Abstract
MicroRNAs (miRNAs) are a novel class of small noncoding RNAs that modulate the expression of genes at the posttranscriptional level. These small molecules have been shown to be involved in cancer, apoptosis, and cell metabolism. In the present study we provide an informative profile of the expression of miRNAs in primary chronic lymphocytic leukemia (CLL) cells using 2 independent and quantitative methods: miRNA cloning and quantitative real-time–polymerase chain reaction (qRT-PCR) of mature miRNAs. Both approaches show that miR-21 and miR-155 are dramatically overexpressed in patients with CLL, although the corresponding genomic loci are not amplified. miR-150 and miR-92 are also significantly deregulated in patients with CLL. In addition, we detected a marked miR-15a and miR-16 decrease in about 11% of cases. Finally, we identified a set of miRNAs whose expression correlates with biologic parameters of prognostic relevance, particularly with the mutational status of the IgVH genes. In summary, the results of this study offer for the first time a comprehensive and quantitative profile of miRNA expression in CLL and their healthy counterpart, suggesting that miRNAs could play a primary role in the disease itself.
Introduction
Chronic lymphocytic leukemia (CLL) is the most common leukemia in the Western world, where it accounts for about 30% of all leukemias and presents a peak in the sixth decade of life. This disease is characterized by the accumulation of relatively mature B cells that display an aberrant coexpression of CD5 with B-cell antigens (CD19, CD20, and CD23). At present, little is known about the molecular mechanisms responsible for the expansion of the leukemic clone; in fact, whereas in the past many reports pointed to a defective apoptosis as the major cause of the pathology,1,2 growing evidence also indicates the presence of an aberrant proliferation,3 thus suggesting that CLL is sustained by a concomitant defect in both cell death and cell proliferation. From a clinical viewpoint, patients with CLL present a very different clinical course. In fact, some individuals have a very indolent disease, whereas others are characterized by a rapid disease progression requiring aggressive treatment.4 This clinical heterogeneity is, in part, sustained by different biologic parameters, such as the mutational status of the immunoglobulin variable genes (IgVH),5,6 ZAP-70 expression,7,8 and specific cytogenetic alterations.9 On the basis of the mutational status, patients with CLL can be classified into 2 major subgroups: the first group has hypermutations of the IgVH genes and a relatively stable disease, whereas the second group shows an unmutated IgVH configuration and is characterized by a more aggressive clinical course. ZAP70 is one of the most discriminative genes between the IgVH-mutated and -unmutated status in patients10 and has been proposed as a surrogate for IgVH status.7,11 Finally, a number of cytogenetic alterations are found in CLL that appear to contribute to the clinical scenario, the most common being the 13q14 deletion, occurring in about 60% of cases and usually associated with a better prognosis.6 Other cytogenetic anomalies are chromosome 12 trisomy, del 17p13, and del 11q23, the latter 2 being associated with a poor prognosis.9
Despite the extensive molecular characterization carried out on CLL cells, a candidate gene responsible for the disease is still lacking, perhaps reflecting its intrinsic nature of multifactor malignancy, probably requiring the accumulation of several genetic defects, none of which is alone sufficient to trigger the disease. Thus, there is a compelling need to identify other mechanisms that may play a major role in CLL.
The microRNAs (miRNAs) are an abundant class of small noncoding RNAs that modulate the expression of their target mRNAs at the posttranscriptional level.12 These RNAs are transcribed as long precursors and processed in various steps to yield RNA molecules that are 20 to 23 nucleotides long.13 In the cytoplasm, mature miRNAs are incorporated in the RNA-induced silencing complex (RISC),14 which is the catalytic engine responsible for target mRNA degradation or translation inhibition. At present, 462 miRNAs have been identified in the human genome, most of them evolutionarily conserved in mouse and rat (http://microrna.sanger.ac.uk/).
In animals, miRNAs recognize their targets by base pairing with a short region of about 7 nucleotides in the 3′ UTR of the target.15,16 On recognition by RISC, target mRNA translation is inhibited or, otherwise, the target itself is committed to degradation.12 Base complementarity between miRNAs and their targets has led to numerous bioinformatic approaches for miRNA target predictions.16,17 Such predictions suggest that about one third of genes are controlled by one or more miRNAs, with an average of about 200 targets for each miRNA.17 Therefore, these small regulators have a great potential in coordinately modulating expression of a large number of genes.18
Recently, many miRNAs have been shown to be involved in cancer, acting either as oncogenes19,20 or tumor suppressors.21 In particular, miR-155 has been shown to be up-regulated in B-cell lymphomas22,23 and indeed induction of up-regulation results in B-cell malignancy in mice.24 Many other reports have described altered expression of miRNAs in cancer samples compared to healthy counterparts, suggesting that these small RNAs could represent novel clinical and prognostic markers.25–27
An miRNA microarray strategy has been used recently to profile miRNA expression in CLL,28,29 and a miRNA signature, associated with different CLL subgroups has been suggested. Moreover, it has been suggested that miR-15a and miR-16 could play a role in the disease.30,31 However, microarray strategy is prone to mishybridization biases that could affect specificity; furthermore, because melting temperatures of different miRNAs vary widely, signal intensities on a DNA microarray are likely to be much more affected by the intrinsic properties of the target miRNA sequences rather than by the miRNA abundance per se, resulting in overestimation or underestimation of miRNA abundance. Careful measurement of miRNAs by a microarray strategy requires probe modifications that can account for differences in melting temperatures of miRNAs.32
In the present work, an informative profile of miRNA expression in CLL cells and their healthy counterparts is provided. Instead of a microarray methodology, a cloning approach was first used to obtain an overview of miRNA expression in these cell types and results were subsequently validated by quantitative real-time–polymerase chain reaction (qRT-PCR).
Patients, materials, and methods
The study was approved by the Institutional Review Board of the Department of Cellular Biotechnologies and Hematology, University “La Sapienza” (Rome, Italy).
Patients and controls
Fifty-six patients with a diagnosis of CLL were evaluated for miRNA profiling. The diagnosis of CLL was based on the presence of more than 5000 lymphocytes/μL expressing a typical CLL phenotype (CD5/CD20+, CD23+, weak surface immunoglobulin [sIg], CD10−) in the peripheral blood. All samples were collected from untreated patients. Five purified CD19+ samples from healthy male donors and 2 CD5/CD19+ samples from cord blood (thereafter referred to as CD5+) were used as controls. Written informed consent for biologic studies was obtained from all patients analyzed, in accordance with the Declaration of Helsinki.
Leukemic and control samples were positively selected using the CD19 human microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany), according to the manufacturer's instructions. This allowed us to achieve a purity of greater than 99%.
IgVH region analysis
IgVH gene rearrangements were amplified with a set of 6 VH gene family-specific primers that hybridize to sequences in the VH leader region in conjunction with a JH primer mix in separate reactions for each VH primer.33–35 A cut-off of more than 2% was established to define IgVH-mutated cases. Genomic DNA was sequenced as described.6
ZAP-70 protein detection
ZAP-70 protein expression was evaluated on mononuclear cells, after fixation and permeabilization, with flow cytometry and immunochemistry methods. For flow cytometry, ZAP-70 was tested by 3-color flow cytometry using the fluorescein-conjugated anti–ZAP-70 antibody (Alexa Fluor 488; Caltag Laboratories, Burlingame, CA), CD19-TC, and CD7-PE and analyzed by flow cytometry. For immunochemistry method, cytospins were prepared with a concentration of 5 × 104 cells/slide, air dried overnight, wrapped in aluminum foil, and stored at −20°C until immunostaining. The unconjugated anti–ZAP-70 clone 2F3.2 (Upstate Biotechnology, Waltham, MA) monoclonal antibody was used at a concentration of 1:60. Positive and negative controls were represented by the Jurkat and Raji cell lines, respectively. The proportion of ZAP-70+ cells was evaluated by light microscopy with oil immersion (original magnification, ×1000) examining 500 cells/sample. Cases were considered as ZAP-70+ if 20% or more of the cells were positive.
Fluorescence in situ hybridization
Fluorescence in situ hybridization (FISH) analysis was performed as previously described.6 The 13q14 region was evaluated with the D13S25 probe (Vysis, Downers Grove, IL), the 11q23 region with the ATM probe (Vysis), chromosome 12 with an α-satellite probe (Oncor, Gaithersburg, MD), and the 17p13 region with a cosmidic probe (Oncor).
Total RNA isolation, small RNA library preparation, sequencing, and sequence annotation
Total RNA was extracted using the TRIzol reagent (Invitrogen Carlsbad, CA); cloning was performed as previously described.36 Total RNA (30 μg) was used for each sample. Roughly 1000 miRNA molecules were independently cloned and sequenced for each sample.
The annotation was based on information from GenBank (http://www.ncbi.nih.gov/Genbank/index.html), a dataset of human tRNA sequences (http://lowelab.ucsc.edu/GtRNAdb/Hsapi/), a dataset of human sn/snoRNA sequences (Small RNA Database,37 snoRNA-LBME-db at http://www-snorna.biotoul.fr/index.php, NONCODE v138 and the data base from http://research.imb.uq.edu.au/rnadb/), a database of miRNAs (http://microrna.sanger.ac.uk/sequences/index.shtml; release version 7.1), and the repeat element annotation of version 17 of the human genome assembly, version 6 of the mouse genome assembly, and version 3 of the rat genome assembly from UCSC (http://genome.cse.ucsc.edu).
Reverse transcription of mature miRNAs
qRT-PCR amplification of miRNAs was performed as described,39 with minor modifications; we used a different adapter sequence for each miRNA instead of the same sequence for all miRNAs. This modification allowed us to perform reverse transcription of all miRNAs in a given sample at the same time.
Three miRNAs (miR-26b, miR-101, and miR-140) were excluded from our analysis because none of the 3 primer couples tested for each miRNA passed our preliminary tests (ie, low PCR efficiency or multiple PCR products detected or both). Moreover, we could not design primers that precisely discriminated between miR-29b and miR-29c; therefore, we decided to use a couple of primers that can amplify both molecules, reasoning that due to the extreme sequence homology those miRNAs are likely to have identical biologic effects, as suggested by target predictions.17
Primers used are listed in Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Total RNA (400 ng) was denatured for 3 minutes at 95°C and reverse transcribed with the Superscript III RNAse H− Reverse Transcriptase (Invitrogen) for 30 minutes at 50°C.
A 10-μL reaction contained each reverse-transcription primer (Table S1) at a final concentration of 0.1 μM. Superscript enzyme was inactivated by incubation at 85°C for 5 minutes.
Each reverse transcription primer consists of 2 different regions: the 3′ region is 7 to 12 nucleotides long and is complementary to the 5′ end of miRNA; the 5′ region (herein referred to as adapter) is a 20- to 22-nucleotide sequence not represented in the mammalian genomes.
qRT-PCR of mature miRNAs
Reverse transcription reaction volume was brought to 800 μL with ddH2O and 10 μL was used for each qPCR. qPCR was performed with iCycler (Bio-Rad, Hercules, CA) using iQ-SYBR-green supermix (Bio-Rad) according to the manufacturer's instructions and primers listed in Table S1.
Forward primers for PCR amplification are 14 to 17 nucleotides long LNA-modified40 DNA oligonucleotides. Three to 5 nonconsecutive nucleotides were LNA modified in the 5′ end of each oligonucleotide. Melting temperature of LNA primers was checked at http://lna-tm.com/. Reverse primers for PCR amplification are the reverse complementary sequence of adapters. PCR primer pair properties were evaluated with the software Perlprimer (http://perlprimer.sourceforge.net/). PCR amplifications were performed in triplicate with a relative error of about 30%.
Mature miRNAs were quantified with the standard curve method. Standard curves were obtained by qRT-PCR amplification of serial dilutions of synthetic DNA templates. U6 small nuclear RNA was measured with the same method and used for normalization. Blank and reverse transcription minus controls were included.
All oligonucleotides were purchased from Sigma-Aldrich (St Louis, MO).
Data analysis
The P values were calculated according to the Wilcoxon rank sum test.
Western analysis of BCL-2 protein
Cells were lysed for 45 minutes on ice in 20 mM Tris-HCL (pH 8), 1% Triton X-100, 1% NP-40, 10% glycerol, 137 mM NaCl, 10 mM EDTA (pH 8), 1 × protease inhibitor cocktail complete mini (Roche, Indianapolis, IN) and 1 mM PMSF. Proteins were separated on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) 12% gels and transferred to Protran nitrocellulose membrane (Schleicher and Schnell Bioscience, Dassel, Germany) by semidry electroblotting for 1 hour. Nitrocellulose filters were blocked with 5% fat-free milk in TBST. Membranes were hybridized using mouse monoclonal anti–Bcl-2 antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) at a final concentration of 2 μg/mL or with mouse monoclonal anti–β-tubulin I (Sigma) at a final concentration of 600 ng/mL, washed twice, and incubated for 45 minutes with goat anti–mouse IgG-HRP–conjugated antibodies (Bio-Rad). Filters were treated with enhanced chemiluminescence (ECL) Plus Western blotting detection system (GE Healthcare, Piscataway, NJ) according to the manufacturer's instructions. Finally, filters were exposed onto hyperfilm (Amersham, Freiburg, Germany). Films were scanned with a HP5500C scanner and densitometric analysis of gel bands was performed with the IMAGE J software.
Results
Patient characteristics
Of the 56 patients evaluated, 31 had mutations for the IgVH genes, whereas 23 had an unmutated configuration; the remaining 2 cases were not evaluated. Within the IgVH-mutated cases, the ZAP-70 protein was evaluated in 27 patients and proved negative in 19 cases and positive in 8. From a clinical standpoint, 13 patients had stable disease and 11 aggressive disease; for the remaining 7 patients the clinical period of observation was too short for evaluation.
Within the 23 IgVH-unmutated cases, 18 expressed ZAP-70 protein, whereas 5 did not. Within the IgVH-unmutated cases, 17 had aggressive disease and 6 had a short period of clinical observation. The clinicobiologic characteristics of the patients are summarized in Table 1.
Clinicobiologic characteristics of the patients
| Patient ID no. . | Disease status . | IgVH mutational status . | ZAP-70 . | del13q14, % . | del17p13, % . | Trisomy 12, % . | del11q23, % . | miRNA profile . | |
|---|---|---|---|---|---|---|---|---|---|
| Cloning . | qRT-PCR . | ||||||||
| 1 | Stable | Mutated | − | NE | NE | NE | NE | − | + |
| 2 | Stable | Mutated | NE | NE | NE | NE | NE | + | + |
| 3 | Progr | Mutated | − | Neg | Neg | Neg | Neg | − | + |
| 4 | Progr | Germline | + | Neg | Neg | Neg | Neg | + | + |
| 5 | ND | Germline | + | 86 | Neg | Neg | 81 | − | + |
| 6 | Progr | Germline | + | Neg | Neg | Neg | Neg | − | + |
| 7 | Progr | Germline | − | NE | NE | NE | NE | − | + |
| 8 | Progr | Germline | + | 94 | Neg | Neg | Neg | + | + |
| 9 | Stable | Mutated | + | Neg | Neg | Neg | Neg | + | + |
| 10 | ND | Germline | − | NE | NE | NE | NE | − | + |
| 11 | Stable | NE | − | NE | NE | NE | NE | − | + |
| 12 | Progr | Germline | + | 87 | Neg | Neg | Neg | − | + |
| 13 | Stable | Mutated | + | 11 | Neg | Neg | Neg | − | + |
| 14 | Stable | Mutated | NE | NE | NE | NE | NE | + | − |
| 15 | Progr | Germline | + | 40 | Neg | Neg | 48 | − | + |
| 16 | Progr | Germline | + | Neg | Neg | Neg | Neg | + | − |
| 17 | Stable | Mutated | − | Pos | Neg | Neg | Neg | + | − |
| 18 | ND | NE | NE | NE | NE | NE | NE | + | − |
| 19 | Progr | Mutated | − | 82 | Neg | Neg | Neg | − | + |
| 20 | ND | Mutated | NE | NE | NE | NE | NE | + | − |
| 21 | ND | Germline | + | NE | NE | NE | NE | − | + |
| 22 | ND | Mutated | + | Neg | 8 | Neg | Neg | − | + |
| 23 | ND | Germline | + | Neg | Neg | Neg | 61 | − | + |
| 24 | Progr | Germline | + | NE | NE | NE | NE | − | + |
| 26 | Stable | Mutated | − | 56 | 21 | Neg | 18 | − | + |
| 27 | ND | Mutated | − | 81 | Neg | Neg | Neg | − | + |
| 28 | Stable | Mutated | − | 41 | Neg | Neg | Neg | − | + |
| 30 | Stable | Mutated | − | Neg | Neg | Neg | Neg | − | + |
| 32 | Progr | Mutated | + | 99 | Neg | Neg | Neg | − | + |
| 35 | Stable | Mutated | − | 19 | Neg | Neg | Neg | − | + |
| 36 | ND | Mutated | − | Neg | NE | NE | NE | − | + |
| 37 | ND | Mutated | + | 74 homozygosis | Neg | Neg | Neg | − | + |
| 43 | Progr | Germline | + | Neg | Neg | Neg | Neg | − | + |
| 50 | Stable | Mutated | NE | NE | NE | NE | NE | − | + |
| 57 | ND | Germline | − | 78 | Neg | Neg | Neg | − | + |
| 58 | ND | Mutated | − | 72 | Neg | Neg | Neg | − | + |
| 59 | Progr | Mutated | − | 49 | Neg | Neg | Neg | − | + |
| 60 | ND | Mutated | − | 89 | Neg | Neg | Neg | − | + |
| 61 | Progr | Mutated | + | Neg | Neg | Neg | Neg | − | + |
| 62 | ND | Germline | − | Neg | Neg | 51 | Neg | − | + |
| 45 | Progr | Mutated | − | 50 | Neg | Neg | Neg | − | + |
| 64 | Progr | Germline | + | Neg | Neg | 75 | Neg | − | + |
| 65 | Progr | Germline | + | Neg | 8 | Neg | 95 | − | + |
| 66 | Progr | Mutated | − | 95 | Neg | Neg | Neg | − | + |
| 67 | Progr | Mutated | − | 89 | Neg | 74 | Neg | − | + |
| 68 | ND | Mutated | − | 63 homozygosis | Neg | Neg | Neg | − | + |
| 69 | Progr | Mutated | + | 84 | Neg | 60 | Neg | − | + |
| 70 | Progr | Germline | + | 80 | Neg | Neg | 95 | − | + |
| 71 | Progr | Germline | + | Neg | Neg | Neg | Neg | − | + |
| 72 | Stable | Mutated | − | Neg | 14 | Neg | Neg | − | + |
| 73 | Progr | Germline | + | Neg | Neg | Neg | 20 | − | + |
| 74 | Progr | Germline | + | Neg | 94 | Neg | Neg | − | + |
| 75 | Progr | Mutated | + | Neg | Neg | Neg | 6 | − | + |
| 76 | Progr | Mutated | − | 85 homozygosis | Neg | 74 | Neg | − | + |
| 77 | Progr | Germline | − | 85 | 8 | Neg | 19 | − | + |
| 78 | Progr | Germline | + | 89 | Neg | Neg | Neg | − | + |
| Patient ID no. . | Disease status . | IgVH mutational status . | ZAP-70 . | del13q14, % . | del17p13, % . | Trisomy 12, % . | del11q23, % . | miRNA profile . | |
|---|---|---|---|---|---|---|---|---|---|
| Cloning . | qRT-PCR . | ||||||||
| 1 | Stable | Mutated | − | NE | NE | NE | NE | − | + |
| 2 | Stable | Mutated | NE | NE | NE | NE | NE | + | + |
| 3 | Progr | Mutated | − | Neg | Neg | Neg | Neg | − | + |
| 4 | Progr | Germline | + | Neg | Neg | Neg | Neg | + | + |
| 5 | ND | Germline | + | 86 | Neg | Neg | 81 | − | + |
| 6 | Progr | Germline | + | Neg | Neg | Neg | Neg | − | + |
| 7 | Progr | Germline | − | NE | NE | NE | NE | − | + |
| 8 | Progr | Germline | + | 94 | Neg | Neg | Neg | + | + |
| 9 | Stable | Mutated | + | Neg | Neg | Neg | Neg | + | + |
| 10 | ND | Germline | − | NE | NE | NE | NE | − | + |
| 11 | Stable | NE | − | NE | NE | NE | NE | − | + |
| 12 | Progr | Germline | + | 87 | Neg | Neg | Neg | − | + |
| 13 | Stable | Mutated | + | 11 | Neg | Neg | Neg | − | + |
| 14 | Stable | Mutated | NE | NE | NE | NE | NE | + | − |
| 15 | Progr | Germline | + | 40 | Neg | Neg | 48 | − | + |
| 16 | Progr | Germline | + | Neg | Neg | Neg | Neg | + | − |
| 17 | Stable | Mutated | − | Pos | Neg | Neg | Neg | + | − |
| 18 | ND | NE | NE | NE | NE | NE | NE | + | − |
| 19 | Progr | Mutated | − | 82 | Neg | Neg | Neg | − | + |
| 20 | ND | Mutated | NE | NE | NE | NE | NE | + | − |
| 21 | ND | Germline | + | NE | NE | NE | NE | − | + |
| 22 | ND | Mutated | + | Neg | 8 | Neg | Neg | − | + |
| 23 | ND | Germline | + | Neg | Neg | Neg | 61 | − | + |
| 24 | Progr | Germline | + | NE | NE | NE | NE | − | + |
| 26 | Stable | Mutated | − | 56 | 21 | Neg | 18 | − | + |
| 27 | ND | Mutated | − | 81 | Neg | Neg | Neg | − | + |
| 28 | Stable | Mutated | − | 41 | Neg | Neg | Neg | − | + |
| 30 | Stable | Mutated | − | Neg | Neg | Neg | Neg | − | + |
| 32 | Progr | Mutated | + | 99 | Neg | Neg | Neg | − | + |
| 35 | Stable | Mutated | − | 19 | Neg | Neg | Neg | − | + |
| 36 | ND | Mutated | − | Neg | NE | NE | NE | − | + |
| 37 | ND | Mutated | + | 74 homozygosis | Neg | Neg | Neg | − | + |
| 43 | Progr | Germline | + | Neg | Neg | Neg | Neg | − | + |
| 50 | Stable | Mutated | NE | NE | NE | NE | NE | − | + |
| 57 | ND | Germline | − | 78 | Neg | Neg | Neg | − | + |
| 58 | ND | Mutated | − | 72 | Neg | Neg | Neg | − | + |
| 59 | Progr | Mutated | − | 49 | Neg | Neg | Neg | − | + |
| 60 | ND | Mutated | − | 89 | Neg | Neg | Neg | − | + |
| 61 | Progr | Mutated | + | Neg | Neg | Neg | Neg | − | + |
| 62 | ND | Germline | − | Neg | Neg | 51 | Neg | − | + |
| 45 | Progr | Mutated | − | 50 | Neg | Neg | Neg | − | + |
| 64 | Progr | Germline | + | Neg | Neg | 75 | Neg | − | + |
| 65 | Progr | Germline | + | Neg | 8 | Neg | 95 | − | + |
| 66 | Progr | Mutated | − | 95 | Neg | Neg | Neg | − | + |
| 67 | Progr | Mutated | − | 89 | Neg | 74 | Neg | − | + |
| 68 | ND | Mutated | − | 63 homozygosis | Neg | Neg | Neg | − | + |
| 69 | Progr | Mutated | + | 84 | Neg | 60 | Neg | − | + |
| 70 | Progr | Germline | + | 80 | Neg | Neg | 95 | − | + |
| 71 | Progr | Germline | + | Neg | Neg | Neg | Neg | − | + |
| 72 | Stable | Mutated | − | Neg | 14 | Neg | Neg | − | + |
| 73 | Progr | Germline | + | Neg | Neg | Neg | 20 | − | + |
| 74 | Progr | Germline | + | Neg | 94 | Neg | Neg | − | + |
| 75 | Progr | Mutated | + | Neg | Neg | Neg | 6 | − | + |
| 76 | Progr | Mutated | − | 85 homozygosis | Neg | 74 | Neg | − | + |
| 77 | Progr | Germline | − | 85 | 8 | Neg | 19 | − | + |
| 78 | Progr | Germline | + | 89 | Neg | Neg | Neg | − | + |
NE indicates not evaluated; Progr, progressive; Neg, negative; ND, not defined.
Cloning of miRNAs reveals a misregulation of several miRNAs in CLL
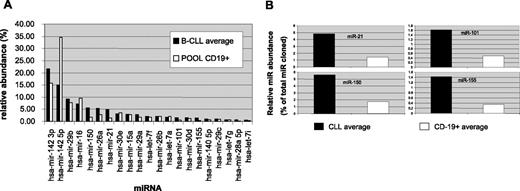
With the aim of characterizing the miRNA expression profile of CD19+ cells purified from the peripheral blood of CLL patients and healthy donors, a set of 9 CLL cases (Table 1), as well as a pool derived from 8 healthy donors (herein referred to as POOL), were evaluated by miRNA cloning following the protocol described by Pfeffer et al.36 This approach allowed us to obtain a semiquantitative profile of miRNAs expressed in these cell types (Figure 1; Table S2).
miRNA expression profile of CLL and CD19+ normal cells. (A) The relative abundance of each miRNA species is plotted as a percentage of total miRNAs cloned. ■ indicates average of the values observed in the 9 CLL samples processed; □, pool of RNA from CD19+ cells from 8 different healthy donors. miRNAs representing at least 0.5% of total miRNAs on average in CLL are reported. (B) The relative abundance of the 4 miRNAs displaying differential expression between CLL samples (■) and POOL (□) is plotted as percentage of total miRNAs cloned.
miRNA expression profile of CLL and CD19+ normal cells. (A) The relative abundance of each miRNA species is plotted as a percentage of total miRNAs cloned. ■ indicates average of the values observed in the 9 CLL samples processed; □, pool of RNA from CD19+ cells from 8 different healthy donors. miRNAs representing at least 0.5% of total miRNAs on average in CLL are reported. (B) The relative abundance of the 4 miRNAs displaying differential expression between CLL samples (■) and POOL (□) is plotted as percentage of total miRNAs cloned.
miRNA cloning indicated that about 20 miRNA species contribute more than 90% of total miRNAs in patients as well as in the POOL sample. Moreover, miR-142-3p and miR-142-5p, originating from the same pre-miRNA, accounted together for about 37% of total miRNA in CLL samples and 50% of total miRNA in the POOL.
The miRNA profiles of the 9 patients were very similar to each other with few exceptions (Table S2). For example, the profile of patient 14 displayed a very low level of miR-15a compared to the other patients and to the POOL. Consistently, the expression of miR-16, whose precursor pre-miR-16-1 is transcribed from the same locus 13q14, was also affected in the same patient.
Comparison of the CLL samples with the POOL was even more informative (Figure 1B); miR-21 and miR-155 were on average 3.4- and 4-fold overexpressed in CLL compared to the POOL, respectively. Moreover, both miR-101 and miR-150 displayed about a 3-fold higher expression in CLL.
qRT-PCR validation of cloning results
To validate the results obtained by miRNA cloning and to acquire a more quantitative profile of the expression of these miRNAs, 22 miRNAs (Table S1) were measured in 4 patients already analyzed by cloning and in 47 additional patients by qRT-PCR amplification of mature miRNAs molecules. Five CD19+ samples from healthy donors were included as controls. Moreover, because many papers in the literature refer to CD5+ cells rather than to CD19+ cells as the genuine counterpart of CLL, 2 CD5+ samples purified from umbilical blood cord were also included in our analysis.
This approach, which requires much smaller amounts of total RNA as a starting material, allowed us, after selection of most expressed miRNAs by the cloning approach, to extend our analysis to a larger number of patients. The miRNAs representing on average more than 0.5% of total miRNAs in CLL were selected for qRT-PCR analysis.
Five miRNAs that did not reach the 0.5% threshold were included: miR-15b, because of its homology with miR-15a and miR-16, possibly involved in the disease; miR-222 and miR-92, due to the possible down-regulation in CLL compared to healthy controls suggested by the cloning data; miR-223, which has been reported to be associated with the IgVH mutational status, as well as ZAP-70 expression, both representing important prognostic factors in CLL28 ; and miR-191, as a control to assess if indeed also qRT-PCR confirms its low abundance detected by cloning.
The results are reported in Table S3.
A comparison between the miRNA cloning and the qRT-PCR results is provided in Table S4; the results obtained are reported for each of the 4 patients whose miRNA profile was assessed with both methods. The correlation between the 2 measures is also reported for each of the 4 patients, highlighting a substantially good agreement.
The main difference between the 2 methods used is represented by the expression levels of miR-142-5p; whereas the cloning suggests that its abundance is roughly comparable to the one of miR-142-3p, qRT-PCR suggests that it is about 7-fold less expressed than miR-142-3p both in patients and controls.
Using the qRT-PCR approach, our data revealed that the expression of several miRNAs was significantly (P < .01) affected in patients compared to healthy controls, regardless of whether CD19+ or CD5+ samples are used as a control (Table 2).
miRNAs significantly deregulated in CLL compared to normal CD19+ cells
| miRNA . | CLL/CD19+ ratio . | P . |
|---|---|---|
| mir-21 | 3.80 | < .001 |
| mir-150 | 2.00 | < .001 |
| mir-155 | 5.34 | < .001 |
| mir-92 | 0.60 | < .001 |
| mir-222 | 0.24 | < .001 |
| miRNA . | CLL/CD19+ ratio . | P . |
|---|---|---|
| mir-21 | 3.80 | < .001 |
| mir-150 | 2.00 | < .001 |
| mir-155 | 5.34 | < .001 |
| mir-92 | 0.60 | < .001 |
| mir-222 | 0.24 | < .001 |
CLL patients were characterized by the significant overexpression of miR-21 (3.8-fold), miR-150 (2-fold), and miR-155 (5.3 fold); furthermore, down-regulation of miR-92 (2-fold) and miR-222 (4-fold) was also significant (Figure 2; Table 2). Overall, these data are in agreement with the cloning approach and indicate that CLL is characterized by deregulation of these miRNAs. It is worth pointing out that most of these miRNAs were deregulated in the vast majority of patients analyzed (eg, 43 of 51 patients displayed an up-regulation of miR-21 > 2-fold, 44 of 51 patients displayed an up-regulation of miR-155 of at least 2-fold).
qRT-PCR analysis of miRNA expression. Expression levels of the miRNAs significantly deregulated in CLL samples compared to CD19+ controls. ■ indicates CLL average expression levels; □, CD19+ average expression levels. Error bars represent the SEM. P values were assessed according to the Wilcoxon rank sum test.
qRT-PCR analysis of miRNA expression. Expression levels of the miRNAs significantly deregulated in CLL samples compared to CD19+ controls. ■ indicates CLL average expression levels; □, CD19+ average expression levels. Error bars represent the SEM. P values were assessed according to the Wilcoxon rank sum test.
Furthermore, 6 patients were characterized by a dramatic down-regulation of miR-15a (about 25-fold; P < .001) and miR-16 (5 fold, P < .001). FISH analysis (Table 1) revealed that 3 of these 6 patients carried a deletion of both 13q14 regions (encompassing the mir-15a/mir16-1 cluster) in a very high percentage (63%-85%) of CLL cells; cytogenetic analysis was not available for 2 patients (nos. 7 and 10) and only a single patient harbored a hemizygous deletion of the 13q14 region. It is worth mentioning that no other patient displayed a double deletion of the 13q14 locus.
It has been reported that miR-15a and miR-16 down-regulation could contribute to BCL-2 overexpression observed in CLL.31 However, Western blot analysis of BCL-2 expression displayed that a dramatic down-regulation of these miRNAs (25-fold) was not paralleled by any significant change in BCL-2 expression (Figure S2).
Comparison between different CLL classes in patients reveals a differential expression of miRNAs
Next, we sought to identify potential differences of the expression levels of each miRNA within the 51 CLL cases analyzed by qRT-PCR.
When the miRNA expression profile of patients with mutated IgVH was compared to the one of unmutated patients, 3 miRNAs displayed a significant difference in expression levels: miR-223, miR-150, and the miR-29b and 29c. However, as shown in Figure 3, whereas all of these miRNAs were on average up-regulated in mutated samples compared to unmutated samples, it is not always true that a patient bearing the mutation has a higher expression of all these miRNAs and vice-versa. For example, patient 43 had a relatively high expression of miR-150, miR-29bc, and miR-223, despite having an unmutated status. In line with this, a higher miR-150 expression was observed in ZAP-70− patients. As pointed out in the case of miR-222, again the qRT-PCR approach, in agreement with cloning, showed that miR-223 is expressed at very low levels.
Three miRNAs are differentially expressed between IgVH-mutated and IgVH-unmutated CLL cases. Data obtained by qRT-PCR amplification of mature miRNAs are plotted. P values for each miRNA are shown. Boxes represent SE. Error bars represent SD.
Three miRNAs are differentially expressed between IgVH-mutated and IgVH-unmutated CLL cases. Data obtained by qRT-PCR amplification of mature miRNAs are plotted. P values for each miRNA are shown. Boxes represent SE. Error bars represent SD.
The genomic loci encoding miR-21 and miR-155 are not amplified
To verify whether miRNAs up-regulation in patients is due to the amplification of the genomic loci encoding for these small RNAs, the DNA copy number of the mir-155 and mir-21 loci has been determined by qPCR of genomic DNA. Genomic DNA from patients and healthy donors was analyzed and showed that an increase in miRNA expression was not paralleled by the corresponding locus amplification (Figure S1), suggesting that deregulation in the expression of these miRNAs occurred at the transcriptional or posttranscriptional level (or both).
Discussion
In this paper, 2 different independent and complementary methods were used to obtain a profile of miRNA expression in patients with CLL compared to healthy donors. The cloning method and the qRT-PCR analysis displayed a striking agreement, identifying the same miRNAs (miR-21, miR-150, miR-155, miR-92, and miR-92) as differentially expressed between pathologic samples and controls. miR-21 and miR-155 appeared to be significantly deregulated in almost every CLL patient sample analyzed, suggesting that the overexpression of these miRNAs is a general characteristic of CLL rather than a phenomenon observed in a subclass of patients.
miR-21, whose up-regulation in CLL was on average 3.8-fold with a maximum of 10-fold compared to normal CD19+ cells, and even greater if purified CD5+ cord blood cells are used as controls, has been demonstrated to be an antiapoptotic factor highly expressed in human glioblastoma.41 This finding is in agreement with the fact that defective apoptosis plays an important role in CLL: overexpression of miR-21 may, therefore, be one of the initiating events occurring in CLL. miR-155 was also up-regulated in CLL (5.3-fold on average, 23-fold in patient 15) consistent with previous reports on miR-155 overexpression in other B-cell malignancies.22,24,42 This observation corroborates the hypothesis that the expression of this miRNA could play an important role in controlling proliferation of B lymphocytes. Analysis of the genomic loci encoding for these 2 miRNAs did not reveal any amplification, thus suggesting that deregulation of miR-21 and miR-155 occurs at a transcriptional or posttranscriptional level. Further analysis is required to elucidate the factors involved in the regulation of miR-21 and miR-155 expression.
miR-150 was also 2-fold overexpressed in CLL; to the best of our knowledge this is the first report linking this miRNA directly to cancer, although its inhibition has resulted in cell growth inhibition in both HeLa and lung carcinoma cells.43 Finally, miR-92 was about 2-fold down-regulated in CLL patients. Based on previous reports, suggesting that the miRNA cluster encompassing it acts as an oncogene, one would rather expect its up-regulation in CLL compared to healthy controls.
As for miR-222, it is of notice that it was expressed at very low levels according to both the cloning and the qRT-PCR results, suggesting that this miRNA may not play a primary role in the pathogenesis of CLL.
Identification of these 4 miRNAs, which are differentially expressed in the patients compared to healthy controls, could represent the first step toward the identification of gene regulation networks whose deregulation could be involved in the disease initiation or progression.
Moreover, both methods showed that the ratio between the abundance of the products of pre-miR-142 in patients and controls is about 0.7. This small but reproducible and significant difference is interesting because this small RNA is a modulator of the hematopoietic lineage differentiation, whose up-regulation during hematopoiesis induces a significant increase of T lymphocytes and a decrease in B lymphocytes.44 Its down-regulation in a B-lymphocyte malignancy is therefore consistent with previous observations.
Previous reports by Calin et al and Cimmino and colleagues28–31 described a down-regulation of miR-15a and miR-16 in the majority of CLL patients; indeed, we also observed a dramatic down-regulation of miR-15a and miR-16, but only in a small subset of patients. In fact, we found a low expression of these miRNAs in 11.7% of patients by qRT-PCR and in a similar percentage (11.1%) by cloning, once again showing a good concordance between the 2 methods. All patients bearing a deletion of both 13q14 regions had a dramatic miR-15a down-regulation, whereas patients bearing a hemizygous 13q14 deletion, in a variable percentage of the CLL clone, were substantially indistinguishable from controls (P = .17). Moreover, at variance from a previous report describing an inverse correlation between miR-15a and BCL-2 levels as well as between miR-16 and BCL-2 levels in CLL,31 we observed that a dramatic down-regulation of both miRNAs was not paralleled by any significant increase in BCL-2 expression levels. These data do not support the hypothesis that in CLL the high levels of BCL-2 expression are secondary to a down-modulation of mir-R15a and mir-16. In any case, this possibility was unlikely also in view of the fact that the 13q14 deletion is detected only in about 60% of CLL patients (58% of the cases hereby analyzed), indicating that this abnormality is not primarily responsible for the disease.
As previously mentioned, another relevant issue in the management of CLL patients is represented by the heterogeneous clinical scenario, that is correlated, among other biologic factors, with the IgVH mutational status5,6 ; in light of this, we sought to identify miRNAs that were associated with this feature and found an overexpression of miR-150, miR-223, and miR-29b, and miR-29c in the IgVH-mutated CLL cases compared to the IgVH-unmutated cases. These findings are in line with the observations by Calin et al,28 who described the up-regulation of miR-223, miR-29b, and miR-29c in patients with IgVH mutations, whereas the up-regulation of miR-150 in IgVH-mutated cases is reported here for the first time. Overall, these results indicate that these miRNAs are closely associated to biologic features with known implications for prognostic factors and may therefore prove useful in patient stratification. Because the number of cases so far evaluated is still relatively limited, further analyses on an extended prospective cohort of patients are required to conclusively define the prognostic relevance of these miRNAs in patients with CLL.
In summary, our work provides novel information about miRNA expression in CLL. On the one hand, the study of the miRNA expression profile identifies a set of miRNAs—miR-21, miR-92, miR-150, and miR-155—that are differentially expressed between patients and healthy donors and therefore represent putative molecules that may play a major role in leukemogenesis initiation. To our knowledge, this is the first report that describes a differential expression of the aforementioned miRNAs between neoplastic and healthy cells. On the other hand, we provide evidence of small RNAs differentially expressed between IgVH-mutated and IgVH-unmutated CLL cases, which could be useful tools whose prognostic significance needs to be further evaluated.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by Fondo Investimenti Ricerca di Base–Ministero Istruzione Università Ricerca 2001 (RBNEO15MPB_001/RBNE01KXC9_006); Associazione Italiana per la Ricerca sul Cancro (AIRC), Milan; Ministero dell'Istruzione, dell'Università e della Ricerca, Rome; and Ministero della Salute, Rome, Italy.
Authorship
Contribution: V.F. designed research, performed miRNA cloning and qRT-PCR amplification of mature miRNAs, analyzed qRT-PCR data, and drafted the manuscript; S.C. designed research, extracted RNA and DNA, and drafted the manuscript; M.G. performed qRT-PCR amplification of mature miRNAs, measured DNA copy number of miR-155 and miR-21 loci, and analyzed qRT-PCR data; G.A. and L.C. designed oligonucleotides and performed qRT-PCR of mature miRNAs; N.C. performed Western blot analyses; S.T. and M.M. extracted RNA and DNA and provided protein pellets for Western blot analyses; A.M. analyzed data; F.C. critically revised the manuscript; R.M. and N.P. performed immunomagnetic purification on samples from patients with CLL as well as from healthy donors and umbilical cord blood; S.S. carried out IgVH sequencing; F.R.M. managed patients and provided follow-up data of the patients described in the manuscript; P.L. provided vital reagents and analyzed cloning data; T.T. provided vital reagents and critically revised the manuscript; D.B.W., M.C., and J.J.R. performed miRNA sequencing; J.J., R.S., C.S., and M.Z. analyzed miRNA sequencing data; and A.G., R.F., and G.M. designed research, analyzed the data, and critically revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
V.F., S.C., and M.G. contributed equally to this work.
Correspondence: Giuseppe Macino, V Clinica Medica, Policlinico Umberto I, Viale Regina Elena, 324 00161, Rome, Italy; e-mail: macino@bce.uniroma1.it.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal