Abstract
Outcomes for older adults with acute myelogenous leukemia (AML) are poor due to both disease and host-related factors. In this phase 2 study, we tested the oral farnesyltransferase inhibitor tipifarnib in 158 older adults with previously untreated, poor-risk AML. The median age was 74 years, and a majority of patients had antecedent myelodysplastic syndrome. Complete remission (CR) was achieved in 22 patients (14%); partial remission or hematologic improvement occurred in 15 patients, for an overall response rate of 23%. The median duration of CR was 7.3 months and the median survival of complete responders was 18 months. Adverse karyotype, age 75 years or older, and poor performance status correlated negatively with survival. Early death in the absence of progressive disease was rare, and drug-related nonhematologic serious adverse events were observed in 74 patients (47%). Inhibition of farnesylation of the surrogate protein HDJ-2 occurred in the large majority of marrow samples tested. Baseline levels of phosphorylated mitogen-activated protein kinase and AKT did not correlate with clinical response. Tipifarnib is active and well tolerated in older adults with poor-risk AML and may impart a survival advantage in those patients who experience a clinical response.
Introduction
Acute myelogenous leukemia (AML) is a clonal disease characterized by proliferation and accumulation of myeloid progenitors in the bone marrow, leading ultimately to hematopoietic failure. In older adults, the prognosis is particularly poor, owing to many factors, including high rates of induction failure, treatment-related mortality, adverse cytogenetics, and multidrug-resistance phenotype.1-7 Even for those patients who achieve complete remissions (CRs) through chemotherapy, the remission duration is generally on the order of only 6 to 9 months.1,6,8-10 For these reasons, novel, targeted, and less toxic agents are necessary to improve outcomes in elderly patients with AML.
Farnesyltransferase inhibitors (FTIs) are potent and selective inhibitors of intracellular farnesyltransferase (FTase), an enzyme that catalyzes the transfer of a farnesyl moiety to the cysteine terminal residue of a substrate protein.11 A host of intracellular proteins are substrates for prenylation via FTase, including Ras, Rho-B, Rac, Rheb, nuclear lamins, and centromeric proteins that interact with microtubules to promote the completion of mitosis.12 Interruption of prenylation may prevent substrates from undergoing maturation, which, in turn, may result in the inhibition of cellular events that depend on the function of those substrates. Although FTIs were initially developed on the premise that FTase inhibition would prevent the posttranslational processing of Ras proteins, they are by no means “selective,” because they can target proteins involved in disparate pathways and thereby exert effects on multiple mechanisms of cellular survival, including angiogenesis, cellular adhesion, and mitosis.13-20
There is rapidly developing experience in the use of FTIs in a wide range of hematologic malignancies, including AML, chronic myelogenous leukemia (CML), myelodysplastic syndromes (MDSs), and myeloproliferative disorders (MPDs).21-26 The oral bioavailability of these agents, along with an acceptable toxicity profile, has fostered their development. Tipifarnib (Zarnestra, R115777) is an oral, nonpeptidomimetic FTI that has been shown to have in vitro antitumor activity across a wide range of malignancies, including tumors with and without mutated ras.27 The first clinical testing of tipifarnib, and of FTIs in general, in hematologic malignancies was a phase 1 trial of tipifarnib administered for 21 days in patients with relapsed or refractory acute leukemias, primarily AML.23 In that study, clinical responses were observed in 29% of patients, along with a low incidence of serious (grade 3-4) nonhematologic toxicities, as well as achievement of therapeutic and sustained drug levels within leukemic bone marrow blasts. Intriguingly, responses were independent of ras mutational status, as none of the 34 leukemic samples demonstrated ras mutations. To follow up those observations, we conducted a multicenter phase 2 trial of tipifarnib in adults with poor-risk, previously untreated AML to further define its antileukemic activity and explore biologic end points that might predict response to this class of agents.
Patients, materials, and methods
Patient eligibility and selection
Adults, age 18 years or older, with pathologically confirmed (World Health Organization),28 previously untreated, “poor-risk” AML, MDS, or chronic myelomonocytic leukemia (CMML) were eligible. “Poor-risk” AML was defined as any of the following: (1) age 65 years or older, (2) adverse cytogenetic profile29 (eg, −5/5q, −7/7q, +8, abn 11q, complex ≥ 3 unrelated abnormalities), (3) AML arising from antecedent hematologic disorder (AHD), and (4) therapy-related AML. Trisomy 8 was included as an adverse karyotypic marker based on US cooperative group data that reported inferior outcomes in patients with this anomaly, particularly when occurring in combination with other abnormalities.30,31 Patients with MDS were eligible if the International Prognostic Staging System (IPSS) score was 1.5 or higher. Patients with CMML were eligible if bone marrow or peripheral blood blasts were 5% to 19%. All patients also had to meet the following criteria: Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0 to 2, normal bilirubin level, hepatic enzymes twice normal or less, serum creatinine 1.5 times normal or less, and left ventricular ejection fraction within the normal range. The trial was initiated in October 2001. In March 2004, the protocol was expanded to enroll 60 to 80 additional patients with poor-risk AML defined by one of the following criteria: (1) age 75 years or older and (2) age 65 years or older with AML arising from an antecedent hematologic disorder (eg, MDS). Results were analyzed as of December 31, 2005.
Patients were ineligible if they had a peripheral blast count of 30 000/μL or higher (cytoreduction with hydroxyurea was permitted up to 24 hours prior to beginning tipifarnib); acute promyelocytic leukemia (M3); disseminated intravascular coagulation; active central nervous system leukemia; concomitant radiation therapy, chemotherapy, or immunotherapy; coexisting medical or psychiatric conditions that could interfere with study procedures; or known allergy to imidazoles. Pregnant or lactating women were ineligible. The study was approved by the institutional review board of each treating center, and informed consent was given according to the Declaration of Helsinki.
Complete history and physical examination were performed within 3 days of study entry. Baseline hematologic and blood chemistry laboratory parameters were obtained within 3 days or sooner before entry. Additional studies (lumbar puncture with cerebrospinal fluid cytospin, computed tomography scans, gallium scans, multiple gated acquisition, or echocardiogram) were performed when clinically indicated.
Treatment schema
After the pretreatment bone marrow aspiration and other appropriate studies, patients received tipifarnib 600 mg twice daily by mouth for 21 days, followed by a rest period of up to 42 days to allow for recovery of peripheral blood counts. After the first cycle of therapy, if stable disease or clinical response (complete, partial, or hematologic improvement [HI]) was observed, patients were eligible to receive a second cycle of therapy. After the second cycle, subsequent cycles of therapy were permitted only if clinical response (complete, partial, or HI) was achieved. For patients achieving a complete remission (CR), up to 4 total cycles of tipifarnib were permitted. Patients achieving partial remission (PR) were eligible to continue to receive tipifarnib on an indefinite basis, provided that disease progression or unacceptable toxicity did not occur. Tipifarnib was permanently discontinued in the event of disease progression or grade 4 nonhematologic toxicity. Tipifarnib was temporarily withheld for any of the following toxicities: grade 2 to 3 neurologic toxicity; grade 3 nonhematologic toxicity (excluding alopecia, nausea and vomiting controlled with appropriate antiemetic therapy, and infection/febrile neutropenia); grade 4 granulocytopenia or thrombocytopenia < 20 × 109/L (20 000/μL) lasting > 3 weeks after completion of each cycle (day 42 of each cycle); and grade 2 to 3 nephrotoxicity. Dose modification of tipifarnib to 400 mg twice daily, and ultimately 200 mg twice a day, was permitted if the toxicities resolved to grade 1 or lower within 2 weeks of drug discontinuation.
Definitions of response
To assess response to tipifarnib, a bone marrow aspiration/biopsy was performed at the completion of each cycle of therapy, typically 7 to 21 days following the last dose of tipifarnib. CR was defined as bone marrow showing less than 5% myeloblasts with normal maturation of all cell lines, absolute neutrophil count (ANC) of 1.0 × 109/L (1000/μL) or higher, and a platelet count of 100 × 109/L (100 000/μL) or higher. PR was defined as the presence of trilineage hematopoiesis in the bone marrow with recovery of ANC and platelets to the stated levels, but with 5% to 19% bone marrow blasts, and at least a 50% decrease in bone marrow blast percentage from baseline. HI was defined as the same as PR, except with recovery of ANC to 0.5 × 109/L to 1.0 × 109/L (500-1000/μL) and platelet count to 20 × 109/L to 100 × 109/L (20 000-100 000/μL). Progressive disease (PD) was defined as any of the following: less than 50% increase in bone marrow blast percentage from baseline (> 5% blasts if baseline < 5%, > 10% blasts if baseline 5%-10%, > 20% blasts if baseline 10%-20%; > 50% increase in circulating blasts; new appearance of circulating blasts [on at least 2 consecutive occasions]); and development of extramedullary leukemia. Stable disease (SD) was defined as any response not meeting CR, PR, HI, or PD criteria.
Laboratory correlative studies
Bone marrow aspirate and buccal mucosal samples were obtained at baseline and at day 8 to assess FTase inhibition and ERK/AKT phosphorylation. Each bone marrow aspirate sample, on collection, was subjected to Ficoll-Hypaque gradient sedimentation for enrichment of the leukemic blast population.
Assessment of FTase inhibition in situ
To determine whether leukemic cell FTase activity was reliably inhibited by tipifarnib, we examined the farnesylation status of HDJ-2, a chaperone protein that undergoes farnesyl-dependent processing that can be detected as an electrophoretic mobility shift when FTase is inhibited.32 Bone marrow mononuclear cells (following Ficoll-Hypaque separation) were washed once with RPMI 1640 containing 10 mM HEPES (pH 7.4 at room temperature), counted, and solubilized for immunoblotting. Aliquots containing 50 μg total cellular protein were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose, and probed with monoclonal anti–HDJ-2 (Neomarkers, Fremont, CA).32 K562 human leukemia cells treated with diluent or R115777 served as negative and positive controls, respectively, for the presence of pre–HDJ-2.
To assay FTase inhibition in corresponding normal tissue, staining for prelamin A, a precursor protein that is processed in a farnesylation-dependent manner, was examined.32,33 Buccal smears obtained prior to treatment and on day 8 were air-dried and fixed in acetone within 3 hours of harvest. Samples were stored in buffer A (10% [wt/vol] powdered milk in 150 mM NaCl, 10 mM Tris-HCl [pH 7.4 at 21°C], 100 U/mL penicillin G, 100 μg/mL streptomycin, and 1 mM sodium azide) and assayed in batches. Samples were simultaneously stained with murine monoclonal anti–lamin A and rabbit anti–pre–lamin A antiserum that detects a peptide removed from prelamin A in a farnesylation-dependent manner.32,33 With each batch of samples, A549 lung cancer cells treated for 24 hours with 1 μM R115777 or diluent were included as positive and negative controls, respectively. After bound antibodies were detected with rhodamine-coupled anti–mouse IgG and fluorescein-coupled anti–rabbit IgG, slides were examined on a model LS310 confocal microscope (Zeiss, Rueil Malmaison, France) and photographed as previously described.32
Phosphorylation of signaling intermediates ERK 1/2 and AKT
To assess ERK 1/2 and AKT phosphorylation, light density bone marrow mononuclear cells were harvested and lysed after Ficoll-Hypaque density gradient centrifugation, lysed with buffer containing 50 nM Tris, pH 8.0, 120 mM NaCl, 0.5% Nonidet P-40, and protease inhibitor cocktail (Roche, Indianapolis, IN). The protein concentration of the lysate was determined by using the Bradford method (Bio-Rad, Hercules, CA). Equal amounts of protein were separated on a Novex 4% to 20% Tris-glycine gel (Invitrogen, Grand Island, NY) and transferred to a BioTrace PVDF membrane. Blots were probed with the designated primary antibody to phospho-AkT, Akt, or phospho-p44/42 MAPK, p44/42 MAPK (Cell Signaling Technology, Beverly, MA). After washing and incubating with secondary antibody, immunoreactive proteins were visualized by using the ECL Plus detection system (Amersham Biosciences, Piscataway, NJ). Densitometric analyses of immunoblots were performed by Scion Image. The results were quantified by densitometry of autoradiograms using National Institutes of Health Image and normalized to β-actin.
Statistical analysis
Best response was summarized using a frequency table. Kaplan-Meier estimates of the event-free survival were calculated for duration of CR and overall survival (OS). Median and the corresponding 95% confidence interval (CI) were provided. For OS, both univariate and multivariate analyses were performed. For univariate analysis, median and the corresponding 95% CI were provided for each risk factor using Kaplan-Meier estimates. For multivariate analysis, the effect of risk factors on OS was examined using the Cox proportional hazards model.
In the analysis to correlate baseline p-ERK and p-AKT expression levels with clinical response, for each outcome, mean, median, standard deviation, and range were calculated for each of the nonresponding and responding groups of patients. The outcomes were compared in nonresponders and responders using a Wilcoxon rank sum. The α level for determining statistical significance was 0.05.
Results
Patient demographics
A total of 171 patients were entered into the study, 158 (92%) of whom had AML. Thirteen of the 171 patients were diagnosed with MDS or CMML, and treatment outcomes in this group of patients will be discussed elsewhere. The characteristics of all 158 patients with AML are listed in Table 1. The marrow blasts ranged from 20% to 90%. None of the patients had favorable cytogenetics. Fourteen patients (9%) presented with peripheral blast counts greater than 30 000/μL and underwent cytoreduction with hydroxyurea prior to beginning tipifarnib. Nonhematologic comorbidities were present in 90% at the time of AML presentation, including 48% of patients with 3 or more comorbidities. Comorbidities consisted predominantly of cardiovascular disorders, but also included diabetes mellitus and genitourinary, gastrointestinal, musculoskeletal, and neurologic disorders. Thirty-eight patients (24%) had prior malignancy.
Response to treatment
All 158 patients with AML were included in the “intent-to-treat” analyses for response, although 13 patients (8%) were considered “not evaluable” due to being withdrawn from the study prior to the completion of one cycle of therapy, for reasons other than PD. The median number of cycles received was one (range, 1-7 cycles), with a median treatment duration of 52 days (range, 3-274 days). The median time from AML diagnosis to treatment was 8 days (range, 1-312 days). The median dose intensity as a percentage of planned dose intensity during cycle 1 was 100%. CR was achieved in 22 patients (14%; Table 2), with 17 achieving CR after cycle 1 and another 5 achieving CR after cycle 2. The median time to CR was 40.5 days (range, 31-121 days) and the median CR duration was 7.3 months (95% CI, 5.1-12.5 months). PR or HI occurred in an additional 15 patients, for an overall response rate of 23%. Fifty patients (32%) experienced stable disease, and 58 (37%) experienced PD during cycle 1 of tipifarnib therapy.
A detailed analysis of the 22 CR patients is delineated in Table 3. CRs were observed in traditional “poor-risk” patients, including those aged 75 years and older, those with MDS/AML, or those with unfavorable karyotypes including complex cytogenetics. Moreover, 15 patients (68%) had one or more nonhematologic comorbidities.
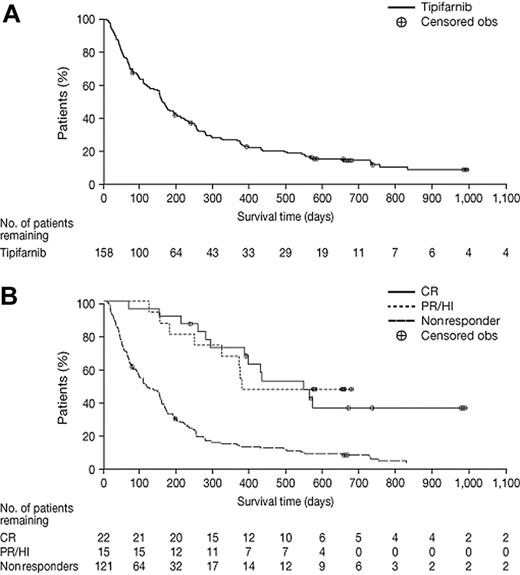
As depicted in Figure 1, the median survival of the entire cohort of 158 patients was 5.3 months (95% CI, 4.5-6.5 months). For patients achieving a CR, the median survival was 18.3 months (95% CI, 12.9-38.7 months). For patients achieving PR or HI, the median OS was 12.6 months. Response and survival by univariate analysis of pretreatment characteristics are summarized in Table 4. In the multivariate analysis, only age 75 years or older (P < .001), ECOG performance status 2 (P = .009), and adverse karyotype (P = .003) negatively affected survival. Interestingly, there was a trend toward favorable survival induced by an antecedent history of MDS (P = .067).
Kaplan-Meier plots of OS for all 158 AML patients and according to response. (A) OS for all 158 AML patients. (B) OS according to response (CR, PR/HI, and nonresponders).
Kaplan-Meier plots of OS for all 158 AML patients and according to response. (A) OS for all 158 AML patients. (B) OS according to response (CR, PR/HI, and nonresponders).
Retreatment with tipifarnib was permitted at the time of relapse for CR patients who had completed all planned cycles of initial therapy. To date, 10 patients have undergone retreatment at a median time of 10.2 months following completion of their initial course (range, 4.7-17.5 months). Three of these 10 achieved a second CR of 5 to 15 months' duration.
Toxicity
All 158 AML patients were evaluable for toxicity (Table 5)Tipifarnib-related nonhematologic serious (Common Toxicity Criteria [CTC] 2.0 grade 3-4) adverse events were observed in 74 patients (47%). Infectious, gastrointestinal (diarrhea, nausea/vomiting), and dermatologic (rash) serious adverse events were among the most frequent. Grade 3 or 4 neurologic toxicity, manifested primarily with confusion or ataxia, was seen in 13 patients (8%) and was rapidly reversible within 24 to 72 hours of drug discontinuation. Other less common serious toxicities included renal failure (4 patients), pancreatic enzyme elevations (6 patients), and fatigue (8 patients). A drug-related adverse event culminating in treatment termination occurred in 19 patients (12%).
Eleven patients (7%) died within 30 days of the final dose of tipifarnib from causes other than PD; however, only 1 patient died with renal failure attributed to tipifarnib toxicity.
A majority of patients (60%) required hospitalization at some point during treatment with tipifarnib (Table 6); however, the median time to hospitalization was 25 days. The median duration of hospitalization for tipifarnib-related complications or toxicities was 14 days (range, 2-58 days).
Biologic correlates
HDJ-2 and lamin A farnesylation.
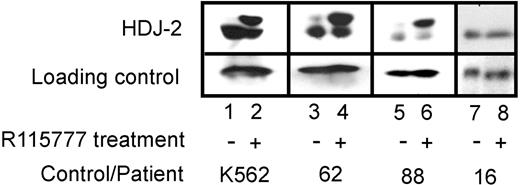
HDJ-2 and lamin A, 2 polypeptides that undergo farnesyl-dependent processing, were examined to assess whether FTase was being inhibited in situ by the administered treatment. Studies in blasts focused on HDJ-2 because lamin A expression in blasts is variable.34 Paired samples (day 0/day 8) from 57 patients contained sufficient cells for this analysis. As depicted in Figure 2, HDJ-2 underwent a clear-cut mobility shift in 43 (75%) of the day 8 samples, consistent with FTase inhibition. In contrast, no mobility shift was observed in 14 samples (25%), as exemplified by sample 88 in Figure 2. No CRs were observed in patients where blast FTase inhibition failed to occur, whereas the CR rate was 16% (7 of 43 patients) when blast FTase was inhibited. Although this difference did not reach statistical significance (χ2 = 2.6, P = .11), the trend suggested that inhibition of FTase might be critical for responses to occur. In an attempt to determine whether lack of FTase inhibition in blasts could also be observed in normal tissue, paired buccal mucosa samples (day 0/day 8) were evaluated for the presence of prelamin A, which is detectable by immunohistochemistry when FTase is inhibited.35,36 Of 49 evaluable samples, 45 (92%) demonstrated an increase in the unfarnesylated form of the product.
Effect of tipifarnib (R115777) on FTase-dependent processing of HDJ-2 in leukemic bone marrow samples. Heparinized bone marrow aspirates on day 0 (prior to tipifarnib treatment) and day 8 of therapy were fractionated on 2-step Ficoll-Hypaque gradients. Mononuclear cells were washed once with RPMI 1640 containing 10 nM HEPES (pH 7.4 at room temperature), counted, and solubilized for immunoblotting. Aliquots containing 50 μg total cellular protein were subjected to SDS-PAGE, transferred to nitrocellulose, and probed with monoclonal anti–HDJ-2 (Neomarkers, Fremont, CA). K562 cells treated with diluent or tipifarnib (R115777) served as negative and positive controls, respectively, for the presence of pre–HDJ-2.
Effect of tipifarnib (R115777) on FTase-dependent processing of HDJ-2 in leukemic bone marrow samples. Heparinized bone marrow aspirates on day 0 (prior to tipifarnib treatment) and day 8 of therapy were fractionated on 2-step Ficoll-Hypaque gradients. Mononuclear cells were washed once with RPMI 1640 containing 10 nM HEPES (pH 7.4 at room temperature), counted, and solubilized for immunoblotting. Aliquots containing 50 μg total cellular protein were subjected to SDS-PAGE, transferred to nitrocellulose, and probed with monoclonal anti–HDJ-2 (Neomarkers, Fremont, CA). K562 cells treated with diluent or tipifarnib (R115777) served as negative and positive controls, respectively, for the presence of pre–HDJ-2.
Inhibition of MAPK and AKT phosphorylation.
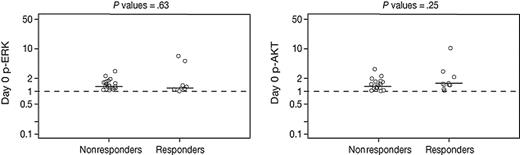
Because the Raf-MEK-MAPK and PI3K-AKT signaling pathways are putative downstream targets of FTIs, we investigated whether tipifarnib had an impact on the phosphorylation (activation) patterns of these signaling intermediates, and whether the baseline phosphorylation levels might correlate with clinical responsiveness to tipifarnib. As shown in Figure 3, there was no significant difference between baseline phosphorylation levels of MAPK and AKT among responders and nonresponders. Compared to baseline, the level of phosphorylated MAPK decreased in only 8 of 24 evaluable patients, whereas the level of phosphorylated AKT decreased in only 11 of 24 evaluable patients (data not shown). A correlation between clinical responsiveness to tipifarnib and a decrease in either phosphorylated MAPK or AKT at day 8 was not detected.
Baseline levels of p-ERK and p-AKT in responders versus nonresponders. Baseline (pretreatment) expression levels of phosphorylated ERK and AKT from bone marrow samples were measured and normalized, as described in “Patients, materials, and methods.” No difference in baseline expression levels of p-ERK or p-AKT was detected between responders and nonresponders. Horizontal bars represent median p-ERK and p-AKT normalized levels.
Baseline levels of p-ERK and p-AKT in responders versus nonresponders. Baseline (pretreatment) expression levels of phosphorylated ERK and AKT from bone marrow samples were measured and normalized, as described in “Patients, materials, and methods.” No difference in baseline expression levels of p-ERK or p-AKT was detected between responders and nonresponders. Horizontal bars represent median p-ERK and p-AKT normalized levels.
Discussion
AML is a heterogeneous disease associated with a poor prognosis. Certain clinical and biologic features of this disease predict for especially poor outcomes, including older age, presence of specific karyotypes, and properties of multidrug resistance.3,4,37,38 In these poor-risk settings, the likelihood of disease-free survival at 1 year is less than 20%. With respect to older patients (≥ 65 years) with AML, particularly those aged 75 years or older, 2 recent large retrospective analyses by the Southwest Oncology Group and the MD Anderson Cancer Center demonstrated very poor median OS durations, particularly in the 75 and older age group, where survival was on the order of 3 to 4 months.39,40 Such poor outcomes reflect low initial remission rates, high treatment-related toxicity and mortality, and a high likelihood of relapse,6,7,10,41-43 clearly highlighting the need for novel therapies in older patients.
Within the cohort of older (≥ 65 years) patients with AML, there are unique subgroups for whom optimal therapy remains to be determined. One such group is the very elderly (75 years and older), who are frequently underrepresented in clinical trials, yet in whom the incidence of AML is higher than other age groups. These very elderly patients, as outlined, appear to have an exceptionally poor prognosis with chemotherapy.39,40 Although systematic exclusion of elderly patients from receiving intensive chemotherapy is not justified, a strong argument for devising alternative therapeutic strategies can be made for this group. A second group of older AML patients with particularly poor prognostic features are those with “secondary AML,” including those with an AHD. Adverse karyotype and multidrug resistance phenotype are common features within this subgroup, which likely contribute to poor prognosis. Some clinical trials and reviews of AML in the elderly have identified the presence of AHD as an independent negative prognostic factor.2,40,44 Although most larger studies of AML in the elderly do not specifically exclude patients with AHD, they comprise a minority of the patients, usually on the order of 20% to 30% of the total population enrolled.
In this unique phase 2 trial, we administered tipifarnib to primarily older patients with previously untreated AML, the large majority of whom carried the notably poor-risk features of age older than 75 years or antecedent history of MDS. As such, this trial stands as one of the few of its kind that has focused nearly exclusively on a poorly served AML population. Interestingly, we observed CRs in 14% of evaluable AML patients, and an additional 10% of patients achieved either PR or HI. Achievement of CR appeared to impart a major survival benefit, particularly in comparison to the short median survival for those patients in whom no response occurred. In addition, patients who achieved a PR or HI appeared to survive for longer than those without any response, although the actual numbers for this analysis are small and will certainly require confirmation in a larger study. In the multivariate analysis, the presence of a worse performance status, age older than 75 years, and adverse karyotype were independent prognostic factors for inferior OS. In addition, there was a trend toward improved survival for patients whose leukemia had evolved from a previous MDS. Nonetheless, meaningful responses, including CRs, were clearly detected in such traditionally poor-risk patients.
Another important end point to this study was the determination of treatment-related toxicity. We observed serious (grade 3 or 4) drug-related nonhematologic toxicity in 42% of patients. Although these toxicities involved several organ systems, the ones more frequently encountered were infection, gastrointestinal disturbances (including diarrhea and pancreatic enzyme elevation), renal insufficiency, and skin rash. Neurologic toxicity was rare, yet rapidly reversible when present. Importantly, the non–disease-related early mortality rate was only 7%, a figure that compares favorably with death rates associated with induction chemotherapy in an elderly cohort of patients. In addition, because tipifarnib was administered orally, the median number of days spent in the hospital was low, and only a minority of patients required hospitalization for reasons that were considered attributable to tipifarnib.
We performed several correlative laboratory studies in an attempt to gain insight into the antileukemic mechanisms of tipifarnib as well as to study potential predictors of response. Specifically, we examined HDJ-2 farnesylation as a surrogate for FTase inhibition and also inspected the patterns of ERK and AKT phosphorylation in pretreatment and posttreatment samples. Whereas HDJ-2 farnesylation was inhibited following drug therapy in the majority of leukemia samples tested, there was a subset of patients in whom blast FTase inhibition could not be detected. Importantly, CRs did not occur in these patients, suggesting that inhibition of FTase is necessary (but not sufficient) for responses to occur. Although there are multiple potential explanations for the failure of FTase to be inhibited, including phenotypic variations in R115777 metabolism or sensitivity of FTase to inhibition, the observation that failure of FTase inhibition occurs in 25% of AML isolates but only 8% of normal tissue samples (χ2 = 5.0, P = .025) raises the possibility of a cell-intrinsic change such as a posttranslational modification of FTase or an alteration in drug accumulation. Further study is required to confirm these observations in a larger cohort of patients and examine potential causes. If these observations are confirmed in subsequent studies, it might become possible to prospectively identify at least one cohort of patients who have a particularly low probability of benefiting from this therapy.
Further studies focused on 2 signaling pathways downstream of Ras. There was no correlation between clinical response and baseline presence of p-ERK and p-AKT. In addition, there was no association between clinical response and a decrease in the amount of p-ERK or p-AKT following treatment. Based on these findings, it appears unlikely that FTIs are targeting a specific signaling pathway, but rather may be influencing a host of intracellular events that are driven by several farnesylated products.
In summary, tipifarnib has clinical activity and is well tolerated in older adults with AML. Importantly, tipifarnib appears to impart a survival advantage in those patients who experience a clinical response. The relatively low toxicity profile may also allow for extended therapy to maintain disease control, quality of life, and possibly survival, even if full response is not achieved. This hypothesis is currently being explored as part of a multicenter, randomized, phase 3 trial in elderly adults with AML, comparing tipifarnib with best supportive care. In reference to its potential use on a prolonged basis, tipifarnib in maintenance therapy during minimal residual disease (ie, CR) is currently being explored in several trials. Much remains to be learned to optimize such therapy in patients with AML. To this end, trials that combine tipifarnib with other agents (including cytotoxics) and that use different dosing schedules are ongoing. Although the precise role of tipifarnib administered with, or in place of, other more established therapies remains to be determined, it appears that its value as a single agent may be important in selected groups of patients.
Authorship
Contribution: J.E.L. and J.E.K. designed the study, were the principal investigators, and wrote the paper; I.G., J. Gotlib, E.J.F., J.L.L., P.L.G., and L.M. are the co-investigators and assisted in writing the paper; S.K., A.A.A., and J.L.L. performed and analyzed laboratory correlates; L.B. performed laboratory correlates; J. Greer coordinated the study; Y.P. and E.G.M. performed statistical analyses; and J.J.W. participated in the design of the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
I.G. and J. Gotlib contributed equally to the paper.
Correspondence: Jeffrey E. Lancet, H. Lee Moffitt Cancer Center & Research Institute, 12902 Magnolia Dr, Tampa, FL 33612; e-mail: lancetje@moffitt.org.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors wish to recognize the important contributions from several outstanding clinical research nurses: Kathy Dugan, RN; Maureen Klein, RN; Patti Messina, RN; Rachel Bartash, RN; and Anthony Young, RN. We also thank the following individuals: Chaohui Lu and Karen Rosell for technical assistance with correlative assays; James Loss for valuable assistance in preparing the manuscript; Mary Ellen Rybak and Alain Thibault, from Johnson & Johnson Pharmaceutical Research and Development, for their support of this trial; Dr Joseph Rosenblatt and Dr Ivan Horak for their key contributions toward the early-phase development of tipifarnib in acute leukemias; and finally, the patients (and their families) who participated in this study.
This work was supported by the National Cancer Institute Cooperative Agreement U01 CA69854 (J.E.K., I.G., J. Greer), National Cancer Institute Cooperative Agreement U01 CA70095 (J.E.K., J. Greer), Douglas Kroll Research Foundation Translational Research Grant of the Leukemia and Lymphoma Society of America N6030-04 (J.L.L., J.E.L.), American Cancer Society grant RSG CCE-102610 (A.A.A.). Johnson & Johnson Pharmaceutical Research & Development provided partial support to the sites for supplemental data collection that was required for completion of the study and submission of the results for review by health authorities.