Abstract
The molecular alterations underlying the pathogenesis of angioimmunoblastic T-cell lymphoma (AITL) and peripheral T-cell lymphoma, unspecified (PTCL-u) are largely unknown. In order to characterize the ontogeny and molecular differences between both entities, a series of AITLs (n = 18) and PTCLs-u (n = 16) was analyzed using gene expression profiling. Unsupervised clustering correlated with the pathological classification and with CD30 expression in PTCL-u. The molecular profile of AITLs was characterized by a strong microenvironment imprint (overexpression of B-cell– and follicular dendritic cell–related genes, chemokines, and genes related to extracellular matrix and vascular biology), and overexpression of several genes characteristic of normal follicular helper T (TFH) cells (CXCL13, BCL6, PDCD1, CD40L, NFATC1). By gene set enrichment analysis, the AITL molecular signature was significantly enriched in published TFH-specific genes. The enrichment was higher for sorted AITL cells than for tissue samples. Overexpression of several TFH genes was validated by immunohistochemistry in AITLs. A few cases with molecular TFH-like features were identified among CD30− PTCLs-u. Our findings strongly support that TFH cells represent the normal counterpart of AITL, and suggest that the AITL spectrum may be wider than suspected, as a subset of CD30− PTCLs-u may derive from or be related to AITL.
Introduction
Peripheral T-cell lymphomas (PTCLs) are uncommon malignancies, representing approximately 12% of all lymphomas.1 In western countries, the most common forms present as nodal tumors and mainly include 3 subtypes: angioimmunoblastic T-cell lymphoma (AITL), anaplastic large cell lymphoma (ALCL), and peripheral T-cell lymphoma, unspecified (PTCL-u).2 AITL, otherwise known as angioimmunoblastic lymphadenopathy with dysproteinemia, is a systemic disease manifested by B symptoms, polyadenopathy, and various immunologic abnormalities. AITL is characterized by a diffuse polymorphous infiltrate including variable proportions of medium-sized neoplastic cells with abundant clear cytoplasm, prominent arborizing blood vessels, proliferation of follicular dendritic cells (FDCs), and the presence of Epstein-Barr virus (EBV)–positive large B-cell blasts.3 ALCL shows a broad morphologic spectrum, unified by the recognition of typical “hallmark” cells strongly expressing CD30. They commonly express epithelial membrane antigen (EMA), cytotoxic molecules, and in up to 85% of cases the anaplastic lymphoma kinase (ALK) protein as a consequence of rearrangement of the ALK gene.4 Conversely, PTCL-u, the most common form of nodal T-cell lymphoma, lacks precise defining features and is diagnosed by exclusion of any recognizable (“specified”) subtype of T-cell lymphoma. Hence, PTCL-u represents a heterogeneous group of tumors that may include cases with borderline features to ALCL and AITL. Nonanaplastic PTCLs usually carry a dismal prognosis, with 5-year overall survival rates averaging 25%.1
The normal cellular derivation of PTCLs is ambiguous.2 The T-cell system is complex, comprising numerous subsets with different effector, regulatory, or memory functions. The immunophenotypic profiles of PTCLs are heterogeneous and not entirely disease specific. Most cases appear to derive from T cells expressing the αβ form of the T-cell receptor (TCR). PTCLs-u more commonly express CD4 than CD8, but a significant proportion of the cases have an aberrant phenotype (CD4+CD8+ or, less commonly, CD4−CD8−).5–7 In contrast, it has been suggested that most AITLs derive from mature helper CD4+CD8− T cells,8 expressing the Th1-associated chemokine receptors CXCR3 and OX40/CD134.9,10 However, based on the expression of single markers (namely the transcription factor BCL6,11 the CXCL13 chemokine,12–14 the membrane receptor PD-1 [also known as PDCD115 ], and CXCR516 ), it has been recently suggested that the neoplastic cells in AITL may derive from a specific subset of T cells normally present in germinal centers with a helper function to follicular B cells (follicular helper T cells, TFH cells).
The genetic alterations and pathogenic mechanisms underlying AITL and PTCL-u are largely unknown. With the exception of ALCL and a small subset of PTCLs with follicular pattern,17 there are no recurrent translocations reported. Complex karyotypes with numeric and structural chromosomal changes are noted in many cases of PTCL-u with recurrent chromosomal losses and gains as demonstrated by genomic profiling.18
In this study, we performed gene expression profiling analysis of a series of AITLs and PTCLs-u in order to delineate their molecular signatures and to gain insight into their ontogeny and molecular mechanisms by an analytic comparison of our data set to available molecular signatures of normal T-cell subsets.19–22
Patients, materials, and methods
Patient characteristics and tumor samples
Thirty-four newly diagnosed, previously untreated patients with nodal PTCL-u (n = 16) and AITL (n = 18) were analyzed in this study, according to a protocol approved by the institutional review board of Hôpital Saint-Louis, Paris. Seventeen patients had been enrolled in clinical protocols of the Groupe d'Etude des Lymphomes de l'Adulte (GELA). There were 18 male and 16 female patients, with a median age at diagnosis of 65 years (range, 21 to 83 years). Patients provided informed consent, in accordance with the Declaration of Helsinki.
All cases were reviewed by 2 hematopathologists (L.d.L. and P.G.). The percentage of malignant cells was evaluated by morphology and CD3 immunostaining as exceeding 30% in all AITL cases, and 50% in all PTCLs-u. Lymphomas were classified according to the criteria of the WHO classification.2 The staining panel included at least CD20 and CD3, and for most AITLs an FDC marker (CD21 and/or CD23 and/or CNA.42) and an EBV marker (latent membrane protein-1 [LMP-1]) and/or EBV-encoded small RNAs (EBERs). FDC expansion and EBV-infected cells were demonstrated in 16 of 17 and 11 of 16 AITLs, respectively. A variable expression of CD10 and BCL6 was demonstrated in 15 of 17 and 10 of 10 AITLs with interpretable staining. CD30 expression in more than 50% of the tumor cells was shown in 6 of 15 PTCLs-u tested. All CD30+ PTCLs-u were EMA-negative large-cell tumors, distinct from ALCL, with a cytotoxic phenotype in 3 of 5 cases tested.
For 17 of 18 AITL patients (S1 to S17) and all PTCL-u patients (S19 to S34), frozen tumor tissue samples were analyzed. For 2 AITL patients with tumor cell suspensions available (S17C and S18C), mononuclear cell suspensions prepared from lymph nodes were enriched in tumor cells by magnetic activated cell sorting. This yielded a 84% CD3+CD10+ cell population for sample S17C and a 82% CD4+CD3−CD10+ cell population for sample S18C.
Microarray procedures
Microarray analyses were performed using 3 μg total RNA as starting material and 10 μg cRNA per hybridization (GeneChip Fluidics Station 400; Affymetrix, Santa Clara, CA). The total RNAs were amplified and labeled following the one-cycle target labeling protocol (http://www.affymetrix.com). The labeled cRNAs were hybridized to HG-U133 plus 2.0 Affymetrix GeneChip arrays (Affymetrix, Santa Clara, CA). The chips were scanned with a Affymetrix GeneChip Scanner 3000 and subsequent images analyzed using GCOS 1.4 (Affymetrix). Except when indicated, all transcriptome analyses were carried out using either an assortment of R system software (v1.9.0; Vienna, Austria) packages including those of Bioconductor23 (V1.1.1) or original R code. Raw feature data were normalized and log2 intensity expression summary values for each probe set were calculated using robust multiarray average (RMA24 ; Bioconductor package affy V1.4.32). Probe sets corresponding to control genes or having an “_x_” annotation were masked yielding a total of 50 406 probe sets available for further analyses. Raw data (Affymetrix U133A/B CEL files) from 6 samples corresponding to 3 T-cell subpopulations (TFH, Th1, and Th2) cited by Chtanova et al22 were normalized, in batch, with another 11 samples of T-cell subpopulations from the same citation using RMA as described above.
Gene expression analyses
Unsupervised classification analyses.
Principle component analysis (PCA) was used to classify the tissue samples.25 The function “prcomp” (R package stats V2.3.0) was used to calculate the 33 principle components for the 33 samples, based on the 25 744 probe sets that yielded a mean intensity level of 3.0 or higher (log2 value). The first 10 components accounted for an accumulated 70% of the cumulative variance. The first 25 components (95% cumulative variance) were used in the hierarchic clustering (complete linkage) of the samples using the R packages cluster (V1.9.3, Bioconductor23 ) and maptree (V1.3.3, Bioconductor23 ). The resulting dendrogram was cut yielding 3 sample groups, and the enrichment of sample annotations across the different groups was calculated using Fisher exact tests. We also classified the samples using a nonsupervised selection of genes and hierarchic clustering (for more details see Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Supervised analyses according to pathological features.
Univariate t tests (BRB ArrayTools v3.4.beta2, http://linus.nci.nih.gov/BRB-ArrayTools.html) were used to define the differentially expressed gene lists with a significance level of each univariate test of P < .002 using a random variance model.26 False discovery rates (FDRs) were calculated based on 1000 sample permutations. Probe sets that yielded a maximal normalized nonlog intensity value of 10 or less were filtered out from further analysis.
Gene ontology (GO) term and KEGG pathways enrichment tests.
We used hypergeometric tests (GOstats, V1.1.1; Bioconductor23 ) to measure the enrichment of GO terms in each of the subgroup-specific gene lists. Independent tests were performed for molecular function (MF), biologic process (BP), and cellular compartment (CC) categories. GeneSpringGX (Agilent Technologies, Santa Clara, CA) was used for testing the overrepresentation of genes in a particular KEGG pathway (168 KEGG pathways in total were tested) for a given list of unique gene identifiers (HUGO gene symbols) versus all of the genes on the microarray mapped to that pathway. This test uses a standard Fisher exact test, and the P value is adjusted with a Bonferroni multiple testing correction.
Gene set enrichment analysis (GSEA).
GSEA was performed as previously described27 to assess the overrepresentation of T-cell–related gene sets in the different groups ofsamples (AITL; PTCL-u; CD30+ PTCL-u, and CD30− PTCL-u). Briefly, this test determines the overrepresentation of a gene set (a list of genes) at the extremes (top or bottom) of the ordered, nonredundant data set (list of all of the genes being used to compare 2 groups of samples). Two different statistics were used to rank the genes for each comparison: “signal-to-noise” ratio (SNR)27 and a classical t test. Fourteen gene sets representative of the Th1 cell, Th2 cell, and TFH subsets derived from literature19–22 were tested using GSEA for an association with the subgroups of tumors (Table 1). Nonredundant data sets were generated specific to each comparison by first filtering out all probe sets that yielded a maximal intensity value of 10 or less for the 2 groups and then averaging data values for multiple probe sets corresponding to the same gene.
Prediction analysis.
Two approaches were used. For the first approach, the sample population was divided into a training group and a validation group. The training group of samples was used for the initial gene selection and building the multigene (1-10 genes) predictors. We restricted this analysis to 4 prediction algorithms: nearest shrunken centroids (PAM, pamr, v1.30), k-nearest neighbors (KNN, class, v7.2-27.1), diagonal quadratic/linear discriminant analyses (DLDA/DQDA, sma, v0.5.15). A bottom-up step approach was used to construct the best multigene predictor for each algorithm (Table S5 legend). As a second approach, BRB ArrayTools (v3.5 beta1) was used to apply a leave-one-out cross validation (LOOCV; Table S6 legend). For this, 7 prediction algorithms were used: compound covariate, diagonal linear discriminant analysis, 1-nearest neighbor, 3-nearest neighbors, nearest centroid, support vector machines, or Bayesian compound covariate. The latter algorithm was based on the method previously described by Wright et al.28
Validation at the protein level
Tissue microarrays (TMAs) containing three 0.6-mm tissue cylinders of each donor block from 13 AITLs and 15 PTCLs-u were constructed using a manual tissue arrayer (Beecher Instruments, Sun Prairie, WI). Adequate control tissues for specific antibodies were also included. Sections (4-μm each) were stained with the following primary antibodies: anti-CD27 (clone 137B4; Novocastra, Newcastle upon Tyne, United Kingdom), anti-CXCL13 (clone 53610; R&D Systems, Minneapolis, MN), antithrombomodulin (clone 1009; Dako, Glostrup, Denmark), anticlusterin (clone 41D; Upstate Biotechnology, Lake Placid, NY), anti-PDCD1 (kindly provided by Daniel Olive), anti-NFATC1 (clone 7A6; Santa Cruz Biotechnology, Santa Cruz, CA). Immunostainings were performed following heat-induced antigen retrieval, using a standard indirect avidin-biotin peroxidase method. For CXCL13, a tyramide signal amplification system (CSAII kit; Dako) was applied.13 For thrombomodulin and clusterin, the distribution and extent of immunostaining were recorded. For other markers, cases were regarded as immunoreactive if clearly neoplastic cells exhibited staining. Differences in marker expression were demonstrated by χ2 analyses.
Results
Unsupervised clustering of PTCLs correlates with pathological features
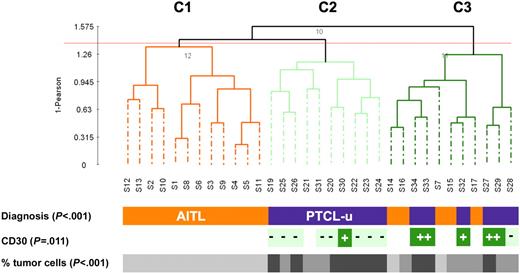
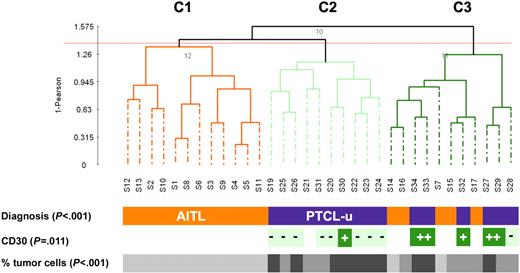
Similar to previous studies,29 we classified our tumor samples using PCA. This method typically relies on the first 3 components to spatially represent the samples in a 3-D plot, often, however, at the expense of the total variation being represented (PC1-3 accounted for only 41% of the variation in our data set). Using a larger number of components (PC1-25, 95% of the variation) in combination with hierarchic clustering, we obtained 3 major groups of tumors (designated C1, C2, and C3; Figure 1), showing striking association with the pathologic classification (P < .001). Clusters C1 and C2 represented 2 homogeneous groups of tumors consisting of 12 AITLs and 10 PTCLs-u, respectively. Cluster C3 was a heterogeneous group of tumors comprising 5 AITLs and 6 PTCLs-u. Among the PTCLs-u, all but one CD30+ tumor clustered within group C3, whereas all but one PTCL-u sample in C2 were CD30− (P = .011). The observed partition of the lymphoma samples also strongly correlated with the percentage of tumor cells (P < .001). When restricting the comparison to the PTCL-u cases distributed across clusters 2 and 3, the percentage of tumor cells (in contrast with the CD30 status) did not correlate with the observed distribution (P = .28). These results were reproducible using different proportions of conserved accumulated variation to cluster the samples (as low as 77% [PC1-13], data not shown). In addition, a similar topology was also obtained using a nonsupervised selection of expression profile, based on the 2.5% most varying expression profiles (644 probe sets, Figure S1 and Table S1).
Unsupervised clustering of 16 PTCL-u and 17 AITL tissue samples. Dendrogram of the 33 lymphoma tissue samples based on PCA of the first 25 components (accumulated variance 95%). CD30: + denotes expression in more than 50% of neoplastic cells; - denotes absence of expression or low level of expression of CD30 in neoplastic cells. The percentage of tumor cells is represented by a gray scale (light gray: 30%-50% tumor cells; medium gray: 50%-70% tumor cells; and dark gray: > 70% tumor cells).
Unsupervised clustering of 16 PTCL-u and 17 AITL tissue samples. Dendrogram of the 33 lymphoma tissue samples based on PCA of the first 25 components (accumulated variance 95%). CD30: + denotes expression in more than 50% of neoplastic cells; - denotes absence of expression or low level of expression of CD30 in neoplastic cells. The percentage of tumor cells is represented by a gray scale (light gray: 30%-50% tumor cells; medium gray: 50%-70% tumor cells; and dark gray: > 70% tumor cells).
AITL and PTCL-u have distinct molecular features
A total of 832 probe sets significantly distinguished AITL tissues from PTCLs-u (P < .002; 10.6% maximal FDR). They corresponded to 678 unique gene identifiers, including 442 genes (545 probe sets) overexpressed in AITL (defining the AITL signature) and 236 genes (287 probe sets) overexpressed in PTCL (see Table S2 for a complete gene list and associated statistics). The genes of the AITL signature belonged to several functional categories (Table 2). The most significant overrepresented GO terms in AITL were related to cell-to-cell communication and adhesion (P < .009), immune response (P < .007), vascular biology (P < .04), and extracellular matrix (P = .005). Compared to PTCL-u, AITL had higher levels of expression of cell adhesion molecules (cadherins, integrins, CD151) and various membrane receptors (CD10, CD40 ligand, CD200, PDCD1) and proteins involved in membrane signaling.30 AITL was also characterized by a striking overrepresentation of B-cell– and plasma cell–related genes, and of FDCs as well as complement factors, and higher level of expression of extracellular matrix components (laminin, collagen, ladinin, fibronectin) and of factors and enzymes involved in matrix synthesis and remodeling (TGFβ, fibroblast growth factor, matrix metalloproteinases). Several genes related to vascular biology—including vascular growth factors, endothelium-related genes, coagulation factors—were also overexpressed in AITL. Conversely, the functional categories of the genes overexpressed in PTCL-u were reflective of nonspecific cellular functions, notably protein ubiquination (TRIM proteins31 ), regulation of transcription, and metabolism (Table S2). Few of the genes overexpressed in PTCL-u (Table 3) were related to immune reactions or chemotaxis; they included CCR7, a chemokine receptor mediating homing to lymph nodes,32 and CD47, an integrin-associated protein involved in interactions between T cells and endothelium.33
The microenvironment component has a significant influence on the AITL signature
In 2 AITL cases, the gene expression profile of highly enriched tumor samples enabled us to delineate the components of the AITL molecular signature (ie, tumor cell versus microenvironment-related genes). To this end, we calculated the fold change between the average gene expression level of each of the 545 probe sets that was significantly overexpressed in AITL (compared to PTCL-u), for the 2 AITL cell samples versus the 17 AITL tissues samples. We found 46 genes that were more associated to the “AITL tumor cells” (fold change [FC] > 1, including 8 genes with a FC > 1.5) and 396 genes that were more associated with the “AITL microenvironment” (FC < 1, including 290 genes with a FC < 0.67) (Table 2; see Table S2 for a complete list of the genes).
The AITL transcriptional profile is enriched in genes characteristic of TFH cells
Using immunohistochemistry, we and others have recently demonstrated that neoplastic cells in AITL express CXCL13,12–14 a chemokine characteristic of TFH cells. Our gene expression profiling data confirmed that AITL expresses higher levels of CXCL13 mRNA than PTCL-u, (Table 2). Moreover, we also identified several other genes overexpressed by AITL (ie, CD200, PDCD1, CD40L, NFATC1, LIF) that had been reported as overexpressed in TFH cells compared to other T-cell subsets.20–22,34 In order to specifically evaluate the significance of the observed similarity between the AITL and the TFH signatures, we used GSEA.27 The TFH signature was defined using set of genes independently identified by Kim et al20 and Chtanova et al21,22 as differentially expressed in CD4+CD57+ cells isolated from tonsils compared to other normal purified T-cell subsets with, respectively, cDNA and oligonucleotide microarrays (Table 1). Having ranked the tissue samples according to the AITL versus PTCL-u distinction, we found that AITLs were significantly enriched in genes up-regulated in TFH cells (GS2_ TFH _up and GS5 TFH _up, P < .014; Tables 4 and S3). Of the 100 genes comprising the GS2_ TFH _up gene set, the GSEA method identified 42 “core” genes that accounted for enrichment signal (Figure 2A) and were distributed among the top 1722 genes in the ranked data set. Next, we assessed the enrichment of these 42 core genes in the AITL samples by ranking the top 1722 genes according to the type of sample (2 cell suspensions versus 17 tissues) by fold change of gene expression. While a meaningful P value cannot be calculated given the limited number of cell suspensions, we observed a higher level of expression in the tumor cell suspensions compared to the tissue samples of these 42 core genes (Figure 2B). The overexpression of TFH genes in AITL tissues and cells is illustrated by a heatmap in Figure 2C.
GSEA ES curves and clustering results for the 2 significant gene signatures significantly overexpressed in AITL compared to PTCL-u. (A-B) ES curve (red) or the running sum of the weighted enrichment score obtained from GSEA software. Vertical blue lines indicate the position of each of the 100 genes (“Hits”) comprising the GS2_ TFH _up gene set. The graph on the bottom of each panel shows the ranked list metric (gray, SNR for A and fold change for B) for each gene as a function of the rank in the ordered data set. This corresponds to the level of correlation with the groups being tested (the far leftmost gene had the highest correlation with the AITL samples, and the far rightmost gene had the highest correlation with PTCL-u samples). (A) Results obtained for GS2_ TFH _up gene set in the comparison of AITL and PTCL-u using the SNR statistic to rank the genes from left to right (highest [0.9] to the lowest [−0.4] real SNR value, respectively). (B) Results obtained for the 42 core (or leading-edge) genes obtained from the analysis in panel A that were distributed in the top 1722 genes. The core genes in the ranked list represent the genes that are at, or before, the point where the running sum reaches its maximum deviation from zero (see Subramanian et al27 for more details). Here, the top 1722 genes obtained in panel A are ranked from highest (left) to lowest (right) fold change between the average of the 2 AITL-sorted cell samples (AITL cell) versus the average of the 17 AITL tissue samples and the hits represent the 42 core genes. (C) Clustering and heatmap of these 42 core genes obtained from the analysis shown in panel A. Data from T-cell subsets (TFH, Th1, and Th2; Chtanova et al21 and this study) were standardized by calculating an independent z-score (mean = 0 and standard deviation = 1) for each data set. Each gene was further standardized by mean-centering its expression profile across all samples. Genes are ordered by their rank calculated in the analysis shown in panel A. DNA-Chip Analyzer (dChip) Version 1.3 (Department of Biostatistics, Harvard School of Public Health, Boston, MA) was used to generate the heatmap and cluster the genes. Standardized expression ranges from −2.0 (blue) to 2.0 (red). Arrows point at the columns representing AITL cell suspensions.
GSEA ES curves and clustering results for the 2 significant gene signatures significantly overexpressed in AITL compared to PTCL-u. (A-B) ES curve (red) or the running sum of the weighted enrichment score obtained from GSEA software. Vertical blue lines indicate the position of each of the 100 genes (“Hits”) comprising the GS2_ TFH _up gene set. The graph on the bottom of each panel shows the ranked list metric (gray, SNR for A and fold change for B) for each gene as a function of the rank in the ordered data set. This corresponds to the level of correlation with the groups being tested (the far leftmost gene had the highest correlation with the AITL samples, and the far rightmost gene had the highest correlation with PTCL-u samples). (A) Results obtained for GS2_ TFH _up gene set in the comparison of AITL and PTCL-u using the SNR statistic to rank the genes from left to right (highest [0.9] to the lowest [−0.4] real SNR value, respectively). (B) Results obtained for the 42 core (or leading-edge) genes obtained from the analysis in panel A that were distributed in the top 1722 genes. The core genes in the ranked list represent the genes that are at, or before, the point where the running sum reaches its maximum deviation from zero (see Subramanian et al27 for more details). Here, the top 1722 genes obtained in panel A are ranked from highest (left) to lowest (right) fold change between the average of the 2 AITL-sorted cell samples (AITL cell) versus the average of the 17 AITL tissue samples and the hits represent the 42 core genes. (C) Clustering and heatmap of these 42 core genes obtained from the analysis shown in panel A. Data from T-cell subsets (TFH, Th1, and Th2; Chtanova et al21 and this study) were standardized by calculating an independent z-score (mean = 0 and standard deviation = 1) for each data set. Each gene was further standardized by mean-centering its expression profile across all samples. Genes are ordered by their rank calculated in the analysis shown in panel A. DNA-Chip Analyzer (dChip) Version 1.3 (Department of Biostatistics, Harvard School of Public Health, Boston, MA) was used to generate the heatmap and cluster the genes. Standardized expression ranges from −2.0 (blue) to 2.0 (red). Arrows point at the columns representing AITL cell suspensions.
CD30+ and CD30− PTCLs-u have distinct molecular features
By 2 different unsupervised clustering methods, CD30+ PTCLs-u tended to cluster together (Figure 1 and Figure S1). Supervised analysis yielded a list of 241 probe sets (186 genes, 24.8% maximal FDR), comprising 73 genes overexpressed in CD30+ PTCLs-u and 113 genes overexpressed in CD30− PTCLs-u (t test, P < .002; Tables 5–6). The only GO terms overrepresented in CD30+ PTCLs-u were related to molecular function (P < .009) and comprised various enzymes with transferase, synthase, and kinase activity. Also overexpressed in CD30+ PTCLs-u were several genes involved in the control of transcription, among which the Jun dimerization protein (acting as an IL2 repressor) showed the highest fold change.35 GO terms overrepresented in CD30− PTCLs-u were mostly related to biologic processes (regulation of physiological and metabolic processes, lymphocyte activation, P < .008). Particularly, CD30− PTCLs-u had overexpression of genes associated with T-cell activation and signal transduction, including the costimulatory receptor CD28 (FC > 6.0), the CD69 activation antigen, CD52, and several molecules involved in TCR signal transduction (Itk, Lyn, and Lck). Accordingly, the TCR signaling pathway was the most significant KEGG pathway overrepresented in CD30− PTCL-u (P < .001; Table S4).
Next, the CD30+ and CD30− subsets of PTCL-u were tested for enrichment in gene sets representative of normal T-cell subsets (Table 1). Although we observed a trend for the signature of CD30− PTCL-u to be associated with the Th2 signature, no significant correlation was found between either of the 2 PTCL-u subgroups and any of the gene sets representative of the Th1 and Th2 subsets (Table S3). Somewhat surprisingly, the molecular signature of CD30− PTCL-u was overlapping with that of TFH cells, although the correlation was statistically weaker than for AITL samples (Tables 4 and S3). This observation raised the question as to whether the TFH signature was present in all or most CD30− PTCLs-u, or if a small subset of PTCL-u cases accounted for this statistical association. All PTCL-u cases were carefully re-examined, and stainings for FDC and EBV were performed for most cases (15/16). Four cases disclosed focal FDC expansion and/or a few EBV-positive large cells (S24, S25, S26, and S28), and, molecularly, these cases (Figure 2C) had some overexpression of the TFH core genes. Leaving out these 4 cases from the GSEA resulted in increased significance of the TFH enrichment of the AITLs (compared to PTCL-u) and reduced significance of the association of the TFH signature to CD30− PTCL-u (Table S3). Thus, the statistical association between CD30− PTCL-u and TFH appeared to be mostly contributed by a small subset of cases.
Prediction analysis
Given the occasional difficulty to distinguish AITL from PTCL-u using current criteria, we addressed the question if AITL and PTCL-u could be molecularly classified. As a first approach, we divided the sample population into a training group (S1, 8 AITLs and 8 PTCLs-u [4 CD30+ and 4 CD30−]) and a validation group (S2, 9 AITLs and 8 PTCLs-u). An initial list of 284 probe sets (P < .001) was selected using the S1 group and used to build a multigene (1-10 genes) predictor to classify the S2 group (see “Patients, materials, and methods” for details). We obtained 4 top predictors (one for each of the prediction algorithms used) that yielded between 82% and 94% success rate in correctly classifying the S2 group (see Table S5 for the list of genes and classification results). The best of the 4 predictors comprised 8 genes (ITIH5, EPIM, THY1, KCNE4, VAV2, LIPT1, C10orf38, MGC16044) and yielded a success rate of 94%. Using a leave-one-out cross-validation (LOOCV) approach, we obtained, at best, an average overall success rate of 88% (see the list of the 220 gene classifier in Table S6).
Immunohistochemical validation
A few genes of the AITL signature were selected for assessment at the protein level by immunohistochemistry on TMAs. Clusterin and thrombomodulin were selected as markers for the microenvironment. CXCL13, PDCD1, and NFATC1 were chosen within the “core” TFH genes, on the basis of available specific antibodies and our experience with these reagents. CD27 was tested because this antigen, routinely assessed by immunohistochemistry, was part of the TFH signature.21
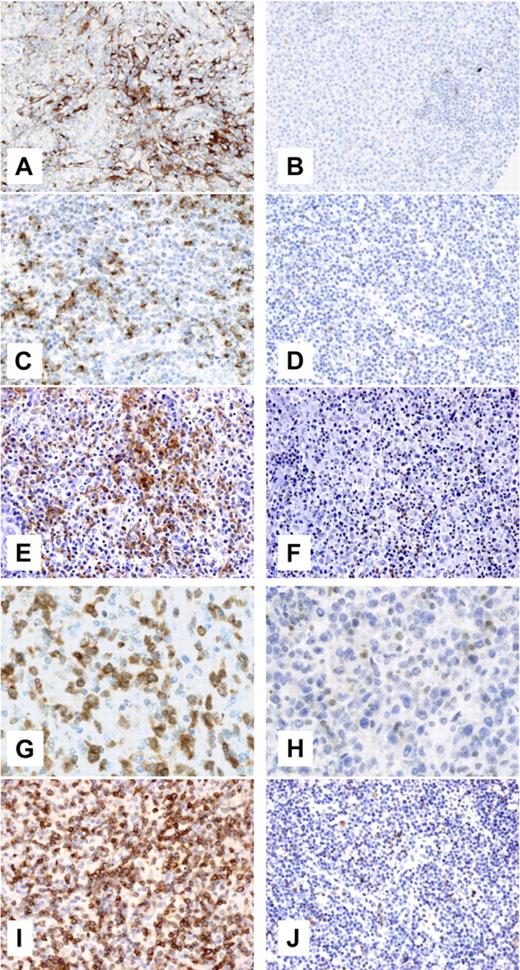
An extensive meshwork of clusterin-positive cells with dendritic morphology was evidenced in 8 of 12 AITLs, but in only 1 of 16 PTCLs-u (P < .001) (Figure 3A-B). Thrombomodulin antibody produced significant interstitial staining in 6 (46%) of 13 AITLs, but in only 1 (7%) of 15 PTCLs-u (P = .016). The neoplastic cells in 12 (92%) of 13 AITL cases stained for CXCL13, whereas only 5 (33%) of 15 PTCLs-u showed some CXCL13 staining in tumor cells (P < .001) (Figure 3C-D). Membrane staining for PDCD1 was evidenced in a fraction of the neoplastic cells in 6 (46%) of 13 AITLs, but in only 1 (7%) of 15 PTCLs-u (P = .016) (Figure 3E-F). In all AITLs, cytoplasmic staining for NFATC1 was evidenced in atypical medium-sized cells, while only 3 of 9 assessable PTCLs-u were positive (P < .001) (Figure 3G-H). Finally, the neoplastic cells were positive for CD27 in 11 (92%) of 12 AITLs and in 5 (66%) of 15 PTCLs-u (P = .002) (Figure 3I-J).
Clusterin, CXCL13, PDCD1, NFTAC1, and CD27 immunohistochemistry in AITL and PTCL-u. Representative AITLs (A, C, E, G, and I) and PTCLs-u (B, D, F, H, and J) were immunostained for clusterin (A-B, original magnification ×200, Zeiss Axioscope; Carl Zeiss, Heidelberg, Germany), CXCL13 (C-D, original magnification ×200, Zeiss Axioscope), PDCD1 (E-F, original magnification ×200, Nikon Eclipse 80i; Nikon, Tokyo, Japan), NFATC1 (G-H, original magnification ×400, Zeiss Axioscope), and CD27 (I-J, original magnification ×200, Nikon Eclipse 80i). For panels A-D and G-H, photographs were taken with a DP70 Olympus camera (Tokyo, Japan); images were acquired using DP Controller 2002 (Olympus) and processed using Adobe Photoshop v7.0 (Adobe Systems, San Jose, CA). For panels E-F and I-J, photographs were taken with a CFW-1310C camera (Scion, Frederick, MD); images were acquired using Histolab 5.131.1 (Alphelys, Plaisir, France) and processed using Adobe Photoshop v7.0. Objectives used were Nikon Plan Fluor 20×/0.50 NA; Zeiss Plan Neufluor 20×/0.40 NA; and Zeiss Plan Neofluor 40×/0.60 NA.
Clusterin, CXCL13, PDCD1, NFTAC1, and CD27 immunohistochemistry in AITL and PTCL-u. Representative AITLs (A, C, E, G, and I) and PTCLs-u (B, D, F, H, and J) were immunostained for clusterin (A-B, original magnification ×200, Zeiss Axioscope; Carl Zeiss, Heidelberg, Germany), CXCL13 (C-D, original magnification ×200, Zeiss Axioscope), PDCD1 (E-F, original magnification ×200, Nikon Eclipse 80i; Nikon, Tokyo, Japan), NFATC1 (G-H, original magnification ×400, Zeiss Axioscope), and CD27 (I-J, original magnification ×200, Nikon Eclipse 80i). For panels A-D and G-H, photographs were taken with a DP70 Olympus camera (Tokyo, Japan); images were acquired using DP Controller 2002 (Olympus) and processed using Adobe Photoshop v7.0 (Adobe Systems, San Jose, CA). For panels E-F and I-J, photographs were taken with a CFW-1310C camera (Scion, Frederick, MD); images were acquired using Histolab 5.131.1 (Alphelys, Plaisir, France) and processed using Adobe Photoshop v7.0. Objectives used were Nikon Plan Fluor 20×/0.50 NA; Zeiss Plan Neufluor 20×/0.40 NA; and Zeiss Plan Neofluor 40×/0.60 NA.
Discussion
Over the past few years, genome-wide expression profiling methods have been applied to most types of B-cell lymphomas, leading to significant advances in our understanding of these diseases.36–39 Yet, few works have been devoted to the molecular profiling of T-cell neoplasms.40–43 In the 3 largest studies comprising up to 62 patients,40,41,43 the series of samples were pathologically heterogeneous, including both precursor and various types of PTCLs, and the hybridization platforms consisted of cDNA arrays, containing at the most 8000 spotted clones. Here, we focused our work on the 2 most common forms of nodal PTCLs, and performed gene expression profiling using a highly standardized pangenomic oligonucleotide Affymetrix microarray.
The clusterings obtained by 2 different unsupervised methods correlated to a large extent with the pathological classification, but also reflected some overlap between AITL and PTCL-u. The molecular distinction between AITL and PTCL-u consisted mostly of an overexpression of genes associated with specific functions in AITL. This finding is in fact in accordance with the current definition of PTCL-u as an exclusion diagnosis. The exceptional availability of 2 AITL enriched-tumor cell samples enabled us to show that nearly 90% of the genes of the AITL signature was contributed by nonneoplastic cells, and included genes reflective of the humoral immune response, and genes encoding various chemokines mediating the recruitment of inflammatory cells, and genes involved in the modulation of vasculogenesis and extracellular matrix. These findings are in accordance with the known pathological features of AITL—including an accumulation of FDCs, B immunoblasts, plasma cells, eosinophils, and vascular proliferation. In addition, we confirmed by immunohistochemistry that clusterin, identified as a marker of AITL microenvironment and otherwise known as a marker for FDCs, was indeed expressed in nonneoplastic stromal cells with FDC morphology, mainly in cases classified as AITL. In agreement with the recent identification of CD10 as a typical marker of AITL,44 we also confirmed overexpression of the CD10 mRNA in the AITL neoplastic cell component.
In order to gain insight into the ontogeny of AITLs, we have applied the recently described GSEA method27 to evaluate the strength of the correlation between the AITL signature and that of TFH cells. The demonstration of a significant enrichment of the TFH gene sets not only in the AITL tumor cells but also in the AITL tissue samples, based on wide-genome expression analysis and robust statistical tools, provides definitive molecular evidence that TFH cells are the normal cellular precursors to AITL. Indeed, in keeping with recent reports,12–15 we showed that the immunohistochemical detection of several TFH markers—especially CXCL13 and PDCD1—help to recognize the (often minimal) neoplastic component of AITL and therefore may provide an important clue for the differential diagnosis with conditions mimicking AITL, including dysimmune reactive conditions.
It may be objected, however, that the profile of AITL tumor cells significantly differs from their postulated normal TFH counterpart. Indeed, whereas TFH cells were initially characterized by their CD57+ phenotype, CD57 expression is not a classical feature of AITL.8,13 This could be explained by recent data showing phenotypic heterogeneity within TFH cells, which, despite a common transcriptional profile,21 comprise a subset of CD57− cells.34 Of interest, CD57 appears to be dispensable to the follicular B-helper activity, which rather relies upon strong ICOS expression.45 This is corroborated by our data set, as ICOS transcripts were more abundant in AITLs than in PTCLs-u (FC = 3.06, P = .08), and more abundant in AITL cells compared to tissues (FC = 5.09).
AITL encompasses a morphologic spectrum, ranging from atypical lymphoproliferations to overtly malignant lymphomas. Three histologic patterns have been described: with hyperplastic follicles, depleted follicles, or without follicles, and interpreted as reflective of earlier to later disease stages.44 These observations in fact fit well the proposed ontogenic model, which intuitively would imply that early disease arises in association with germinal centers and that extrafollicular extension parallels disease progression. Our findings also reinforce the hypothesis of the relationship of AITL to a peculiar form of PTCL-u with a follicular growth pattern and suspected to be derived from germinal center T cells,46 and perhaps to another form of PTCL with a parafollicular pattern of growth47 or with involvement of the follicular mantle zone.48
It is remarkable that a minor subset of normal T cells34 would be the cell of origin of one of the most frequent type of T-cell lymphoma. In view of the major role of the B-cell follicle in B-cell lymphomagenesis, with more than half of mature B-cell lymphomas deriving from germinal center cells, it is questionable whether mechanisms of genetic alterations similar to those operating in B-cell transformation may also target TFH cells.
Analysis of the molecular features of PTCLs-u considered as a whole has provided deceptively little information. The molecular features of PTCL-u were associated with common biologic functions, and GSEA failed to disclose any correlation with normal TFH, Th1, and Th2 subsets. These results may have been anticipated given the known heterogeneity within the PTCL-u category and the limited number of samples analyzed in this study, and in light of the complexity and variability of normal T-cell subsets, as well as their phenotypic modulation upon activation. The heterogeneity of PTCLs at the molecular level has been highlighted in a previous microarray study.43 Further larger studies are warranted to better understand the ontogeny of PTCL-u.
The distinct clustering of CD30+ and CD30− PTCLs-u suggested that these may be associated with distinct molecular features, and, indeed, we found that the CD30+ subset had reduced expression of genes with specific functions, in particular T-cell activation and TCR signal transduction factors such as Lck, Fyn, and Lyn. Of interest, lack of TCR expression is a feature of both ALK+ and ALK− subsets of ALCL, and it has been suggested that this characteristic may provide a unifying feature for ALCL.49 Expanding on this concept, our findings raise the question as to whether CD30 expression may be an objective criteria to delineate a meaningful PTCL category. The clinical and biologic relevance of this distinction should be investigated in larger series of cases. The reduced transcript levels of CD52 in CD30+ PTCL-u is of particular clinical interest, since pilot studies have confirmed the potential interest of the humanized anti-CD52 monoclonal antibody (alemtuzumab) for the treatment of T-cell lymphoproliferations,50 and the level of target expression may be an important determinant of response to therapy.51
Of importance, the results of our study provide novel pieces of information to substantiate the debate on the pathological classification of PTCLs, and more specifically the borders of the PTCL-u spectrum as opposed to AITL. Indeed, we found that the molecular signature of CD30− PTCL-u overlaps with that of TFH, although this is less significant than for AITL. Upon review of the cases, we identified a few PTCL-u cases with minimal AITL-like characteristics and molecular TFH-like features, which largely accounted for this statistical association. This suggests that CD30− PTCLs-u include some lymphomas derived from AITLs. Accordingly, the spectrum of AITL may be wider than suspected. Alternatively, it cannot be formally eliminated that a subgroup of PTCLs-u, distinct from AITL, may also derive from TFH cells but develop along a distinct pathogenic pathway. This raises the questions whether the classic diagnostic criteria used to differentiate both entities may be too stringent,2 and how to define the border between AITL and PTCL-u. Of interest, we were able to construct molecular predictors for the AITL versus PTCL-u distinction, and an 8-gene predictor yielded a very high success rate. Although these results need to be expanded validated on a larger series of samples, they lay the premise that the molecular classification may supplement other approaches for the classification of PTCLs.
The major finding presented herein is the identification of the putative normal cellular counterpart of AITL. In the future, this might provide a basis for a more precise diagnosis of AITL, and possibly for the elaboration of TFH-specific targeted therapies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Institut National de la Santé et de la Recherche Médicale (INSERM), by the Ligue Contre le Cancer and the program “Carte d'Identité des Tumeurs,” and by the Belgian National Fund for Scientific Research (FNRS). L.d.L. is a senior researcher and C.T. is a research assistant of the FNRS.
The authors wish to thank C. Coppeaux, S. Sousa, N. Martin-Garcia, and J. Marquet for their technical assistance; V. Fattaciolli for tumor banking; and F. Petel for submitting the data to EBI. We also thank our colleagues Drs N. Brousse, F. Charlotte, C. Chassagne-Clément, C. Lavignac, P. Pocachard, and I. Theate for providing samples.
Authorship
Contribution: L.d.L., L.X., and P.G. designed the research; C.T. and Y.-L.H. performed the research; L.d.L., C.T., Y.-L.H., G.D., L.L., K.L., J.B., T.M., F.B., C.G., and P.G. collected the data; L.d.L., D.S.R., C.T., A.R., C.G., L.X., and P.G. analyzed the data; D.S.R. and A.R. contributed vital analytical tools; and L.d.L., D.S.R., L.X., and P.G. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
L.d.L. and D.S.R. equally contributed to this work.
Correspondence: Laurence de Leval, Department of Pathology, CHU Sart-Tilman, Tour de Pathologie, +1, 4000 Liège, Belgium; e-mail: l.deleval@ulg.ac.be.

![Figure 2. GSEA ES curves and clustering results for the 2 significant gene signatures significantly overexpressed in AITL compared to PTCL-u. (A-B) ES curve (red) or the running sum of the weighted enrichment score obtained from GSEA software. Vertical blue lines indicate the position of each of the 100 genes (“Hits”) comprising the GS2_ TFH _up gene set. The graph on the bottom of each panel shows the ranked list metric (gray, SNR for A and fold change for B) for each gene as a function of the rank in the ordered data set. This corresponds to the level of correlation with the groups being tested (the far leftmost gene had the highest correlation with the AITL samples, and the far rightmost gene had the highest correlation with PTCL-u samples). (A) Results obtained for GS2_ TFH _up gene set in the comparison of AITL and PTCL-u using the SNR statistic to rank the genes from left to right (highest [0.9] to the lowest [−0.4] real SNR value, respectively). (B) Results obtained for the 42 core (or leading-edge) genes obtained from the analysis in panel A that were distributed in the top 1722 genes. The core genes in the ranked list represent the genes that are at, or before, the point where the running sum reaches its maximum deviation from zero (see Subramanian et al27 for more details). Here, the top 1722 genes obtained in panel A are ranked from highest (left) to lowest (right) fold change between the average of the 2 AITL-sorted cell samples (AITL cell) versus the average of the 17 AITL tissue samples and the hits represent the 42 core genes. (C) Clustering and heatmap of these 42 core genes obtained from the analysis shown in panel A. Data from T-cell subsets (TFH, Th1, and Th2; Chtanova et al21 and this study) were standardized by calculating an independent z-score (mean = 0 and standard deviation = 1) for each data set. Each gene was further standardized by mean-centering its expression profile across all samples. Genes are ordered by their rank calculated in the analysis shown in panel A. DNA-Chip Analyzer (dChip) Version 1.3 (Department of Biostatistics, Harvard School of Public Health, Boston, MA) was used to generate the heatmap and cluster the genes. Standardized expression ranges from −2.0 (blue) to 2.0 (red). Arrows point at the columns representing AITL cell suspensions.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/11/10.1182_blood-2006-10-055145/4/m_zh80110703680002.jpeg?Expires=1767779700&Signature=JS2~aHfOXRfbL0-K7lpQ2qDSz~-c3nNDXT7AiUpTyHAzbPCc0NBcF446~vkMgXhRjzdD-eong3vgpkZOlmF75AhTT6eQwHG8MQD6DbI1JErRis2OJGq2xL1o9q~NhyMgMkNyMS7Pujwp1qrQlW9Nh5Hxt8s7lc-TS43CE31OelAnbDewkr4phpnfgAmPNCMDgFvxoAB-o9~RVCA12etM0TmVY0V4KycmiZMVTzVET4bfwSIeazqsKNuKpNOz9Be8q~AuSsw9WOypM74Cif9afH1O6pghO~dym4vpLMYOvGKfcv-VNUVvFBLgRV2Vcnfm579JJ-VFvr5Umdu351exXQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 2. GSEA ES curves and clustering results for the 2 significant gene signatures significantly overexpressed in AITL compared to PTCL-u. (A-B) ES curve (red) or the running sum of the weighted enrichment score obtained from GSEA software. Vertical blue lines indicate the position of each of the 100 genes (“Hits”) comprising the GS2_ TFH _up gene set. The graph on the bottom of each panel shows the ranked list metric (gray, SNR for A and fold change for B) for each gene as a function of the rank in the ordered data set. This corresponds to the level of correlation with the groups being tested (the far leftmost gene had the highest correlation with the AITL samples, and the far rightmost gene had the highest correlation with PTCL-u samples). (A) Results obtained for GS2_ TFH _up gene set in the comparison of AITL and PTCL-u using the SNR statistic to rank the genes from left to right (highest [0.9] to the lowest [−0.4] real SNR value, respectively). (B) Results obtained for the 42 core (or leading-edge) genes obtained from the analysis in panel A that were distributed in the top 1722 genes. The core genes in the ranked list represent the genes that are at, or before, the point where the running sum reaches its maximum deviation from zero (see Subramanian et al27 for more details). Here, the top 1722 genes obtained in panel A are ranked from highest (left) to lowest (right) fold change between the average of the 2 AITL-sorted cell samples (AITL cell) versus the average of the 17 AITL tissue samples and the hits represent the 42 core genes. (C) Clustering and heatmap of these 42 core genes obtained from the analysis shown in panel A. Data from T-cell subsets (TFH, Th1, and Th2; Chtanova et al21 and this study) were standardized by calculating an independent z-score (mean = 0 and standard deviation = 1) for each data set. Each gene was further standardized by mean-centering its expression profile across all samples. Genes are ordered by their rank calculated in the analysis shown in panel A. DNA-Chip Analyzer (dChip) Version 1.3 (Department of Biostatistics, Harvard School of Public Health, Boston, MA) was used to generate the heatmap and cluster the genes. Standardized expression ranges from −2.0 (blue) to 2.0 (red). Arrows point at the columns representing AITL cell suspensions.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/11/10.1182_blood-2006-10-055145/4/m_zh80110703680002.jpeg?Expires=1767803582&Signature=36tIrN8K9ODheVosxAjRXXXRrE0Gedh3llkA4zmFl7~z7mRkpQHLD021rA4k1p~jE5TvySGjRn-yT~gqRHSsCtq0idSQoGHtSj9lJmA39uAWv5fGNcbvHfqn1ndKf5J-FndszGXmrPrfwbOnxoyYwzFT-Nhu-VFpBbRaCjKmaFNl2-DZMjlzOkRG2Yhum0X6qD1Op4EOo4t82mT~Y1InedHv~busEcYgxylTP3eb~4Uw8tdKAtMoasctmH0BA1Pjctn-4oInH7CK8iSA8mHuv8wgWMZsUlrhOCizxDxyV-8IZmIdHhQYhYz2blFdwImo4yRYau8A-2OrWPkJz8Cwtg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
