Abstract
Lymphocyte extravasation requires that emigrating cells process chemoattractant signals, typically mediated by chemokines, encountered on endothelial surface (apical) and subendothelial (basal) compartments. These signals are delivered under conditions of hemodynamic shear, a fundamental feature of all physiologic leukocyte–endothelial interactions. To analyze lymphocyte responsiveness to spatially distributed chemokines and their effects on transendothelial migration (TEM) under hydrodynamic shear, we constructed a transwell-based flow assay. We observed that the inflammatory chemokine CCL5 (RANTES) induces negligible human T-cell migration across inflamed human umbilical vascular endothelial cells (HUVECs) when displayed alone in the subendothelial compartment under static or hemodynamic shear conditions or when combined with apical CXCL12 (SDF-1α) under static conditions. However, under shear stress, T cells encountering apically presented CXCL12 were primed to undergo robust LFA-1–dependent TEM toward subendothelial CCL5. Notably, locomotive T cells arriving at endothelial junctions were retained and extended pseudopodia into and through the junctions, thereby increasing sensitivity to subendothelial CCL5. These findings provide the first evidence that lymphocytes integrate, conditional to shear forces, permissive apical chemokine deposits, and integrin engagement signals, resulting in morphologic changes and amplified chemotaxis to an otherwise weak subendothelial chemokine signal.
Introduction
The multistep paradigm of leukocyte recruitment holds that extravasating leukocytes receive biochemical signals in the milieu that trigger integrin-dependent leukocyte arrest and locomotion on and across the endothelium under fluid shear conditions.1 These key signals are typically delivered by chemokines that engage their relevant G-protein–coupled receptors on the leukocyte surface.2,3 The discrete distribution of chemokines in luminal and perivascular areas provides critical tissue-specific signals directing transendothelial migration (TEM) of leukocytes.2,4-7 Although it is widely believed that leukocyte TEM occurs at or near their original site of recruitment on the vessel surface, little is known about how physiologic shear forces regulate diapedesis or how apical (endothelial surface) and subendothelial chemokine deposits affect this process temporally and spatially.
Most studies of leukocyte TEM make use of the transwell/Boyden chamber assay, which measures the movement of leukocytes under static (ie, no flow) conditions through a membrane or endothelial barrier in response to chemokines typically placed in the lower well. Although this system is useful for assessing chemotactic responses, its inability to recapitulate hemodynamic shear makes it a poor model for analyzing chemokine effects on leukocyte–endothelial interactions. Other reports have used the parallel-plate flow chamber system to study leukocyte arrest and TEM under simulated blood flow conditions,8-10 but this approach is limited in that chemokines can only be presented apically. Several reports have indicated that chemokines may function in a promigratory or a repulsive fashion, depending on the particular chemokine and its local concentration.11-14 These findings unveil an increasing complexity of chemokine biology, with resultant leukocyte responses reflecting a tapestry of chemokine effects. Recent studies showing that CXCL12 is displayed on the endothelial cell surface in association with heparan sulfate,15 together with studies detailing the in vivo presentation of chemokines along and beneath microvessels,7 suggest that certain chemokines may preferentially induce cell adherence interactions on the endothelium while others expressed beneath the endothelium and in cell junctions may promote TEM. Although intravital microscopy and other in vivo imaging techniques show that chemokines and adhesion molecules combine to direct transmigration,16,17 these in vivo techniques cannot reconcile how chemoattractants cooperate to promote tissue and leukocyte subset–specific TEM.
In this study, we sought to analyze the effect of apical CXCL12 on T-cell TEM in response to a subendothelial chemokine signal under hemodynamic shear conditions. To this end, we developed a transwell-based flow assay for studying the effects of fluid shear forces and apical and subendothelial chemokines on lymphocyte TEM in real time (Figure 1A). With this system, we observed that the inflammatory chemokine CCL5 (RANTES) induces little human T-cell migration across TNFα–stimulated human umbilical vascular endothelial cells (HUVECs) when displayed alone in the subendothelial compartment under static or flow conditions. However, deposits of apical CXCL12 at levels that do not promote significant T-cell TEM under shear or static conditions permissively synergize with subendothelial CCL5 to stimulate highly efficient TEM, but only under shear stress. Conspicuously, fluid shear stress triggers formation of pseudopodial projections of T cells into endothelial junctions, resulting in β2 integrin engagement and efficient chemotaxis toward the subendothelial chemokine. These results expand our current understanding of chemokine and integrin mechanosignaling and provide the first evidence that physiologic shear flow promotes combinatorial responses to sequential heterologous chemokine signals during TEM.
Materials and methods
Antibodies and reagents
Murine mAbs to human CCR5 (2D7, IgG2a) and CXCR4 (12G5, IgG2a) were obtained from BD Biosciences (San Jose, CA). TNF-α, anti–human ICAM-1 (AF720, sheep IgG), anti-CD49d (2B4, a VLA-4 function–blocking mouse IgG1) mAbs, and the recombinant human chemokines CCL5 and CXCL12 were obtained from R&D Systems (Minneapolis, MN). Murine anti–human CD11a mAb (TSI/22, an LFA-1 function–blocking IgG1) were obtained from Pierce (Rockford, IL). CFDA SE Cell Tracer was obtained from Molecular Probes (Eugene, OR). Fibronectin was obtained from Sigma (St Louis, MO), and pertussis toxin (PTX) was obtained from Calbiochem (La Jolla, CA). Blood was obtained from healthy volunteers after informed consent in accordance with the Declaration of Helsinki and under a protocol approved by the institutional review board of the Brigham & Women's Hospital (Partners Human Research Committee).
Cell isolation and culture
Human peripheral blood lymphocytes (PBLs) were isolated from citrated whole blood by Histopaque-1077 (Sigma) density-gradient centrifugation under pathogen-free conditions. CD3+ T cells were enriched on sterile nylon-wool columns (Polysciences, Warrington, PA), as described,18 or were isolated using a pan-T-cell negative selection system and AutoMACS separator (Miltenyi Biotec, Bergisch Gladbach, Germany; greater than 99% CD3+ cells by flow cytometry). T cells were maintained in RPMI-1640 (Gibco Life Technologies, Grand Island, NY) with 10% fetal bovine serum (FBS) (Sigma), penicillin (100 U/mL), and streptomycin (100 μg/mL; Life Technologies), as described.8 HUVECs, isolated as described,19 were obtained from the Vascular Biology core facility of the Department of Pathology of Brigham & Women's Hospital and were cultured in medium 199 (BioWhittaker, Walkersville, MD) supplemented with 20% FBS (Sigma), 2 mM L-glutamine (BioWhittaker), 100 U/mL K-penicillin G and 100 μg/mL streptomycin sulfate (Gibco), 100 μg/mL heparin (Sigma), and 50 μg/mL endothelial cell growth supplement (ECGS; Biomedical Technologies, Cambridge, MA).
Transwell chemotaxis assays
HUVECs were cultured in the upper chamber of a fibronectin-coated transwell insert (5.0-μM pores; Costar, Corning, NY). Before each experiment, monolayer confluence was confirmed by fluorescence staining with CFDA SE Cell Tracer, and cells were stimulated with TNF-α (2 ng/mL; 200 U/mL) for 12 hours. CCL5 (100 ng/mL) was added to the lower chamber (100 ng/mL), and T cells were immediately added in TEM medium (Hanks balanced salt solution [Gibco], 1 mM Ca2+, 1 mM Mg2+, 10 mM HEPES [Cellgro; Mediatech, Herndon, VA], and 2 μg/mL BSA [Sigma]) to the upper chamber, at 5 × 104 per well. Medium from the lower chamber was collected after 3 hours at 37°C, and harvested cells were counted.
Real-time transendothelial migration analysis in a transwell flow chamber
The chamber is depicted in Figure 1A (for detailed descriptions, see Videos S1 and S2, available on the Blood website; see the Supplemental Videos link at the top of the online article). HUVECs (passage 2 or 3) were plated at 50% confluence and then cultured on the underside (Figure 1B) of fibronectin-coated transwell inserts (transwell-clear, 0.4-μM pores) for an additional 48 hours to allow complete confluence. Confluent HUVECs (verified by microscopy) were stimulated for 12 hours before each experiment with TNF-α (2 ng/mL). HUVEC-coated transwell inserts (Figure 1A) were then placed into the flow chamber and mounted on the stage of an overhead phase-contrast microscope (Eclipse E-600; Nikon, Tokyo, Japan). The insert was then rinsed and filled with TEM medium alone or with 100 ng/mL of the chemokine of interest. The HUVEC-coated side was overlaid with CXCL12 (apical presentation at 0, 5, or 10 ng/mL, as stated in the figure legends, for 2 minutes) and was washed extensively before T-cell perfusion (3 × 106/trial) at defined shear stress generated by syringe pump (Harvard Apparatus, Natick, MA). All experiments were performed at 37°C. The perfusion period was recorded in real time (SVO-2100 VHS video; Sony, Tokyo, Japan). For all experiments, T cells were perfused in TEM medium into the chamber and allowed to settle for 1 minute over the HUVEC monolayer (settling phase), thus allowing proximate apposition of the cells to the endothelium within the dimensions of the flow chamber but of insufficient duration to elicit any adherence. Shear stress was then adjusted to 1.0 dyne/cm2 for 2 minutes to allow lymphocyte accumulation on the monolayer (accumulation phase) and then was increased to 2.0 dyne/cm2, a physiologic level previously found to support TEM.8 This level of shear stress either was maintained for 7 more minutes (continuous shear conditions) or was halted for the same period (shear-free conditions). Motion analysis and TEM determination were performed manually, as described,8 by an observer blinded to the experimental conditions used. Four distinct categories of T cells were described: (1) detaching or (2) stationary/arrested during the migration phase and locomotive from the site of an initial stationary attachment (3) without or (4) with subsequent TEM, as previously described.8 Coordinate trajectories of these locomotive T cells were analyzed from stacked digital AVI images (ImageJ [nonproprietary] and Excel [Microsoft, Redmond, WA] software) (Figure 4A). T cells not present in the field of view (550 μm × 370 μm) for the duration of the trial were not scored. Transwell chemotaxis assays (static) and transwell flow chamber assays were performed in parallel for T cells obtained from each donor.
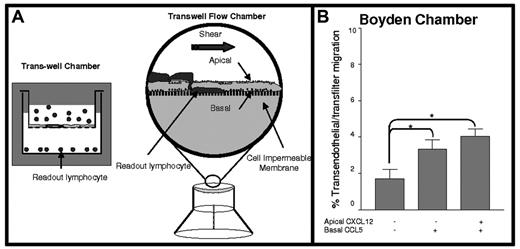
Schematic presentation of modified transwell chamber assay system for monitoring lymphocyte TEM under flow. (A, left) Classical Boyden assay setup (Trans-well Chamber) wherein lymphocyte chemotaxis across endothelial barrier (or filter) toward a chemoattractant in the lower chamber is quantified (lymphocyte readout). (A, right) Modified transwell chamber wherein lymphocytes are perfused over an endothelial monolayer with or without apical chemokines, with additional chemoattractant(s) introduced beneath the endothelium (basal presentation). TEM is measured in real time by video microscopy under fluid shear conditions (TEM is quantified as lymphocytes collecting underneath the endothelium). (B) T-cell chemotaxis across TNF-α–activated HUVECs toward 100 ng/mL CCL5 introduced in the lower chamber under static conditions. Apical CXCL12 (10 ng/mL) was overlaid for 5 minutes and then was washed before lymphocyte placement. Data are mean ± SEM from 3 independent experiments. *P < .05.
Schematic presentation of modified transwell chamber assay system for monitoring lymphocyte TEM under flow. (A, left) Classical Boyden assay setup (Trans-well Chamber) wherein lymphocyte chemotaxis across endothelial barrier (or filter) toward a chemoattractant in the lower chamber is quantified (lymphocyte readout). (A, right) Modified transwell chamber wherein lymphocytes are perfused over an endothelial monolayer with or without apical chemokines, with additional chemoattractant(s) introduced beneath the endothelium (basal presentation). TEM is measured in real time by video microscopy under fluid shear conditions (TEM is quantified as lymphocytes collecting underneath the endothelium). (B) T-cell chemotaxis across TNF-α–activated HUVECs toward 100 ng/mL CCL5 introduced in the lower chamber under static conditions. Apical CXCL12 (10 ng/mL) was overlaid for 5 minutes and then was washed before lymphocyte placement. Data are mean ± SEM from 3 independent experiments. *P < .05.
Transmission electron microscopy
To analyze the morphology of T cells adherent to HUVECs, T cells were perfused according to the settling and accumulation phases (described in “Real-time transendothelial migration analysis in a transwell flow chamber”) followed by 2 minutes of exposure to either physiologic shear (2.0 dyne/cm2) or 2 minutes of shear-free conditions on chemokine-overlaid monolayers. Subsequently, chambers were perfused with 3% glutaraldehyde, 2% paraformaldehyde in 0.1 M cacodylate buffer. Sample preparation and electron microscopy were performed as described.8 Analysis of T cell–endothelial cell interactions was performed only where lymphocyte morphology was confirmed by nuclear identification using AnalySIS software (Soft-Imaging Software, Münster, Germany).
Statistical analysis
Data and SEM were compiled using GraphPad (San Diego, CA) Prism 2.01 software. All comparisons were carried out using one-way ANOVA, and statistical significance of P below .05, or .01 was denoted by asterisks as appropriate.
Results
Boyden chamber studies
T-cell chemotaxis induced by CXCL12 and CCL5—2 chemokines known to support lymphocyte chemotaxis and TEM20 —was measured in the Boyden chamber assay. By flow cytometry, the expression of CXCR4 and CCR5 on freshly isolated peripheral blood T cells ranged from approximately 60% to 80% and approximately 15% to 30%, respectively, with dual expression ranging from approximately 15% to 25% of cells. CCL5 (100 ng/mL) introduced in the subendothelial compartment triggered low-level migration of T cells across filters coated with TNF-α–stimulated HUVEC monolayers (Figure 1B). Significantly, the introduction of apical CXCL12 at levels known to trigger lymphocyte motility over endothelial surfaces under shear conditions8 did not enhance T-cell migration toward CCL5 under static conditions (Figure 1B).
Real-time PBL migration analysis using a novel flow chamber
To investigate how shear stress and combinatorial chemokine signals contribute to TEM, we used a new transwell system allowing real-time imaging under flow conditions (Figure 1A). Viewed under real time with video microscopy and recording for off-line analysis, the response of T cells to chemokine signaling was evaluated by discrete parameters: detachment, stationary arrest, locomotion, and TEM. Detachment was defined by T cells that made rolling or stationary contact with the endothelial surface and then was released during the period of observation. Stationary arrest was defined by T cells that made rolling contact, adhered firmly, and remained fixed for the duration of the assay. Locomotion was defined by T cells that made rolling contact, adhered to the endothelium, and displayed motility (ameboid movement) across the surface of the endothelium during the assay. TEM was defined by T cells that made rolling contact, adhered to the endothelium, underwent locomotion, and migrated beneath the surface of the endothelium through an intercellular junction.
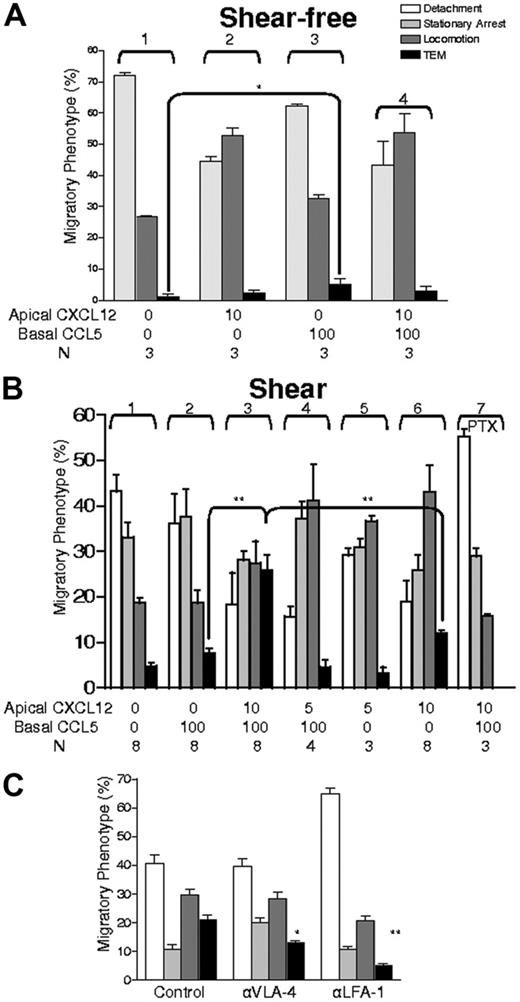
We observed that in the absence of shear stress, the level of TEM obtained in the flow chamber was not different from that found in the Boyden assay (compare Figures 1B and 2A; Video S1). Notably, as viewed by microscopy, subendothelial CCL5 did not increase T-cell arrest or locomotion over the endothelial surface (Figure 2A; compare groups 1 and 3). In contrast, the introduction of apical CXCL12 increased T-cell locomotion over the level observed with control (no chemokine) or with subendothelial CCL5 under shear-free (static) conditions (Figure 2A; compare groups 1-3) and under shear conditions (eg, Figure 2B; compare groups 1 and 2 with 5 and 6). Despite increased locomotion in the presence of apical CXCL12, there was no evident increased transmigration under both static conditions. Importantly, enhanced T-cell localization at intercellular junctions was observed with apical CXCL12 under both static and continuous shear conditions, yet there was no increase in T-cell TEM toward subendothelial CCL5 under static conditions (Figure 2A; compare groups 3 and 4). Thus, in contrast to a report indicating that monocyte locomotion to intercellular junctions correlates with enhanced TEM,21 facilitated locomotion of T cells over apical endothelial surfaces and scanning of potential interendothelial sites of diapedesis are insufficient to promote endothelial crossing. However, when T cells and endothelial monolayers were exposed to continuous physiological shear stress, high levels of TEM were observed within a 7-minute period (compare Figure 2A group 4 with Figure 2B group 3; Video S2). This robust TEM required the presence of subendothelial CCL5 (Figure 2B; compare groups 3 and 6; P < .05), together with a critical (more than 5 ng/mL) input density of apical CXCL12 (Figure 2B; compare groups 3 and 4). Notably, this rapid and efficient TEM was not observed when the apical CXCL12 level was below a threshold level despite optimal subendothelial CCL5 and despite significant CXCL12-triggered locomotion of T cells toward endothelial junctions (Figure 2B; groups 3-5). The use of a 10 ng/mL concentration of apical CXCL12 was based on initial titration studies showing that intermediate concentrations between 5 and 10 ng/mL yielded inconsistent responses in transmigration observed among T-cell populations from different donors, likely reflecting the individual T-cell variability of CXCR4 expression. However, the effects on transmigration were uniformly observed at 10 ng/mL CXCL12 among T cells from all donors, thus defining an operational threshold for native T-cell populations as a whole and eliminating donor-to-donor variability. Pretreatment of T cells with the CXCR4-neutralizing antibody 12G5 resulted in the abrogation of transmigration to levels equivalent to those observed in the absence of CXCL12, with markedly diminished adherence and locomotion (data not shown). Consistent with results previously reported,9 perfusion of the CXCL12-bearing endothelium with buffer before the introduction of T cells did not induce TEM under shear-free conditions, ruling out the redistribution of apical CXCL12 to subendothelial compartments or shear-induced changes in endothelium as the reason for the observed increased TEM under continuous shear stress. TEM triggered by apical CXCL12 and subendothelial CCL5 was abrogated by PTX pretreatment (100 ng/mL) of T cells (Figure 2B; group 7), indicating that Gi-protein signaling downstream of chemokine receptor engagement is crucial for TEM.
Effect of apical CXCL12 and shear on lymphocyte TEM toward subendothelial (basal) CCL5 and contribution of α4 or αL integrins. (A) T cells in contact with TNF-α–activated HUVECs under shear were observed during a shear-free (static) period in the presence or absence of specified chemokines (note that rate of detachment was not measured in this analysis because the objective was to assess TEM of adherent cells). (B) Lymphocytes contacting endothelium were analyzed for the indicated migratory categories under continuous shear flow. Concentrations of apical CXCL12 are indicated and were presented with or without basal CCL5 (100 ng/mL). For inhibition of Gi proteins, lymphocytes were incubated overnight in culture medium with 100 ng/mL PTX. (C) Effects of blocking α4 (VLA-4) or αL (LFA-1) integrins on the indicated adhesive and migratory phenotypes of T cells. Cells were pretreated with indicated blocking mAbs (10 μg/mL) for 10 minutes and were perfused into the chamber in medium containing 1 μg/mL of the respective mAbs and chemokine configurations identical to those used in panel B group 3. Migratory phenotypes developing under persistent shear stress were analyzed as in Figure 1. Data are mean ± SEM from 4 experiments that used different lymphocyte donors and HUVEC preparations. *P < .05; **P < .01.
Effect of apical CXCL12 and shear on lymphocyte TEM toward subendothelial (basal) CCL5 and contribution of α4 or αL integrins. (A) T cells in contact with TNF-α–activated HUVECs under shear were observed during a shear-free (static) period in the presence or absence of specified chemokines (note that rate of detachment was not measured in this analysis because the objective was to assess TEM of adherent cells). (B) Lymphocytes contacting endothelium were analyzed for the indicated migratory categories under continuous shear flow. Concentrations of apical CXCL12 are indicated and were presented with or without basal CCL5 (100 ng/mL). For inhibition of Gi proteins, lymphocytes were incubated overnight in culture medium with 100 ng/mL PTX. (C) Effects of blocking α4 (VLA-4) or αL (LFA-1) integrins on the indicated adhesive and migratory phenotypes of T cells. Cells were pretreated with indicated blocking mAbs (10 μg/mL) for 10 minutes and were perfused into the chamber in medium containing 1 μg/mL of the respective mAbs and chemokine configurations identical to those used in panel B group 3. Migratory phenotypes developing under persistent shear stress were analyzed as in Figure 1. Data are mean ± SEM from 4 experiments that used different lymphocyte donors and HUVEC preparations. *P < .05; **P < .01.
Successful lymphocyte TEM across inflamed endothelium has been shown to depend on both VLA-4 and LFA-1 for adherence and motility.22-24 Using the combination of apical CXCL12 (10 ng/mL) and subendothelial CCL5 (100 ng/mL) under continuous fluid shear, function-blocking antibodies to VLA-4 and LFA-1 eliminated T-cell TEM and decreased resistance of adherent cells to detachment (data not shown). Applied separately, blocking mAb to VLA-4 diminished TEM approximately 30%, whereas blocking mAb to LFA-1 resulted in approximately 80% inhibition of TEM (Figure 2C). These data suggest a dominant role for LFA-1 in TEM triggered by sequential chemotactic stimuli under shear conditions.
Shear stress increases lymphocyte retention in and TEM through intercellular endothelial junctions without affecting locomotion to these sites
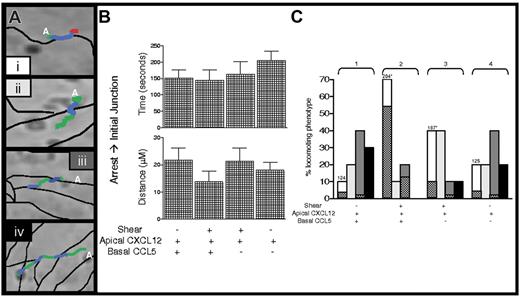
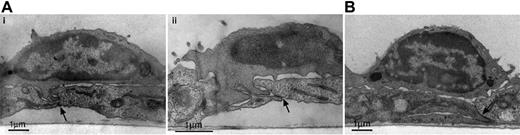
Lymphocyte TEM triggered by apical CXCL12 occurs exclusively in paracellular junctions,25 and it has recently been reported that locomotion to junctions is a critical step in leukocyte TEM.21 To assess whether shear stress facilitates lymphocyte movement to paracellular sites of diapedesis, detailed dynamic analysis was performed to measure T-cell locomotion from sites of initial transient arrest to nearby junctions in the presence or absence of continuous shear flow (Figure 3A). This analysis showed that, in the presence of apical CXCL12, shear or subendothelial chemokine did not influence either the time required or the distance traveled by T cells reaching an initially encountered junction (Figure 3B). However, further kinetic analysis revealed that T cells were retained longer in the initial junction, where most TEM occurred (Figure 3C; group 2), under fluid shear (mean, 204 ± 20 seconds; Figure 3C; group 2) than under shear-free (mean, 124 ± 20 seconds; Figure 3C; group 1) conditions. Electron microscopy analysis of T cells fixed in situ at this time indicated that, in the presence of shear stress, 39% of the total endothelial adherent T cells (total analyzed, 67 lymphocytes) developed extensive pseudopodial protrusions within junctions (Figure 4A). In contrast, in the absence of shear stress, only 11% of endothelial adherent T cells (total analyzed, 63 lymphocytes) protruded into junctions, and most T cells traveled on the junction without any overt morphologic changes (Figure 4B). It has been reported8 that shear and apical CXCL12 alone are sufficient to induce pseudopodial protrusions. The present data extend these results by demonstrating that, in the absence of continuous shear, these protrusions are not maintained despite accumulation on the endothelium in the presence of shear flow. The relative proportions of T cells shown to develop junctional protrusions are identical to the proportions of cells capable of completing TEM with respect to the presence or absence of shear, respectively. Interestingly, shear prolonged T-cell retention in the initial junction regardless of whether subendothelial CCL5 was present (Figure 3; compare groups 2 and 3 with 1 and 4), but prolonged retention did not translate to increased TEM in the absence of CCL5 (Figures 3; compare groups 2 and 3). A small percentage of the locomotive lymphocytes also reached second junctions and could complete TEM toward subendothelial CCL5, but only in the presence of continuous shear.
Dynamics of T-cell locomotion toward, within, and through endothelial junctions. (A) Four categories of T-cell locomotion patterns are characterized from initial transient arrest sites (letter A) over the apical endothelial surface and junctions (thin black lines). (i) Representative T cell locomotes toward the first junction (green line denotes T-cell trajectories, as analyzed by digital imaging; see “Materials and methods”) and within that junction (blue line) before TEM (red line). (ii, iv) Representative T cells locomote toward the first junction (green line), cross them (blue line), and continue to locomote beyond the first (ii) and the second (iv) junctions (green lines). (iii) Representative T cell locomotes through the first junction (green, blue, green) and is retained within the second junction (blue). (B) T cells analyzed in Figure 2A-B for experiments using 10 ng/mL apical CXCL12, basal CCL5 (as indicated), or both, were tracked and categorized as in panel A. Times and distances from the site of initial transient arrest to the first junction (green lines) were calculated and are displayed. Error bars represent SEM. (C) All T cells analyzed in panel B were tracked and monitored by time and distance from the site of initial transient arrest until the end of the experimental period and were categorized as patterns i, ii, iii, or iv, as described in panel A. Shaded bars correspond to the different locomotion patterns shown in panel A, and checkered bars denote the percentage of TEM within the first and second junctions (i and iii, respectively). Note that T cells in group 2 did not locomote beyond the second junction. Average retention times within the first junction are indicated above the bars for i. Data are presented as percentages of the indicated locomotion pattern from analysis of 30 locomotive cells. *P < .05.
Dynamics of T-cell locomotion toward, within, and through endothelial junctions. (A) Four categories of T-cell locomotion patterns are characterized from initial transient arrest sites (letter A) over the apical endothelial surface and junctions (thin black lines). (i) Representative T cell locomotes toward the first junction (green line denotes T-cell trajectories, as analyzed by digital imaging; see “Materials and methods”) and within that junction (blue line) before TEM (red line). (ii, iv) Representative T cells locomote toward the first junction (green line), cross them (blue line), and continue to locomote beyond the first (ii) and the second (iv) junctions (green lines). (iii) Representative T cell locomotes through the first junction (green, blue, green) and is retained within the second junction (blue). (B) T cells analyzed in Figure 2A-B for experiments using 10 ng/mL apical CXCL12, basal CCL5 (as indicated), or both, were tracked and categorized as in panel A. Times and distances from the site of initial transient arrest to the first junction (green lines) were calculated and are displayed. Error bars represent SEM. (C) All T cells analyzed in panel B were tracked and monitored by time and distance from the site of initial transient arrest until the end of the experimental period and were categorized as patterns i, ii, iii, or iv, as described in panel A. Shaded bars correspond to the different locomotion patterns shown in panel A, and checkered bars denote the percentage of TEM within the first and second junctions (i and iii, respectively). Note that T cells in group 2 did not locomote beyond the second junction. Average retention times within the first junction are indicated above the bars for i. Data are presented as percentages of the indicated locomotion pattern from analysis of 30 locomotive cells. *P < .05.
Shear stress applied on T cells approaching endothelial junctions trigger membrane protrusions within junctions and into neighboring endothelial cells. T cells locomotive on HUVECs in the presence of apical CXCL12 (10 ng/mL) and basal CCL5 (100 ng/mL) were subjected to continuous application of shear or to shear-free conditions (for 2 minutes), as described in “Materials and methods” (see also Figure 2). After 2 minutes, HUVEC monolayers and adherent T cells were immediately fixed and processed for transmission electron microscopy. (A, i-ii) Cross-sections of 2 representative T cells reaching endothelial junctions under continuous shear flow. (B) Representative T cell under shear-free conditions, showing contact over the junction without pseudopodial extensions. Arrows denote the endothelial junctions in each panel. Scale is indicated by the 1-μM bar in each panel. The magnification of panels A, B, and C is 11 000×, 16 000×, and 11 000×, respectively.
Shear stress applied on T cells approaching endothelial junctions trigger membrane protrusions within junctions and into neighboring endothelial cells. T cells locomotive on HUVECs in the presence of apical CXCL12 (10 ng/mL) and basal CCL5 (100 ng/mL) were subjected to continuous application of shear or to shear-free conditions (for 2 minutes), as described in “Materials and methods” (see also Figure 2). After 2 minutes, HUVEC monolayers and adherent T cells were immediately fixed and processed for transmission electron microscopy. (A, i-ii) Cross-sections of 2 representative T cells reaching endothelial junctions under continuous shear flow. (B) Representative T cell under shear-free conditions, showing contact over the junction without pseudopodial extensions. Arrows denote the endothelial junctions in each panel. Scale is indicated by the 1-μM bar in each panel. The magnification of panels A, B, and C is 11 000×, 16 000×, and 11 000×, respectively.
Discussion
Although the identification and characterization of chemokines has expanded in the past decade,6,7 similar advances toward an understanding of the physiologic interplay of chemokines has been impeded by poor availability of in vivo and in vitro functional model systems. Because of the apparent fine control of inflammatory responses provided by chemokines,7 there is a need to define causal relationships between chemokine display and lymphocyte homing patterns. In addition, because of the complexity of these interactions in vivo and the fact that these signals are delivered to circulating cells under hemodynamic shear stress conditions, we developed a novel apparatus that can now be used to dissect the interactions between lymphocytes and endothelium under flow in the presence of multiple chemokines. Because the display of CXCL12 on the surfaces of endothelial cells is well recognized15 and recent data suggest that platelets store CXCL12 and can deposit this chemokine dynamically onto the endothelium,26 we decided to use CXCL12 as the apical chemokine in these initial experiments with this system. The application of CXCL12 to the endothelial surface was limited to an exposure time of 2 minutes. The endothelial surface was then washed in media extensively before placement in the chamber and then was subjected to additional washing by the application of shear flow. We specifically chose this short time frame and the subsequent extensive washing/perfusion to prevent diffusion into the subendothelial compartment, thus defining CXCL12 presentation as apical. Consistent with this intent, CXCL12 was retained sufficiently to uniformly induce increased locomotion (Figure 2A-B). In contrast, we used CCL5 as a representative subendothelial chemokine. Although it is known that CCL5 can be synthesized by endothelial cells and is also released by platelets, CCL5 is produced robustly by many cells in the extravascular compartment, including fibroblasts, epithelial cells, and, significantly, tissue-infiltrating lymphocytes and monocytes.27-29 Indeed, in studies of human skin, extravascular injection of CCL5 itself induces a potent T-lymphocyte recruitment.30,31 Accordingly, we sought to determine how this agent affected lymphocyte TEM when presented in the subendothelial compartment in our system.
Our results showed that apical chemokine stimulation by CXCL12 promotes locomotion on the endothelium and positioning at endothelial cell junctions, but fluid shear forces induced T-cell morphologic changes at junctions, resulting in efficient chemotaxis toward a subendothelial chemoattractant. The absence of effective TEM under shear-free conditions, despite optimal presentation of apical and subendothelial chemokines, indicates that integration by lymphocytes of multiple endothelial chemokine signals is conditional on the application of continuous shear stress on the cells. Arrival at intercellular boundaries alone does not ensure T-cell TEM in the absence of appropriate shear and chemotactic stimuli. Analysis of locomotive T cells by transmission electron microscopy suggests that the application of shear flow enables the formation of pseudopodia that can project into and through intercellular junctions. It is possible that T cells may localize specific chemokine receptors in microdomains within these processes, affecting the level or intrinsic signaling of CCL5 receptors such as CCR1 and CCR5 within these shear-triggered projections. Additional studies are warranted to define the kinetics of the appearance of pseudopodia relative to the transition between locomotion on the endothelium and subsequent transmigration and to elucidate the topography and activity of chemokine receptors on these structures. Similarly, our studies highlight a critical role for LFA-1 in mediating transmigration, but the localization of LFA-1 and other effector adhesion molecules within the pseudopod–endothelial “synapse” requires further investigation.
Results described here using the transwell flow chamber provide important new insights into how 3-dimensional chemokine presentation, integrin occupancy events, and shear-transduced mechanical signals combine to direct T-cell transendothelial migration. The data show clearly that although the use of the Boyden chamber has advanced our basic understanding of the role of chemokines as chemoattractants, this model, as applied to the study of leukocyte recruitment, is significantly constrained by the absence of hemodynamic shear, a fundamental feature of physiologic leukocyte–endothelial interactions. A greater understanding of how molecular effectors (adhesion molecules and chemoattractants) combine with shear forces to promote lymphocyte recruitment should translate into strategies to optimize T-cell trafficking for therapeutic purposes.
Authorship
Contribution: T.H.S. performed the research, collected and analyzed the data, and wrote the paper. V.S. performed the research and collected and analyzed the data. D.W.C. collected and analyzed the data. R.A. contributed vital new analytical tools, designed the research, analyzed the data, and wrote the paper. R.S. designed the research, contributed vital new reagents and analytical tools, analyzed the data, supervised all experimentation, funded the research, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert Sackstein, Harvard Institutes of Medicine, 77 Ave Louis Pasteur, Rm 671, Boston, MA 02115; e-mail: rsackstein@rics.bwh.harvard.edu.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health grants RO1 HL060528 (R.S.) and HL073714 (R.S.) and was partially supported by the Israel Science Foundation and the EU6 Program for Migration and Inflammation (R.A.).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal