Abstract
The proapoptotic function of p53 is thought to underlie most anticancer modalities and is also activated in response to oncogenic insults, such as overexpression of the Myc oncoprotein. Here we generated tractable B lymphomas using retroviral transduction of the MYC oncogene into hematopoietic cells with 2 knock-in alleles encoding a fusion between p53 and 4-hydroxytamoxifen (4OHT) receptor (p53ERTAM). In these polyclonal tumors, Myc is the only oncogenic lesion, and p53ERTAM status can be rapidly toggled between “off” and “on” with 4OHT, provided that the Trp53 promoter has been independently activated. Although 4OHT can trigger widespread apoptosis and overt tumor regression even in the absence of DNA-damaging agents, in tumors with high levels of Mdm2 these responses are blunted. However, cotreatment with proteasome inhibitors fully restores therapeutic effects in vivo. Similarly, human Burkitt lymphomas with wild-type p53 and overexpression of Hdm2 are highly sensitive to proteasome inhibitors, unless p53 levels are reduced using the HPV-E6 ubiquitin ligase. Therefore, proteasome inhibitors could be highly effective as a monotherapy against Myc-induced lymphomas, with no need for adjuvant chemotherapy or radiation therapy. On the other hand, their efficacy is crucially dependent on the wild-type p53 status of the tumor, placing important restrictions on patient selection.
Introduction
Tumor chemoresistance is often associated with inactivation of the proapoptotic p53 tumor suppressor.1,2 In many cases, this occurs through point mutations or chromosomal deletions or both, making the tumor amenable to gene or protein therapy.3 However, in other tumors, such as Myc-overexpressing Burkitt lymphomas (BLs), the p53 gene is often intact and its inactivation occurs at the posttranslation level.4–6 Such tumors can in principle undergo apoptosis in response to p53 activation with genotoxic agents, such as cyclophosphamide, hydroxydaunomycin, Oncovin (vincristine), prednisone (CHOP regimen) or methotrexate, bleomycin, Adriamycin (doxorubicin), cyclophosphamide, Oncovin, dexamethasone (m-BACOD regimen).7 In reality, however, they are usually defective in the p53-mediated apoptotic pathway, owing to deregulation of its upstream regulators and downstream effectors.8
The main negative regulator of p53 stability and activity is mouse double-minute 2 (Mdm2, Hdm2 in humans), an oncoprotein that antagonizes p53 function by 3 mechanisms: (1) acceleration of p53 nuclear export and its sequestration in the cytoplasm9–12 ; (2) masking of the N-terminal transactivation domain13,14 ; and most importantly, (3) acting as an E3 ubiquitin ligase to target p53 for proteasomal degradation.15–17 Although other E3 ligases can ubiquitinate p53,18–20 overexpression of Mdm2 is the best-documented alternative to p53 mutations in nonviral tumors,21,22 including BL.23 In preneoplastic cells, the oncogenic effects of Mdm2 are counteracted by the Arf protein, which inhibits Mdm2-dependent ubiquitination of p53.24–27 Interestingly, common oncogenic insults, such as Myc-induced hyperproliferation, are strong inducers of Arf and therefore of p53.28,29 Given the propensity of p53 to induce apoptosis, the “Arf-Mdm2” pathway is commonly disrupted in Myc-induced tumors even prior to genotoxic therapy.29–31
This observation implies that many types of neoplastic cells maintain functional levels of p53, be it through intrinsic DNA damage, or oncogene activation, or another type of stress. To address this hypothesis, a knock-in mouse model has recently been developed wherein the endogenous Trp53 promoter is driving expression of p53ERTAM, a p53-estrogen receptor fusion protein whose function is strictly dependent on 4-hydroxytamoxifen (4OHT).32 However, mobilization of p53 with 4OHT does not occur unless the Trp53 promoter is independently activated. Using this model, it has been demonstrated that the treatment of mice bearing preneoplastic cells with 4OHT affords a degree of protection against lymphomas induced by either radiation33 or overexpression of the Myc transgene.34 Proapoptotic activity of p53ERTAM in normal tissues is balanced by endogenous Mdm2.35 Whether this basal activity can be unmasked toward therapeutic ends in tumors with Arf/Mdm2 aberrations remained to be seen.
We had previously developed and extensively characterized a murine B-lymphoma model based on retroviral transduction of the Myc oncoprotein into p53-null hematopoietic cells.36–39 Transduction of Myc into bone marrow cells of naïve p53ERTAMmice also yields transplantable tumors of B-cell lineage.40 In some of them, activation of p53ERTAM with 4OHT causes overt tumor regression, whereas others are largely refractory to treatment with 4OHT. Here, using 4OHT-resistant neoplasms, we demonstrate the ability of proteasome inhibitors to overcome Mdm2/Hdm2-dependent resistance in both murine and human lymphomas and reveal the role of p53 status in therapeutic responses to these compounds.
Materials and methods
Animal models
The p53ERTAM mice have been described previously.32 Retroviral transduction of bone marrow cells was performed as described.36 Resultant tumors were propagated using subcutaneous injections into syngeneic recipients (F1 hybrid B6129PF1/J mice, stock no. 100492, Jackson Laboratories, Bar Harbor, ME) as described previously.38 Alternatively, 107 neoplastic cells were injected into the tail vein of a syngeneic mouse. Human B-lymphoma cells Ly47, Ly91, and Ramos were injected into scid mice (National Cancer Institute, Frederick, MD) subcutaneously, 2 × 106 cells/mouse. Treatment with 4OHT has been described earlier.38 Alternatively, in vivo experiments were carried out using tamoxifen (T5648, Sigma-Aldrich, St Louis, MO), of which 4OHT is a metabolite. Bortezomib (Millennium Pharmaceuticals, Cambridge, MA) was dissolved in saline and delivered via intraperitoneal injections (1 mg/kg). Epoxomicin (BioMol International, Plymouth Meeting, PA) was dissolved in 10% DMSO and also delivered via intraperitoneal injections (1 μmol/kg).
Analysis of tumor specimens
The analysis of tumor clonality was performed using the detection of D-J recombination events as described previously.36 Hematoxylin and eosin stainings were carried out using standard histologic techniques. TUNEL staining was performed using the fluorescein-labeled In Situ Cell Death Detection Kit (Roche Diagnostics, Indianapolis, IN) per the manufacturer's recommendations. Immunohistochemical staining for p53 was carried out using the antibodies from Santa Cruz Biotechnology (Santa Cruz, CA, catalog no. sc-6243) essentially as described.38
In vitro culturing and analyses of B-lymphoid cells
Neoplastic cells were cultured either on the feeder layer of S17 cells or in the medium supplemented with LPS as described previously.37 All BL cell lines were cultured in RPMI 1640 medium supplemented with 10% FBS. Where indicated, 4OHT, epoxomicin, bortezomib, or cycloheximide (Sigma-Aldrich) was added to culture medium at the concentrations of 0.25 μM, 0.5 μM, 10 nM, and 30 μg/mL, respectively. Flow cytometry for green fluorescent protein (GFP) was performed using the FACSCalibur apparatus (Becton Dickinson, Mountain View, CA) and the data analysis software CellQuest (Becton Dickinson).
Real-time PCR
Total RNAs were isolated using TRI reagent (Sigma-Aldrich) and treated with a TURBO DNA-free kit (Ambion, Austin, TX). cDNAs were prepared using SuperScript First-Strand Synthesis System for reverse transcription-polymerase chain reaction (RT-PCR; Invitrogen, Carlsbad, CA). Amplification reactions were performed using titanium Taq DNA polymerase (BD Biosciences, Palo Alto, CA), SYBR green I (Roche) in LightCycler (Roche). The following cycling parameters were used: 1 second at 95°C for denaturation and 15 seconds at 70°C for annealing and extension for the total of 35 cycles. Primer sequences are available on request.
Immunoblotting
Standard immunoblotting techniques were applied. Where indicated, subcellular fractionation was performed using Nuclear/Cytosol Fractionation Kit (Biovision, Mountain View, CA, catalog no. K266-100). The following Santa Cruz Biotechnology primary antibodies were used: anti-p53 (sc-6243), anti-Mdm2 (sc-812), anti-Arf (sc-32748), and anti-PCNA (sc-9857). The anti–β-actin antibody was obtained from Sigma-Aldrich (clone TAC-40).
Generation of and infection with Arf- and E6-encoding retroviruses
To generate the MIGR1-Arf retrovirus, the full-length p19ARF cDNA (catalog no. 7057366, GenBank ID no. BC058190) was purchased from the American Type Culture Collection (Manassas, VA), subcloned into the pSL1180 phagemid vector (Amersham Pharmacia, Piscataway, NJ) using KpnI and XbaI restriction enzymes, and subsequently inserted into the MIGR1 retrovirus using the EcoRI restriction site. For in vitro infection with the Arf retrovirus, GP293-packaging cells were transfected with either MIGR1 or MIGR1-Arf using Lipofectamine 2000 (Invitrogen, San Diego, CA). Conditioned media were harvested and added to p53ERTAM lymphoma cells for 12 hours. Percentages of GFP+ cells in infected cultures were enumerated every 24 hours using flow cytometry. For HPV-E6 infections,41 recombinant DNAs were transfected into GP293-packaging cells and conditioned media containing retroviruses (E6 or empty LXSN vector) were used to infect BL cells. Infected cells were then selected in medium containing 1 mg/mL G418.
Results
Reactivation of p53ERTAM in Myc-induced lymphomas fails to regress tumors with high Mdm2 levels
To generate neoplasms bearing 2 p53ERTAM alleles, bone marrow cells from several homozygous mice were harvested, admixed with LMycSN retrovirus-producing packaging cells, and reinjected subcutaneously in syngeneic hosts, as described previously.36,40 Mice receiving transplants were kept off 4OHT, to prevent p53 from being precociously active. Four to 5 weeks later, animals engrafted with Myc-transduced bone marrow cells developed tumors of B-cell lineage (data not shown). The experiment was repeated twice, and the arising neoplasms were additionally passaged in naïve recipients. All tumors with immunoglobulin heavy-chain rearrangements were polyclonal, as judged by the analysis of their D-J junctions, suggesting that no selection for single-cell clones had taken place (Figure 1A). Nevertheless, independent transduction experiments yielded both ArfHIGHMdm2LOW and ArfLOWMdm2HIGH neoplasms (immunoblotting in Figure 1B insert). We hypothesized that the former (dubbed Mp53ERSEN) would be more sensitive to p53-dependent apoptosis than the latter (dubbed Mp53ERRES).
Molecular and cellular analyses of p53ERTAM lymphomas. (A) Analysis of tumor clonality through PCR amplification and detection of D-J junctions by Southern blotting. A, B, and C refer to 3 independent p53ERTAM tumors. gDNAs from rodent fibroblasts and pro-B cells without D-J rearrangements were used as negative controls. gDNAs from total bone marrow and a previously characterized Myc-induced polyclonal lymphoma were used as positive controls. (B) Tumor volumes versus days of 4OHT treatment. Plots with squares and circles refer to Mp53ERSEN and Mp53ERRES tumors, respectively. Solid lines/filled symbols and dashed lines/open symbols refer to untreated and 4OHT-treated mice, respectively. Error bars represent SD. The inset depicts immunoblotting on Mp53ERSEN and Mp53ERRES cells, using anti-Arf and anti-Mdm2 antibodies. β-Actin was used as a loading control. (C, top) Histologic staining of representative specimens from panel B. Tissues were harvested 3 hours after the commencement of 4OHT treatment. (Middle) TUNEL staining of adjacent sections. (Bottom) Immunohistochemical staining for p53. Brown color refers to p53 expression, and blue color is counterstaining with hematoxylin. In the last 4 panels, insets represent tumors from untreated animals. Sections were analyzed using Axioskop 40 microscope (Carl Zeiss, Maple Grove, MN) equipped with a 20×/0.45 NA Achroplan objective lens and 10× eyepieces. Images were captured using Evolution QEi camera (MediaCybernetics, Houston, TX) and Phase 3 ImagePro software (Imaging Systems, Bridgewater, NJ) and further processed using Adobe Photoshop CS v8.0 (Adobe Systems, San Jose, CA).
Molecular and cellular analyses of p53ERTAM lymphomas. (A) Analysis of tumor clonality through PCR amplification and detection of D-J junctions by Southern blotting. A, B, and C refer to 3 independent p53ERTAM tumors. gDNAs from rodent fibroblasts and pro-B cells without D-J rearrangements were used as negative controls. gDNAs from total bone marrow and a previously characterized Myc-induced polyclonal lymphoma were used as positive controls. (B) Tumor volumes versus days of 4OHT treatment. Plots with squares and circles refer to Mp53ERSEN and Mp53ERRES tumors, respectively. Solid lines/filled symbols and dashed lines/open symbols refer to untreated and 4OHT-treated mice, respectively. Error bars represent SD. The inset depicts immunoblotting on Mp53ERSEN and Mp53ERRES cells, using anti-Arf and anti-Mdm2 antibodies. β-Actin was used as a loading control. (C, top) Histologic staining of representative specimens from panel B. Tissues were harvested 3 hours after the commencement of 4OHT treatment. (Middle) TUNEL staining of adjacent sections. (Bottom) Immunohistochemical staining for p53. Brown color refers to p53 expression, and blue color is counterstaining with hematoxylin. In the last 4 panels, insets represent tumors from untreated animals. Sections were analyzed using Axioskop 40 microscope (Carl Zeiss, Maple Grove, MN) equipped with a 20×/0.45 NA Achroplan objective lens and 10× eyepieces. Images were captured using Evolution QEi camera (MediaCybernetics, Houston, TX) and Phase 3 ImagePro software (Imaging Systems, Bridgewater, NJ) and further processed using Adobe Photoshop CS v8.0 (Adobe Systems, San Jose, CA).
We directly tested responses of Mp53ERSEN and Mp53ERRES tumors to 4OHT, which on its own does not inhibit lymphomagenesis.34 Palpable tumors were allowed to form, and animals were randomized into 2 groups. Mice in the first group were kept off 4OHT, whereas mice in the second group began to receive daily injections of 4OHT. To ensure statistical significance, no less than 6 tumors were tested in each treatment group. Tumor volumes were measured and recorded daily. Beginning 24 hours after randomization, all Mp53ERSEN tumors in 4OHT-treated animals sharply decreased in volume and subsequently shrank further, becoming too small to measure (Figure 1B). This decrease was highly significant, per Student t test (P = .001). In contrast, all Mp53ERRES decreased in volume during the first 24 hours but then stabilized and resumed progressive growth.
To characterize the cellular mechanisms of resistance, hematoxylin-eosin and TUNEL stainings were performed on representative tumor specimens. As evidenced by hematoxylin-eosin staining, prior to 4OHT treatment, both Mp53ERSEN and Mp53ERRES tumors were mostly composed of viable cells and lacked large apoptotic areas (data not shown). However, 3 hours after 4OHT treatment, only cells in Mp53ERRES tumors remained viable, but Mp53ERSEN neoplasms contained almost exclusively dying cells (Figure 1C, top row). This finding was corroborated by TUNEL staining. Untreated tumors contained isolated clusters of TUNEL+, apoptotic cells that were embedded into largely healthy tumor tissues (Figure 1C middle row insets). On treatment with 4OHT, widespread apoptosis was apparent in Mp53ERSEN tumors, whereas Mp53ERRES neoplasms exhibited only a small increase in the number of TUNEL+ cells (Figure 1C, middle row).
Resistance to 4OHT correlates with rapid degradation of p53ERTAM in the nucleus
To determine the molecular mechanisms of resistance to 4OHT, we compared representative Mp53ERSEN and Mp53ERRES tumors using immunohistochemical (IHC) staining with an anti-p53 antibody. This approach revealed similar levels of p53ERTAM in untreated tumors (Figure 1C bottom row insets). However, following 4OHT treatment, p53ERTAM levels were sustained in Mp53ERSEN neoplasms but were virtually undetectable in Mp53ERRES tumors (Figure 1C, bottom row). To facilitate further analysis, we briefly cultured Mp53ERSEN and Mp53ERRES in vitro on the monolayer of γ-irradiated S17 stromal cells and subsequently in feeder layer-free conditions in media supplemented with LPS.37 The cultures retained both the LMycSN and the p53ERTAM transgenes (genomic PCR data not shown), indicating that they represent bona fide neoplastic cells.
We next examined the expression levels of p53ERTAM before and after stimulation with 4OHT. Immunocytochemistry (Figure 2A) and immunoblotting (Figure 2B) using an anti-p53 antibody confirmed that prior to 4OHT treatment, Mp53ERSEN and Mp53ERRES cells had similar levels of p53ERTAM, which was mostly cytoplasmic and presumably in a complex with heat-shock proteins.42 After several hours of treatment with 4OHT, p53ERTAM levels in the cytoplasm decreased. However, whereas in Mp53ERSEN cells p53ERTAM was stably expressed in the nucleus, almost no nuclear p53ERTAM was detectable in Mp53ERRES cells (Figure 2A-B), fully consistent with the IHC results (Figure 1B, bottom row).
Analyses of Mp53ERSEN and Mp53ERRES cells in vitro. (A) Immunocytochemical detection of p53ERTAM in acetone-fixed cells. Cells were counterstained with DAPI to visualize nuclei. Where indicated, cells were pretreated with 4OHT for 6 hours. Images were captured and processed as described in Figure 1C, except that the fluorescent light source was used. (B) Immunoblotting detecting p53ERTAM in whole-cell lysates and cytosolic and nuclear fractions. Actin and PCNA were used as control cytosolic and nuclear proteins, respectively. The duration of 40HT treatment was 3 hours. (C) Quantitation of the real-time RT-PCR experiment comparing expression levels of Puma and Noxa mRNAs in 40HT-treated versus untreated cells. Error bars represent SD. (D) Flow cytometric analyses of tumor cells stained with annexin V (left) and TMRE (right). Numbers refer to percentages of cells committed to apoptosis.
Analyses of Mp53ERSEN and Mp53ERRES cells in vitro. (A) Immunocytochemical detection of p53ERTAM in acetone-fixed cells. Cells were counterstained with DAPI to visualize nuclei. Where indicated, cells were pretreated with 4OHT for 6 hours. Images were captured and processed as described in Figure 1C, except that the fluorescent light source was used. (B) Immunoblotting detecting p53ERTAM in whole-cell lysates and cytosolic and nuclear fractions. Actin and PCNA were used as control cytosolic and nuclear proteins, respectively. The duration of 40HT treatment was 3 hours. (C) Quantitation of the real-time RT-PCR experiment comparing expression levels of Puma and Noxa mRNAs in 40HT-treated versus untreated cells. Error bars represent SD. (D) Flow cytometric analyses of tumor cells stained with annexin V (left) and TMRE (right). Numbers refer to percentages of cells committed to apoptosis.
To confirm low levels of functional p53ERTAM in nuclei of Mp53ERRES cells, we performed real-time RT-PCR on transcripts of proapoptotic genes known to be direct p53 targets: Puma43–45 and Noxa.46 These mRNAs were induced in both tumor types. However, whereas in Mp53ERSEN cells gene activation was very robust (10- to 15-fold), only a minimal (< 2-fold) increase in steady-state levels was observed in Mp53ERRES cells (Figure 2C). This deficiency could translate into impaired 4OHT-dependent apoptosis. Indeed, when cells were stained with annexin V (to identify the loss of membrane asymmetry) and TMRE (to identify the loss of membrane potential in the mitochondria), Mp53ERRES cells proved to be largely viable, whereas Mp53ERSEN cells were highly prone to apoptosis (Figure 2D).
Lack of nuclear p53ERTAM was likely due to its rapid degradation. To confirm this mechanism, we directly measured p53ERTAM stability in the absence and the presence of 4OHT. Control and 4OHT-treated cells were exposed to cycloheximide (CHX) to block new protein synthesis, and p53ERTAM steady-state levels were followed using Western blotting. In the absence of 4OHT, p53ERTAM levels did not change appreciably even after 4 hours of CHX treatment. However, when CHX was added along with 4OHT, p53ERTAM levels decreased drastically as early as in 30 minutes (Figure 3A.) This short half-life is typical of wild-type, nuclear p53.47 We thus concluded that in Mdm2-overexpressing tumor cells p53ERTAM undergoes rapid degradation in conjunction with nuclear translocation.
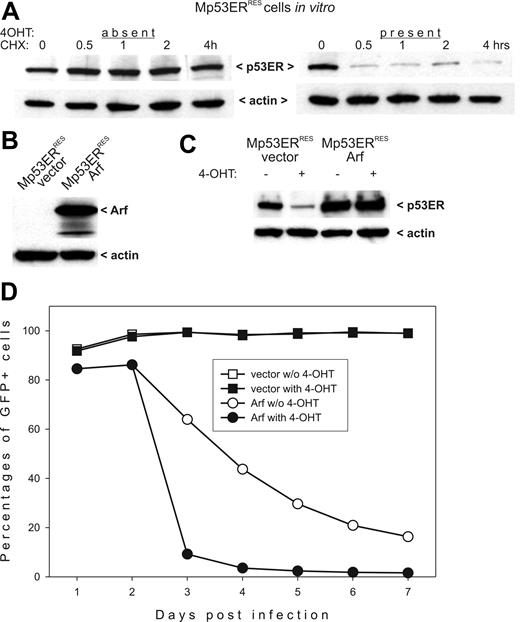
p53ERTAM stability in Mdm2-and Arf-overexpressing cells. (A) Immunoblotting detecting p53ERTAM in cycloheximide (CHX)–treated Mp53ERRES cells in the absence (left panel) and the presence (right panel) of 4OHT. (B) Immunoblotting detecting Arf in Mp53ERRES cells infected with the control (“vector”) and the Arf-transducing (“Arf”) retroviruses. (C) Immunoblotting detecting p53ERTAM in the same cultures. Where indicated, cells were pretreated with 4OHT for 3 hours. (D) Selection against Arf-overexpressing Mp53ERRES cells in mixed cultures. When indicated, 4OHT was added on day 2 after infection.
p53ERTAM stability in Mdm2-and Arf-overexpressing cells. (A) Immunoblotting detecting p53ERTAM in cycloheximide (CHX)–treated Mp53ERRES cells in the absence (left panel) and the presence (right panel) of 4OHT. (B) Immunoblotting detecting Arf in Mp53ERRES cells infected with the control (“vector”) and the Arf-transducing (“Arf”) retroviruses. (C) Immunoblotting detecting p53ERTAM in the same cultures. Where indicated, cells were pretreated with 4OHT for 3 hours. (D) Selection against Arf-overexpressing Mp53ERRES cells in mixed cultures. When indicated, 4OHT was added on day 2 after infection.
Overexpression of Arf in Mdm2HIGH tumors restores p53ERTAM levels and salvages responses to 4OHT in vitro
To determine whether apoptotic responses in Mp53ERRES cells are salvageable, we attempted to restore p53ERTAM levels using overexpression of the Mdm2 antagonist Arf. We cloned the Arf cDNA into the MigR1 bi-cistronic retrovirus coexpressing, through the IRES element, the GFP. The Arf/GFP and the control viruses were used to infect Mp53ERRES cells. The expression of exogenous Arf in transduced cells was confirmed by immunoblotting (Figure 3B). Importantly, when Arf-transduced cells were treated with 4OHT, no degradation of p53ERTAM was apparent (Figure 3C).
To determine whether restored p53ERTAM levels compromised the viability of Arf-overexpressing cells, we used the previously developed selection assay.39 According to this experimental procedure, retrovirally transduced cultures are passaged in vitro and percentages of GFP+ cells are tracked. A sharp decline in transduction rates is indicative of cell cycle arrest or apoptosis, both of which are attributable to p53. Indeed, as evidenced by data in Figure 3D, cultures initially containing about 90% of Arf/GFP-expressing cells eliminate the majority of them within 2 days, as long as 4OHT is present in culture medium. Interestingly, we observed a loss of transduced cells, albeit at a much slower rate, even without 4OHT administration, possibly indicative of p53-independent effects of Arf. In contrast, there was no significant change in the numbers of GFP+ cells in the control group infected with the “empty” retrovirus, with or without 4OHT treatment (Figure 3D).
Proteasome inhibitors restore p53ERTAM levels, salvage apoptotic responses, and cause tumor regression
Experiments with the Arf-transducing retrovirus provided a proof of principle that Mdm2 effects of p53ERTAM can be overcome at a posttranslational level. We thus tested whether p53 levels in Mp53ERRES cells can be restored using 2 proteasome inhibitors: epoxomicin,48 which had been successfully used in an in vivo animal model,49 and bortezomib (Velcade50 ), which has been approved by the Food and Drug Administration for the treatment of multiple myeloma. In vitro, incubation of Mp53ERRES cells with either epoxomicin (not shown) or bortezomib (Figure 4A, left panel) for 3 hours had no effect on basal p53 levels but efficiently prevented its 4OHT-dependent degradation.
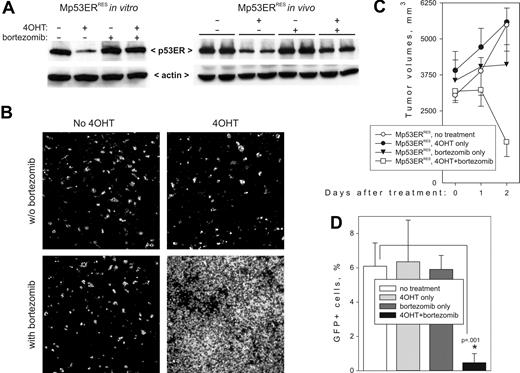
Restoration of apoptotic responses and tumor regression by bortezomib. (A) Immunoblotting detecting p53ERTAM in Mp53ERRES cells (left) and tumors (right) treated with 4OHT or bortezomib or both. For the in vivo experiment, 2 independent tumors were included in each treatment group. (B) TUNEL staining of the same tumors. Images were captured and processed as described in Figure 2A. (C) Kinetics of tumor growth in 4OHT- or bortezomib-treated animals. Each group contained 4 mice; error bars represent SD. (D) The effects of 4OHT and bortezomib on circulation of neoplastic cells in peripheral blood following tail vein injections. Each group contained 5 mice; error bars represent SD.
Restoration of apoptotic responses and tumor regression by bortezomib. (A) Immunoblotting detecting p53ERTAM in Mp53ERRES cells (left) and tumors (right) treated with 4OHT or bortezomib or both. For the in vivo experiment, 2 independent tumors were included in each treatment group. (B) TUNEL staining of the same tumors. Images were captured and processed as described in Figure 2A. (C) Kinetics of tumor growth in 4OHT- or bortezomib-treated animals. Each group contained 4 mice; error bars represent SD. (D) The effects of 4OHT and bortezomib on circulation of neoplastic cells in peripheral blood following tail vein injections. Each group contained 5 mice; error bars represent SD.
We then asked whether p53 status affects biologic responses to bortezomib. Mp53ERRES tumor-bearing mice were treated with bortezomib, alone or in combination with 4OHT. We found that administration of bortezomib effectively restored p53 levels in 4OHT-treated animals (Figure 4A, right panel). Importantly, although neither drug alone induced significant cell death, the combination of bortezomib and 4OHT led to widespread apoptosis after only 24 hours (TUNEL staining data in Figure 4B). Moreover, after 48 hours, overt tumor regression was apparent in mice treated with bortezomib plus 4OHT, but not in those treated with bortezomib alone (Figure 4C).
To assess the effects of proteasome inhibitors on systemic disease, we also injected Mp53ERRES cells into tail veins of syngeneic recipients, which results in colonization of bone marrow and viscera, as documented previously.38 Mice receiving the injections were kept untreated for several weeks, until neoplastic cells have appeared in the bloodstream (as judged by flow cytometry for GFP). Then animals were randomized into treatment groups. 4OHT was administered on days 21, 22, and 23, and bortezomib on days 21 and 22. Elimination of neoplastic cells was assessed using flow cytometry 1 week later. As in the experiments with subcutaneous tumors, neither 4OHT alone nor bortezomib alone was effective. However, the combined action of the 2 drugs reduced the number of circulating cells by at least 90% (Figure 4D).
p53 dictates apoptotic responses to bortezomib in BLs
To extend our discoveries to spontaneous B-cell neoplasms, we investigated the efficacy of bortezomib as a monotherapy against BLs with Hdm2 overexpression. In a pilot experiment, we measured Hdm2 levels in lysates of Ly91, Ly47, Ag876 (all wild-type p5323,51,52 ), and Ramos (mutant p5353 ) cells. As controls, we used EBV-immortalized nontumorigenic lymphoblastoid cell lines GM607 and LCL1. We found that, as described previously,23,51 Ly91 and Ly47 (but not Ag876) express high levels of Hdm2. Curiously, Ramos cells were also found to overexpress Hdm2, perhaps as a vestige of their original wild-type p53 status (Figure 5A). Subsequent experiments were performed with Ly91, Ly47, and Ramos cells, which have similar levels of Hdm2 but different p53 status. Additionally, we generated Ly47 and Ly91 derivatives, expressing the human papillomavirus (HPV) E6 protein, another E3 ligase capable of p53 ubiquitination20 but refractory to common proteasome inhibitors.53
Elicitation of apoptotic responses in BLs by bortezomib. (A) Immunoblotting detecting Hdm2 levels in lysates of BL Ly 91, Ly47, Ag876, and Ramos, and lymphoblastoid cell lines GM607 and LCL1. (B) Immunoblotting detecting p53 levels in Ly47 and Ramos cells in vitro. Ly47 cells were additionally infected with either empty vector or the E6-encoding retrovirus. Bar graphs below blots represent quantitation of p53-specific bands. (C) TUNEL staining of Ly47 and Ramos tumors from mice treated with bortezomib or vehicle alone. (D) Quantitation of tumor cell apoptosis. Bars refer to average numbers of TUNEL+ cells per microscopic field. No less than 4 fields were counted for each tumor specimen. Error bars represent SD. The asterisk refers to a statistically significant difference in apoptosis between Ly47/vector and Ly47/E6 tumors.
Elicitation of apoptotic responses in BLs by bortezomib. (A) Immunoblotting detecting Hdm2 levels in lysates of BL Ly 91, Ly47, Ag876, and Ramos, and lymphoblastoid cell lines GM607 and LCL1. (B) Immunoblotting detecting p53 levels in Ly47 and Ramos cells in vitro. Ly47 cells were additionally infected with either empty vector or the E6-encoding retrovirus. Bar graphs below blots represent quantitation of p53-specific bands. (C) TUNEL staining of Ly47 and Ramos tumors from mice treated with bortezomib or vehicle alone. (D) Quantitation of tumor cell apoptosis. Bars refer to average numbers of TUNEL+ cells per microscopic field. No less than 4 fields were counted for each tumor specimen. Error bars represent SD. The asterisk refers to a statistically significant difference in apoptosis between Ly47/vector and Ly47/E6 tumors.
Treatment of Ly91 (not shown) and Ly47 (Figure 5B) cells with bortezomib resulted in an approximately 2-fold increase in p53 levels. Additional overexpression of the E6 protein decreased both basal and induced p53 levels. As a result, p53 levels in bortezomib-treated Ly47/E6 cells were similar to those in untreated Ly47/vector cells. Treatment of Ramos cells with bortezomib caused minimal up-regulation of p53.
Ly47 and Ramos cells were implanted in immunocompromised mice and palpable neoplasms were allowed to form. Animals were then treated with bortezomib for 24 hours and tumors were harvested and subjected to TUNEL staining. Ramos tumors were largely refractory to treatment with bortezomib, but in vector-transduced Ly47 tumors bortezomib caused massive apoptosis, with the vast majority of cells becoming nonviable. However, in Ly47/E6 tumors with diminished capacity to activate p53, numbers of dying cells were drastically reduced (Figure 5C-D).
Discussion
The success of most anticancer therapies is predicated on the induction of tumor cell apoptosis, which often depends on p53.1 Thus, activation of p53 by DNA-damaging agents such as cancer chemotherapeutics and ionizing radiation is seen as germane to these treatments. However, it remains unclear whether chemotherapy and radiation therapy are able to activate p53 in a sustained fashion. On the other hand, p53 can also be activated by a variety of stress signals including, but not limited to, oncogene overexpression.2 The prime example of such p53 activator is the Myc oncoprotein, which has been reported to directly activate the p53 promoter.54 Myc also regulates p53 at the posttranslational level, through up-regulation of Arf that counteracts Mdm2-assisted degradation of p53.28 Thus, an intriguing possibility is that in Myc-overexpressing tumors, the trp53 is constitutively turned on and as long as the p53 protein is functional (ie, not ubiquitinated by Mdm2), tumor cell apoptosis is the likely outcome. This idea is given credence by extensive studies on Eμ-myc lymphomas,55 wherein both alleles of p53 or Arf need to be inactivated before frankly malignant neoplasms develop.29,30 On the contrary, haploinsufficiency for Mdm2 is known to inhibit lymphomagenesis.56 That p53-mediated antitumor surveillance is a major block to the ability of Myc to induce B lymphomagenesis is also illustrated by frequent expression in BL of mutant Myc variants that are unable to activate the BH3-only proapoptotic protein Bim.57 Yet direct evidence implicating Myc as a major p53 activator in vivo is still lacking.
Another under-explored aspect of p53 biology is its role as a target for proteasome inhibitors.58 Although in rodent Rat-1 fibroblasts stabilization of p53 does cause apoptosis,59 in primary human fibroblasts exposure to the proteasome inhibitors causes transcriptional activation of the cdk-inhibitor p21 and therefore growth arrest.60,61 Similar observations were made using human colon carcinoma cells.62 In several tumor cell lines (primary effusion lymphoma,63 adult T-cell lymphoma,64 multiple myeloma,65 and prostate cancer66 ), bortezomib-induced apoptosis was accompanied by up-regulation of p53 but also by inhibition of NF-κB and bcl-2 pathways and direct activation of the caspase cascade. These pleiotropic effects mask the role of stabilized p53 in therapeutic apoptosis. Furthermore, in yet other tumor cell lines (mantle cell lymphoma,67 glioblastoma multiforme,68 leukemia,69 colon cancer,70 and prostate cancer71 ) bortezomib-induced apoptosis can occur in the absence of p53. However, with some exceptions (eg, Kamat et al72 ), most studies have been performed in vitro and have not directly addressed the role of p53 as a proteasome inhibitor target in vivo. Consequently, the choice between using proteasome inhibitors as a monotherapy or in combination with chemotherapy remains largely empirical.
Our data and another recently published report34 argue that Myc-induced lymphomas can succumb to apoptosis and overtly regress on activation of p53 in the absence of any extrinsic DNA damage. Moreover, even in apoptosis-resistant tumors p53ERTAM is abundantly produced but is targeted for proteasomal degradation following nuclear translocation. When proteasomal degradation is blocked, through either Arf overexpression or epoxomicin/bortezomib treatment, p53 appears to be fully functional and readily triggers apoptosis in vivo. Conversely, when p53 levels in bortezomib-sensitive human BL cells are compromised using HPV-E6 overexpression, therapeutic apoptosis is dramatically reduced. Our results have important implications for the field of proteasome inhibitors. We believe that their use against BL might have to be limited to lymphomas with the wild-type TP53 gene. However, in those tumors a proteasome inhibitor such as bortezomib might be highly effective as a monotherapy.
Note added in proof:
The same week this article was published as a First Edition manuscript, 2 papers appeared in Nature demonstrating therapeutic effects of restoring p53 function in the mouse models of liver carcinoma,73 T lymphoma, and sarcoma.74
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health grants CA 097932 and CA 102709 and Commonwealth of Pennsylvania Health Research Formula Fund no. 4100020574 (A.T.-T.).
We are indebted to Drs Gerard Evan and Maria Christophorou (University of California, San Francisco) for providing us with p53ERTAM mice, sharing data prior to publication, and insightful comments on the manuscript. We are grateful to Dr Denise Galloway (Fred Hutchinson Cancer Research Center, Seattle, WA) for the gift of the LXSN/E6 retrovirus. We thank Dr Joelle Wiels (Institut Gustave Roussy, Villejuif, France) for Ly47 and Ly91 and Dr Riccardo Dalla-Favera (Columbia University, New York, NY) for Ag876 and Ramos Burkitt lymphoma cell lines. Drs Carlo Croce (Ohio State University, Columbus) and Earl Robertson (University of Pennsylvania, Philadelphia) are acknowledged for the gift of GM607 and LCL1 lymphoblastoid cell lines, respectively. We also thank Drs Ravi Amaravadi and Craig Thompson (University of Pennsylvania, Philadelphia) and members of our laboratories for many stimulating discussions.74
National Institutes of Health
Authorship
Contribution: D.Y. codesigned and performed the experiments; M.C. designed experiments with bortezomib; and A.T.-T. codesigned the experiments and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andrei Thomas-Tikhonenko, Department of Pathobiology, University of Pennsylvania, M/C 6051 3800 Spruce St, Philadelphia, PA 19104; e-mail: andreit@mail.vet.upenn.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal