells cultured on control IgG terminally differentiated. Mature T cells were not generated at any concentration of Delta1ext-IgG or at any time point, as indicated by the absence of cells with a mature CD3+ T-cell phenotype (data not shown). However, we found a significant increase in the percentage of cells with a phenotype characteristic of a DN2 (CD44+CD25+) early T-cell progenitor when cells were cultured in the presence of higher density of Delta1ext-IgG as compared with lower densities (Figure 1).
Density-dependent effect of Delta1ext-IgG on generation of T-cell progenitors. LSK cells were cultured for 28 days with Delta1ext-IgG plated at increasing concentrations and were analyzed by FACS to determine the percentage of cells expressing CD44 and CD25. Numbers in right upper corner represent the percent of CD44+CD25+ cells plus or minus the standard error of the mean (SEM) of 5 separate experiments.
Density-dependent effect of Delta1ext-IgG on generation of T-cell progenitors. LSK cells were cultured for 28 days with Delta1ext-IgG plated at increasing concentrations and were analyzed by FACS to determine the percentage of cells expressing CD44 and CD25. Numbers in right upper corner represent the percent of CD44+CD25+ cells plus or minus the standard error of the mean (SEM) of 5 separate experiments.
We found that LSK cells cultured with the highest density of Delta1ext-IgG (10 μg/mL) contained the greatest numbers of early Sca-1+c-kit+ progenitors and DN2 early T-cell precursors and had the highest in vivo T-cell reconstitution potential (data not shown). Based on these results, plates coated with Delta1ext-IgG at 10 μg/mL were used for all subsequent experiments.
Accelerated T-cell reconstitution by Delta1ext-IgG-cultured LSK cells compared with noncultured LSK cells
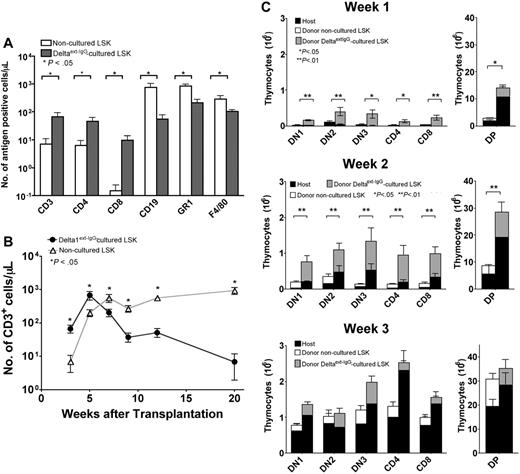
To determine whether Delta1ext-IgG-cultured cells can accelerate T-cell reconstitution compared with noncultured LSK cells, we used a competitive repopulation assay. Lethally irradiated Ly5.1-congenic mice received noncultured Ly5.2 LSK BM cells (5 × 103) or Ly 5.2 LSK cells cultured with 10 μg/mL Delta1ext-IgG (1 × 106). Both groups also received syngeneic Ly5.1 BM cells (1 × 105). At 3 weeks after HCT, blood samples from recipients of Delta1ext-IgG-cultured cells contained significantly fewer Ly5.2+ donor-derived B (CD19+) and myeloid (GR1+ and F4/80+) cells than samples from recipients of noncultured LSK cells (Figure 2A). At 3 and 5 weeks after HCT, respectively, blood samples from recipients of Delta1ext-IgG-cultured cells contained 9- and 3-fold higher numbers of Ly5.2+ donor-derived CD3+ cells compared with the numbers in blood samples from recipients of noncultured LSK cells (Figure 2B). By 7 weeks, however, blood samples from recipients of Delta1ext-IgG-cultured cells contained one-third as many Ly5.2+ donor-derived CD3+ cells compared with samples from recipients of noncultured LSK cells. At 9 weeks after HCT, the number of Ly5.2+ donor-derived CD3+ cells from recipients of Delta1ext-IgG-cultured cells decreased, whereas the numbers of Ly5.2+ donor-derived CD3+ cells in blood samples from recipients of noncultured LSK cells were maintained. By 20 weeks, blood samples from recipients of Delta1ext-IgG-cultured cells contained only one-tenth as many Ly5.2+ donor-derived CD3+ cells compared with samples from recipients of noncultured LSK cells (Figure 2B). The data show that during the first 5 weeks after HCT, higher numbers of Ly5.2+ donor-derived CD3+ cells were generated in mice injected with Delta1ext-IgG-cultured cells as compared with noncultured LSK cells. Delta1ext-IgG-cultured cells generated progenitors that significantly accelerated early T-cell reconstitution compared with noncultured LSK cells.
T-cell reconstitution by LSK cells cultured on immobilized Delta1ext-IgG compared with noncultured LSK cells. (A) Lethally irradiated Ly5.1 C57BL/6 recipients received 5 × 103 noncultured LSK cells or 1 × 106 Ly5.2 Delta1ext-IgG-cultured cells along with 1 × 105 Ly5.1 BM cells. FACS analysis and CBCs were performed on blood samples obtained at 3 weeks after HCT to determine the number of Ly5.2+ CD3+, CD4+, CD8+, CD19+, GR1+, and F4/80+ cells in mice that received LSK cells cultured on Delta1ext-IgG (closed bars) compared with noncultured LSK cells (open bar). (B) FACS analysis and CBCs were performed on blood samples obtained at various times after HCT to determine the number of Ly5.2+ CD3+ cells in mice that received LSK cells cultured on Delta1ext-IgG (•) compared with noncultured LSK cells (▵). (C-E) FACS analysis was performed on thymuses at 1 week (C), 2 weeks (D), and 3 weeks (E) after HCT to determine the total number of Ly5.1 host and Ly5.2 donor DN1, DN2, DN3, CD4+, CD8+, or DP cells in mice that received noncultured Ly5.2 LSK cells or Ly5.2 LSK cells cultured on Delta1ext-IgG. Data represent mean plus or minus SEM of 3 separate experiments (n = 10-15).
T-cell reconstitution by LSK cells cultured on immobilized Delta1ext-IgG compared with noncultured LSK cells. (A) Lethally irradiated Ly5.1 C57BL/6 recipients received 5 × 103 noncultured LSK cells or 1 × 106 Ly5.2 Delta1ext-IgG-cultured cells along with 1 × 105 Ly5.1 BM cells. FACS analysis and CBCs were performed on blood samples obtained at 3 weeks after HCT to determine the number of Ly5.2+ CD3+, CD4+, CD8+, CD19+, GR1+, and F4/80+ cells in mice that received LSK cells cultured on Delta1ext-IgG (closed bars) compared with noncultured LSK cells (open bar). (B) FACS analysis and CBCs were performed on blood samples obtained at various times after HCT to determine the number of Ly5.2+ CD3+ cells in mice that received LSK cells cultured on Delta1ext-IgG (•) compared with noncultured LSK cells (▵). (C-E) FACS analysis was performed on thymuses at 1 week (C), 2 weeks (D), and 3 weeks (E) after HCT to determine the total number of Ly5.1 host and Ly5.2 donor DN1, DN2, DN3, CD4+, CD8+, or DP cells in mice that received noncultured Ly5.2 LSK cells or Ly5.2 LSK cells cultured on Delta1ext-IgG. Data represent mean plus or minus SEM of 3 separate experiments (n = 10-15).
Thymuses of lethally irradiated Ly5.1-congenic recipients injected with Ly5.2 Delta1ext-IgG-cultured cells (1 × 106) or noncultured Ly5.2 LSK cells (5 × 103) were examined at various times after HCT to evaluate thymic reconstitution. The total number of Ly5.1 host and Ly5.2 donor thymic subpopulation for DN, double positive (DP), and single positive (SP) thymocytes was determined at 1, 2, and 3 weeks after HCT (Figure 2C–E). At 1 and 2 weeks, the total number of DN, DP, and SP thymocytes was significantly higher in mice injected with Delta1ext-IgG-cultured cells as compared with noncultured LSK cells (Figure 2C–D). Furthermore, the numbers of Ly5.2 donor thymocytes and the number of Ly5.1 host thymocytes were higher in mice injected with Delta1ext-IgG-cultured cells compared with the number of Ly5.2 donor thymocytes and the number of Ly5.1 host thymocytes in mice injected with noncultured LSK cells. At 3 weeks, the total numbers of DN, DP, and SP thymocytes in mice injected with Delta1ext-IgG-cultured cells versus noncultured LSK cells showed no statistically significant differences (Figure 2E). By 4 weeks, Delta1ext-IgG-derived T-cell precursors decreased substantially and by 15 weeks, Delta1ext-IgG-derived T-cell precursors were barely detectable in the thymus (data not shown). The data demonstrate that for early thymic repopulation, Delta1ext-IgG-cultured cells were more efficient than noncultured LSK cells.
Culture of LSK cells with Delta1ext-IgG increases the number of progenitors capable of thymic reconstitution
We used LDA to quantitate the change in the total number of progenitors capable of T-cell reconstitution generated by culturing LSK cells with Delta1ext-IgG. In 3 separate experiments, LSK cells (1 × 103) cultured with Delta1ext-IgG generated an approximate 108-fold increase in the total number of cells (1.96 × 1011 ± 1.1 × 1011 progeny generated). Noncultured LSK cells and Delta1ext-IgG-cultured cells in limiting doses were injected together with syngeneic Ly5.1 BM cells (1 × 105) into lethally irradiated Ly5.1-congenic mice, and blood was examined after 3 weeks for successful T-cell reconstitution. Based on the precursor frequency estimates, we determined that the number of progenitors with T-cell reconstitution potential in 1 × 103 LSK cells was 2.3; after culture with Delta1ext-IgG, the number of progenitors with T-cell reconstitution potential in the 1011 expanded product was 1.9 × 109, representing a 5 × 108-fold increase (Table 1)
To determine the clonality of donor T cells in the recipient mice, TCR Vβ expression was analyzed by FACS. Lethally irradiated Ly5.1-congenic mice received 1 × 105 syngeneic Ly5.1 BM cells and 1 × 106 Ly5.2 cells cultured with 10 μg/mL Delta1ext-IgG. At 3 weeks after HCT, blood samples showed broad Vβ repertoire in donor CD3+ (Figure 3).
Analysis of the TCR Vβ expression by FACS. Lethally irradiated Ly5.1 C57BL/6 recipients received 1 × 106 Ly5.2 Delta1ext-IgG-cultured cells along with 1 × 105 Ly5.1 BM cells. Blood samples obtained at 3 weeks after HCT were stained with monoclonal antibody against CD3 and TCR Vβ. The percentage of Vβ-positive cells among donor CD3+ lymphocytes in mice that received Delta1ext-IgG-cultured cells (closed bars) and compared with control C57BL/6 mice (open bars) is shown. Data shown are the mean plus or minus SEM of 2 separate experiments (n = 7-10).
Analysis of the TCR Vβ expression by FACS. Lethally irradiated Ly5.1 C57BL/6 recipients received 1 × 106 Ly5.2 Delta1ext-IgG-cultured cells along with 1 × 105 Ly5.1 BM cells. Blood samples obtained at 3 weeks after HCT were stained with monoclonal antibody against CD3 and TCR Vβ. The percentage of Vβ-positive cells among donor CD3+ lymphocytes in mice that received Delta1ext-IgG-cultured cells (closed bars) and compared with control C57BL/6 mice (open bars) is shown. Data shown are the mean plus or minus SEM of 2 separate experiments (n = 7-10).
T-cell reconstitution by LSK cells cultured on Delta1ext-IgG is independent from BM conditioning
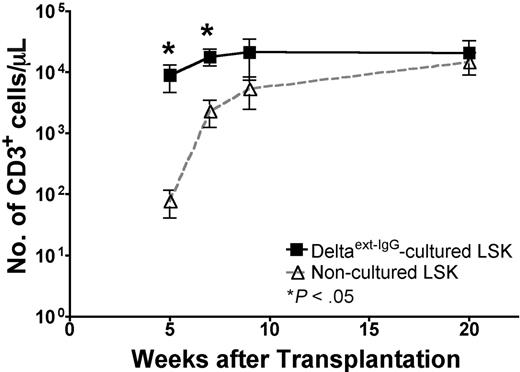
Thymopoiesis after HCT may occur transiently by progenitors that home directly to the thymus.17,18 Alternatively, transplanted cells can migrate to the BM and provide progenitors that seed the thymus.17,18 To minimize BM engraftment and BM-derived progenitors seeding the thymus, we transplanted Delta1ext-IgG-cultured cells into nonirradiated mice. To allow for thymic engraftment we used lymphoid-deficient Rag−/− recipients. Ly5.1 Rag−/− mice received injections of noncultured LSK BM cells (1 × 103) or Ly5.2 cells cultured with Delta1ext-IgG (1 × 106). At 5 and 7 weeks after HCT, respectively, blood samples from recipients of Delta1ext-IgG-cultured cells contained 4- and 5-fold increased numbers of CD3+ cells compared with the numbers in blood samples from recipients of noncultured LSK cells (Figure 4). By 20 weeks, the numbers of CD3+ cells in blood samples were similar in the 2 groups. During the first 20 weeks after HCT, B cells and myeloid cells derived from Delta1ext-IgG-cultured cells were not observed in recipients of Delta1ext-IgG-cultured cells (data not shown). The data demonstrate that during the first 7 weeks after HCT, recipients of Delta1ext-IgG-cultured cells contained significantly higher numbers of CD3+ T cells compared with recipients of noncultured LSK cells. Furthermore, significant numbers of T cells derived from Delta1ext-IgG-cultured cells were sustained for up to 20 weeks after HCT.
T-cell reconstitution in nonirradiated lymphoid cell–deficient mice by LSK cells cultured on immobilized Delta1ext-IgG compared with noncultured LSK cells. Nonirradiated Ly5.1 Rag−/− recipients received 1 × 103 noncultured LSK cells or 1 × 106 Ly5.2 Delta1ext-IgG-cultured cells. FACS analysis and CBCs were performed on blood samples obtained at various times after HCT to determine the number of Ly5.2+ CD3+ cells in mice that received LSK cells cultured on Delta (▪) compared with noncultured LSK cells (▵). Data represent the mean plus or minus SEM of 3 separate experiments (n = 5-10)
T-cell reconstitution in nonirradiated lymphoid cell–deficient mice by LSK cells cultured on immobilized Delta1ext-IgG compared with noncultured LSK cells. Nonirradiated Ly5.1 Rag−/− recipients received 1 × 103 noncultured LSK cells or 1 × 106 Ly5.2 Delta1ext-IgG-cultured cells. FACS analysis and CBCs were performed on blood samples obtained at various times after HCT to determine the number of Ly5.2+ CD3+ cells in mice that received LSK cells cultured on Delta (▪) compared with noncultured LSK cells (▵). Data represent the mean plus or minus SEM of 3 separate experiments (n = 5-10)
We used LDA in nonirradiated recipients to quantitate the number of T-cell progenitors generated by culturing LSK cells with Delta1ext-IgG. Noncultured LSK cells and Delta1ext-IgG-cultured cells in limiting doses were injected into nonirradiated Rag−/− recipients, and blood was examined after 5 weeks for successful T-cell reconstitution. Based on the precursor frequency estimates, we determined that the number of progenitors with T-cell reconstitution potential in 1 × 103 LSK cells was 1.3; after culture with Delta1ext-IgG, the number of progenitors with T-cell reconstitution potential in the 1 × 1011 expanded product was 3.7 × 105, representing a 3 × 105-fold increase (Table 2). Thus, the increase in the number of T-cell progenitors generated by culturing with Delta1ext-IgG can be observed in recipients in which thymic seeding by BM progenitors was minimized.
Augmenting BM graft with Delta1ext-IgG-cultured LSK cells accelerates T-cell reconstitution
Because residual host T cells persist in the T-cell compartment even after lethal irradiation of C57BL/6 recipients,38 we investigated the kinetics of T reconstitution in lymphoid cell–deficient recipients where competing effects of host T cells are not present. Since T cells were derived only from transplanted Ly5.1 BM or Ly5.2 Delta1ext-IgG-cultured cells, we were able to assess any augmentation of de novo T-cell generation gained by adding Delta1ext-IgG-cultured cells to a BM graft. Furthermore, based on recent work suggesting that the kinetics of T-cell reconstitution depends on the cell dose,11 we tested whether the enhancement of T- cell reconstitution by Delta1ext-IgG-cultured cells is observed in mice infused with larger numbers of noncultured whole BM cells. Lethally irradiated Ly5.1-congenic Rag−/− mice received Ly5.1-syngeneic (1 × 105, 1 × 106, or 1 × 107) BM cells alone or supplemented with Ly5.2 Delta1ext-IgG-cultured cells (1 × 107) in 3 separate experiments. At 3 and 5 weeks after HCT, blood samples from lethally irradiated Rag−/− recipients injected with Delta1ext-IgG-cultured Ly5.2 cells (1 × 107) and Ly5.1 BM cells (1 × 105, 1 × 106, or 1 × 107) exhibited significantly higher proportions of Ly5.2 CD3+ and CD4+ T cells (Figure 5A–B). The proportion of CD8+ T cells derived from Delta1ext-IgG-cultured Ly5.2 cells was also significantly higher in mice injected with 1 × 105 Ly5.1 BM cells but not in those injected with 1 × 106 or 1 × 107 Ly5.1 BM cells (Figure 5C). At subsequent time points, CD3+, CD4+, and CD8+ cell reconstitution derived from Delta1ext-IgG-cultured cells was replaced to a significant extent by cells derived from noncultured BM cells. CD19+ B (Figure 5D) and myeloid (Figure 5E) cells were derived mostly from noncultured BM cells, with a limited contribution from Delta1ext-IgG-cultured cells. Over time, the percent of B-cell and myeloid engraftment derived from Delta1ext-IgG-cultured cells declined significantly. However, analysis of the BM at 15 weeks showed low levels of donor chimerism (< 0.5%), which could account for persistent low levels of myeloid cells present in the peripheral blood.
T-cell reconstitution by Delta1ext-IgG-cultured cells transplanted with increasing doses of whole BM. (A) Lethally irradiated Rag−/− recipients received 1 × 105, 1 × 106, or 1 × 107 Ly5.1 BM cells augmented with 2.5 × 107 Ly5.2 Delta1ext-IgG-cultured cells. FACs analysis and CBCs were performed on blood samples obtained at various times after HCT. Panels A to E show the absolute number of cells expressing CD3+, CD4+, CD8+, CD19+, and Gr1+ cells contained in blood samples from Rag−/− recipients that received, respectively, 1 × 105, 1 × 106, or 1 × 107 Ly5.1 BM cells augmented with 1 × 107 Ly5.2 Delta1ext-IgG-cultured cells. Graphs depict engraftment from BM cells (▵) and engraftment from Delta1ext-IgG-cultured cells (•). Data represent the mean plus or minus SEM of 3 separate experiments (n = 10-15).
T-cell reconstitution by Delta1ext-IgG-cultured cells transplanted with increasing doses of whole BM. (A) Lethally irradiated Rag−/− recipients received 1 × 105, 1 × 106, or 1 × 107 Ly5.1 BM cells augmented with 2.5 × 107 Ly5.2 Delta1ext-IgG-cultured cells. FACs analysis and CBCs were performed on blood samples obtained at various times after HCT. Panels A to E show the absolute number of cells expressing CD3+, CD4+, CD8+, CD19+, and Gr1+ cells contained in blood samples from Rag−/− recipients that received, respectively, 1 × 105, 1 × 106, or 1 × 107 Ly5.1 BM cells augmented with 1 × 107 Ly5.2 Delta1ext-IgG-cultured cells. Graphs depict engraftment from BM cells (▵) and engraftment from Delta1ext-IgG-cultured cells (•). Data represent the mean plus or minus SEM of 3 separate experiments (n = 10-15).
These data show that accelerated T-cell kinetics of CD3+ and CD4+ T cells by Delta1ext-IgG-cultured cells occurs when mice are given larger numbers of noncultured BM cells, suggesting that initial T-cell recovery can be improved even when large doses of HSCs are infused.
Identification of populations capable of thymic reconstitution
Recently, LSK cells have been identified as circulating progenitors with T-lineage potential, and these cells may represent the principal circulating progenitors with T-lineage potential.20 Thus, we tested the T-cell reconstituting potential of the cultured Sca-1+c-kit+ population. LSK cells cultured for 28 days with 4GF and Delta1ext-IgG were sorted into a fraction containing Sca-1+c-kit+ cells and another fraction containing the rest of the population (Figure 6A), and each population was then injected together with syngeneic Ly5.1 BM cells (1 × 105) into lethally irradiated Ly5.1-congenic C57BL/6 mice. At 3 and 5 weeks after HCT, both fractions were able to reconstitute T cells with no significant difference between the 2 groups (Figure 6B). The data show that T cells generated from the population enriched for Sca-1+c-kit+ cells were able to maintain the number of CD3+ cells in the peripheral blood up to 7 weeks, whereas the population of cells depleted of Sca-1+c-kit+ declined 5 weeks after HCT.
T-cell potential of Sca-1+c-Kit+ cells generated by culturing with Delta1ext-IgG. (A) Cultured LSK (Ly5.2) cells were stained for Sca-1 and c-Kit and sorted into a fraction containing Sca-1+ c-Kit+ cells and another fraction containing the rest of the population (Sca-1− cKit+, Sca-1+c-kit−, and Sca-1−c-kit−) and injected into lethally irradiated C57BL/6 mice with 1 × 105 Ly5.1-competing BM cells. (B) CBCs and FACS analysis were performed on blood samples at 3 weeks after HCT to determine the number of CD3+ cells generated in mice receiving either Sca-1+ c-Kit+ cells (○) or Sca-1−cKit+, Sca-1+c-kit−, and Sca-1−c-kit− (▿). Data represent the mean plus or minus SEM. (C) Cultured cells were gated for Sca-1– and c-kit–expressing cells and sorted according to the presence or absence of B220, CD25, Thy1, or Il7Rα expression. (D) Lethally irradiated C57BL/6 recipients received 1 × 106 Ly5.2 SK-lymphoid–positive cells and 1 × 105 Ly5.1 BM cells (closed bar) or 1 × 104 Ly5.2 SK-lymphoid–negative cells and 1 × 105 Ly5.1 BM cells (open bar). FACs analysis and CBCs were performed on blood samples obtained at various times after HCT. At 5 weeks, lymphoid (CD3, CD4, CD8, and CD19) and myeloid (GR1 and F4/80) engraftment were compared between the 2 groups. Data represent the mean plus or minus SEM.
T-cell potential of Sca-1+c-Kit+ cells generated by culturing with Delta1ext-IgG. (A) Cultured LSK (Ly5.2) cells were stained for Sca-1 and c-Kit and sorted into a fraction containing Sca-1+ c-Kit+ cells and another fraction containing the rest of the population (Sca-1− cKit+, Sca-1+c-kit−, and Sca-1−c-kit−) and injected into lethally irradiated C57BL/6 mice with 1 × 105 Ly5.1-competing BM cells. (B) CBCs and FACS analysis were performed on blood samples at 3 weeks after HCT to determine the number of CD3+ cells generated in mice receiving either Sca-1+ c-Kit+ cells (○) or Sca-1−cKit+, Sca-1+c-kit−, and Sca-1−c-kit− (▿). Data represent the mean plus or minus SEM. (C) Cultured cells were gated for Sca-1– and c-kit–expressing cells and sorted according to the presence or absence of B220, CD25, Thy1, or Il7Rα expression. (D) Lethally irradiated C57BL/6 recipients received 1 × 106 Ly5.2 SK-lymphoid–positive cells and 1 × 105 Ly5.1 BM cells (closed bar) or 1 × 104 Ly5.2 SK-lymphoid–negative cells and 1 × 105 Ly5.1 BM cells (open bar). FACs analysis and CBCs were performed on blood samples obtained at various times after HCT. At 5 weeks, lymphoid (CD3, CD4, CD8, and CD19) and myeloid (GR1 and F4/80) engraftment were compared between the 2 groups. Data represent the mean plus or minus SEM.
Evaluation of the Sca-1+c-kit+ population in our cultures showed that most (98%) of these cells expressed the lymphoid-associated antigens B220, IL7Rα, CD25, and/or Thy1 (Figure 6C) and are referred to as SK-lymphoid–positive cells as contrasted with SK-lymphoid–negative cells. To determine whether the T-cell reconstituting progenitors were enriched in either population, we transplanted proportionate numbers of SK-lymphoid–positive or SK-lymphoid–negative cells into lethally irradiated congenic C57BL/6 mice. Mice receiving sorted SK-lymphoid–positive cells (1 × 106) and syngeneic Ly5.1 BM cells (1 × 105) had significantly higher numbers of blood CD3+ cells than those receiving sorted SK-lymphoid–negative cells (1 × 104) and syngeneic Ly5.1 BM cells (1 × 105). The numbers of CD19+, GR1+, or F4/80+ cells did not differ significantly in the 2 groups (Figure 6D). Therefore, despite injecting 100-fold fewer cells, the SK-lymphoid–negative cells generated similar numbers of CD19+, GR1+, or F4/80+ cells as SK-lymphoid–positive cells. Although the SK-lymphoid–negative cells were capable of effective T-cell reconstitution, the SK-lymphoid–positive cells contributed significantly more CD3+ T cells since larger numbers of the SK-lymphoid–positive cells were generated.
Discussion
The data presented in this report describe a novel and clinically feasible ex vivo culture system using the Notch ligand Delta1ext-IgG for the generation of T-cell progenitors as a means to accelerate initial T-cell recovery after HCT. We observed accelerated thymic engraftment and higher numbers of T cells early after HCT in recipients of LSK cells cultured with Delta1ext-IgG compared with recipients of noncultured LSK cells. Augmenting the HSC graft with Delta1ext-IgG-cultured cells provided additional B and myeloid cells but the enhanced T-cell recovery was more profound. These results suggest that the accelerated early T-cell reconstitution by Delta1ext-IgG-cultured cells resulted from the generation of progenitor cells induced toward T-cell differentiation. Furthermore, T-cell reconstitution by Delta1ext-IgG-cultured cells transplanted with increasing doses of whole BM showed consistent acceleration in the generation of CD3+ cells and thymus-dependent CD4+ cells by Delta1ext-IgG-cultured cells. Since the slow recovery of marrow-derived CD4+ T cells directly correlates with increased risk of infection,8 acceleration of initial CD4+ recovery may have significant clinical implications. Although increasing the number of HSCs transplanted can accelerate the rate of T-cell reconstitution, our data suggest that the addition of progenitors differentiating toward the lymphoid lineage may provide further benefit.
The rate-limiting step for initial T-cell reconstitution after HCT could be either the number of thymic progenitors produced by the BM early after HCT or the number of thymic progenitors in the graft.9-11 In a murine model, Uchida et al10 showed that the most rapid engraftment was observed with a dose of at least 5000 syngeneic purified HSCs. Similarly, Chen et al11 demonstrated that the rate of T-cell recovery during the first 7 weeks after HCT depended on the dose of HSCs infused. After 8 weeks, the dose-dependent effect of T-cell reconstitution was not observed. Furthermore, despite injection of 5000 purified HSCs, significant numbers of donor-derived T cells were not detected in the blood until 4 weeks after HCT. These findings suggest that higher doses of HSCs may accelerate T-cell reconstitution, but there may be an upper limit for this effect.10,11 Alternatively, the limitation could be the capacity of the thymus to accept progenitors.39,40 However, our study clearly demonstrates that increasing the number of progenitors in the graft by the addition of Delta1ext-IgG-cultured progenitors to 5000 LSK cells accelerated T-cell reconstitution. We conclude that the accelerated recovery of T cells results from more input of precursors with T-cell potential into the thymus. This implies, however, that the acceleration of immune reconstitution with the use of cultured T-cell progenitors depends on thymic function. Accordingly, we would expect that the benefits of adding committed T-cell progenitors to the graft will be greater in younger individuals than in older individuals.
It is possible that the accelerated thymic reconstitution may have occurred because of the presence of multiple DN stages in the cultured cells. Studies by others have suggested that competition for a limited number of niches for each DN subset in the thymus may limit thymopoiesis.39-43 Thus, generation of several DN cell types at different stages during culture with Delta1ext-IgG may confer a competitive advantage over noncultured LSK cells for thymic reconstitution by engrafting additional niche sites. Furthermore, consistent with the competition for specific thymic niches is the improved rapid T-cell production in the SK-lymphoid–positive population, which contains the CD25+CD44+ DN cells.
LDA studies in irradiated mice show that culture with Delta1ext-IgG produced a multilog increase in the number of T-cell progenitors. The analysis of an irradiated recipient was limited by the continual seeding of donor-origin progenitors from the chimeric BM to the thymus, which obscured the quantitation of T-cell progenitors in the graft. Similar studies in nonirradiated recipients showed that T-cell reconstitution could be accelerated under circumstances where Delta1ext-IgG-cultured cells had a limited ability to repopulate the BM. In these experiments, we did not detect donor B-cell or myeloid cells in the blood samples from recipients of Delta1ext-IgG-cultured cells even when tested at 5 months after HCT, indicating that high levels of cultured cell engraftment in the BM had not occurred. We cannot exclude low-level engraftment of Delta1ext-IgG-cultured cells in the BM of nonirradiated recipients.
We found that the benefits of Delta1ext-IgG-cultured cells were significant during the first 5 weeks after HCT and then declined after 7 weeks, since noncultured BM cells were able to generate a significant number of T cells by this time. In lymphoid cell–deficient recipients, Delta1ext-IgG-cultured cells sustained significant numbers of T cells for up to 20 weeks after HCT. These results are consistent with data showing that the expansion of peripheral T cells was down-regulated in the presence of a functional thymus.44 Our data suggest that the decline of T cells derived from Delta1ext-IgG-cultured cells resulted from competition with marrow-derived progenitor cells. Furthermore, the addition of Delta1ext-IgG-cultured cells had no detrimental effect on the long-term generation of T cells by the BM graft. Thus, despite the short-term improvement, the rapid rise in the number of T cells generated by Delta1ext-IgG-cultured cells early after HCT may provide a means of improving early T lymphopenia until the BM graft can provide larger numbers of progenitors.
We found that both Sca-1+c-kit+–enriched and –depleted populations of cultured cells were capable of T-cell reconstitution. There is growing evidence that many discrete BM progenitors have the potential to become T cells,12,16,19,20,45 but the identity of cells that normally seed the thymus remains elusive. Our data are consistent with findings that multiple progenitors retain potential for T-cell development. We found that transplantation of the Sca-1+c-kit+–enriched population maintained higher levels of T cells (up to 7 weeks) than did transplantation of the Sca-1+c-kit+-depleted population, suggesting that the Sca-1+c-kit+ population had the potential for more sustained T-cell production. It has been reported that primitive LSK cells can sustain T-cell production after intravenous injection, whereas more committed progenitors cannot sustain T-cell production.17,18 Furthermore, BM LSK cells have a potential for a 1 000 000-fold population expansion after intrathymic injections, whereas more committed progenitors are capable of 100 000-fold population expansion.18,32,43,46 These data suggest that the Sca-1+c-kit+ population contained a more primitive precursor compared with the other subsets in our cultures.
Most of the cultured Sca-1+c-kit+ cells expressed lymphoid-associated antigens: B220, Thy1, IL7Rα, and/or CD25, (SK-lymphoid–positive cells). The SK-lymphoid–negative cells were rare, representing 1 in 100 cells in culture. The data imply, on a per cell basis, that the SK-lymphoid–negative population was the most efficient population for T-cell reconstitution and may have a higher expansion potential compared with the SK-lymphoid–positive population. Alternatively, the SK-lymphoid–negative population may be able to reconstitute the BM and provide additional progenitors to the thymus. This hypothesis is supported by the data that the SK-lymphoid–negative population had enhanced B-lymphoid and myeloid reconstitution potential on a per cell basis compared with the SK-lymphoid–positive population. Although most of the early T-cell reconstitution originated from the larger number of SK-lymphoid–positive population, this population may have limited BM repopulation and expansion potential. Nonetheless, SK-lymphoid–positive cells are potent T-cell progenitors and serve as more committed progenitors that preferentially reconstitute T cells, since B-cell enhancement was not observed with these cells. The data imply that the SK-lymphoid–negative population is less mature than the SK-lymphoid–positive population.
We have developed a method to increase the number of early progenitors that preferentially commit toward T-cell differentiation. Hematopoietic progenitors expanded ex vivo by culturing with Delta1ext-IgG have profound in vivo effects on accelerating T-cell reconstitution early after HCT. Our approach has clinical promise because it does not generate mature T cells ex vivo and does not increase the risk of graft-versus-host disease. In a clinical setting, augmentation of the graft with expanded progenitors induced toward T-cell differentiation may improve initial immune reconstitution in patients undergoing HCT.
Authorship
Contribution: M.H.D. designed and performed research, analyzed data, and wrote the paper; and B.V.F., P.J.M., and I.D.B. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Irwin D. Bernstein, Clinical Research Division, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D2-373, Seattle, WA 98109; e-mail: ibernste@fhcrc.org.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Stacey Dozono, David Flowers, Cynthia Nourigat, and Amanda Egge for expert technical assistance, and Colleen Delaney and Roland Walter for helpful criticism of the manuscript. We also thank Ted A. Gooley for statistical analysis. This work was supported by grants P50 HL54 881, T32CA09 351, and K12 CA076 930 from the National Institutes of Health. I.D.B. is an American Cancer Society–F. M. Kirby Clinical Research Professor.