Abstract
Although much is known about the transcriptional profiles of dendritic cells (DCs) during maturation, the molecular switches critical for the induction of a tolerogenic program in DC subsets are still obscure. We examined the gene-expression profiles of murine splenic CD8+ DCs rendered highly tolerogenic by interferon-γ (IFN-γ), which activates the enzyme indoleamine 2,3-dioxygenase (IDO, encoded by Indo) and thus initiates the immunosuppressive pathway of tryptophan catabolism. By examining the expression of a series of relevant genes in IDO+ compared with IDO- DCs, we found consistent and selective association of the IDO-competent phenotype with down-modulation of the Tyrobp gene, encoding the signaling adapter DAP12, which typically associates with activating receptors. Down-modulation of Tyrobp involved IFN consensus sequence binding protein (ICSBP), a transcription factor also known as IRF-8. In murine and human monocyte-derived DCs, silencing DAP12 expression imparted IDO functional competence to IDO- cells, whereas silencing IRF-8 in IDO+ counterparts abolished IDO expression and function. Thus, IRF-8 is required in tolerogenic DCs for the positive regulation of Indo and the negative regulation of Tyrobp. Overall, these studies reveal the occurrence of a simple and evolutionarily conserved code in the control of tolerance by an ancestral metabolic enzyme.
Introduction
Dendritic cells (DCs) represent a heterogeneous population that plays a critical role in the induction of immunity and tolerance. The decision between these 2 opposite outcomes depends on several factors, among them the occurrence of specialized DC subsets and the maturation or activation state of the DCs.1-4 One mechanism exploited by tolerogenic DCs involves indoleamine 2,3-dioxygenase (IDO), a tryptophan-catabolizing enzyme. IDO-competent DCs exert regulatory effects on T cells that are mediated by tryptophan depletion and by the production of metabolic byproducts collectively known as kynurenines.5-7 The control of IDO transcription is complex and cell-type specific,6 though IFN-γ can be considered the principal inducer.8 In addition, IDO activity is also regulated at the posttranslational level.6
Through use of a series of stimuli, we previously demonstrated that the default functional program of murine splenic CD8- and CD8+ DCs in the presentation in vivo of a poorly immunogenic antigen is highly flexible.9 In particular, IL-610 and CD28-Ig,11 a soluble form of CD28, would similarly affect the functional phenotype of both types of cell through increased stimulatory capacity in CD8- DCs and the ablation of the tolerogenic ability of CD8+ DCs. In contrast, interferon-γ (IFN-γ), though capable of potentiating the tolerogenic program of CD8+ DCs in an IDO-dependent fashion,12 could not modify the default immunogenic function of CD8- DCs. The cytokine, indeed, does not confer tryptophan-catabolizing activity on CD8- DCs, although the resultant IDO expression is comparable in the 2 DC subtypes.13 However, cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), either in a soluble form (ie, CTL0-4-Ig) or anchored to the membrane of regulatory T cells, does activate an IFN-γ/IDO-dependent tolerogenic program in CD8+ and CD8- DCs.9,14,15
In human DCs, it has recently been suggested that CTL0-4-Ig is unable to activate IDO and requires high levels of IFN-γ for full expression of enzyme activity.16 However, we have recently demonstrated that the fusion protein will induce immunosuppressive IDO activity in human DCs matured by LPS but not by CD40 ligand (CD40L).17 Contrasting results have been found regarding the occurrence of a subset of monocyte-derived DCs that might constitutively express IDO and suppress allogeneic T-cell responses.18-20 Overall, these data suggest that tryptophan catabolism in human DCs could result from a complex integration of multiple environmental factors, including experimental conditions and maturation signals.
In the present study, we analyzed the gene-expression profile of murine CD8+ DCs treated with IFN-γ. By comparing the expression of relevant genes in IDO+ and IDO- DCs and using gene silencing in combination with DC-mediated priming, we found that the turning on of an IDO-dependent tolerogenic program in murine DCs requires tight comodulation of a restricted number of genes. The same “tolerogenic signature” characterizes the IDO+ phenotype of human DCs.
Materials and methods
Mice and reagents
Female DBA/2J (H-2d) mice were obtained from Charles River Laboratories (Calco, Milan, Italy). The source and characteristics of murine rIFN-γ and rIL-6 were as described previously.10 Human rIFN-γ (hIFN-γ) was from PeproTech (Inalco, Milan, Italy). CTL0-4-Ig and CD28-Ig consisted, respectively, of a fusion protein between the extracellular domain of mouse CTLA-4 or CD28 and the Fc portion of a mouse IgG3 antibody, as described.11,14 hCTL0-4-Ig, consisting of the extracellular domain of human CTLA-4 and the Fc portion used for the generation of mouse CTL0-4-Ig, was prepared and purified as described.17 Human rFlt3-L and trimeric CD40L were from Immunex (now Amgen; Seattle, WA). The synthetic peptide NRP-A7 (KYNKANAFL) was synthesized and purified as described.21
DC purification
Murine splenic DCs were prepared and fractionated according to CD11c/CD8α expression using positive selection columns in combination with CD11c and CD8α MicroBeads (Miltenyi Biotec, Bergish Gladbach, Germany), as described previously.14,15 Before purification of CD8+ DCs to be used in microarray experiments (but not for the rest of the experiments), donor mice were injected intraperitoneally with 10 μg Flt3-L daily for 9 days. After DC purification, the recovered cells were greater than 98% CD11c+, greater than 99% MHC I-A+, greater than 98% B7-2+, less than 0.1% CD3+, and less than 0.5% B220+. After cell fractionation, the recovered CD8- cells typically contained less than 0.5% contaminating CD8+ DCs, whereas the CD8+ fraction was made up of greater than 95% CD8+ DCs. Human blood mature CD11c+ DCs were obtained as described.17 Briefly, positively selected CD14+ monocytes (Miltenyi Biotec) were cultured for 5 days in the presence of 50 ng/mL human GM-CSF (Schering-Plough, Milan, Italy) and 200 U/mL human IL-4 (PeproTech). The resultant monocyte-derived immature DCs were washed and cultured for 24 hours with 1 μg/mL LPS or CD40L to obtain mature DCs. FACS analysis revealed that mature DCs were CD1a+, CD11c+, CD11b+, CD14low, CD8-, HLA class IIhigh, CD80high, and CD86high (Vacca et al17 and data not shown).
DC treatments
Unless otherwise stated, murine CD8+ and CD8- DCs were exposed overnight to 200 U/mL rIFN-γ,12,13 10 ng/mL rIL-6,10 20 μg/mL CTL0-4-Ig,14 or 40 μg/mL CD28-Ig.11,22 Human mature DCs were exposed overnight to 100 U/mL hIFN-γ or 20 μg/mL hCTL0-4-Ig.17 In all experiments involving treatment with fusion proteins, control treatments were performed with Ig-Cγ3, a construct consisting of the same Fc fragment used in the generation of all fusion proteins,11 and no effects were found (data not shown). For immunization of mice, cells were washed between and after incubations before peptide loading (5 μM, 2 hours at 37°C), irradiation, and intravenous injection into recipient hosts. CD8- (3 × 105) DCs were injected either alone or in combination with 5% CD8- DCs subjected to different treatments. The final viability of DC cultures to be used for in vitro or in vivo experiments always exceeded 90%.
cRNA preparation, microarray hybridization, and data analysis
Two independent microarray experiments, using murine CD8+ DCs purified from a pool of 20 Flt3-L-treated mice for each experiment, were performed. Purified CD8+ DCs were incubated with IFN-γ, and RNA was extracted at each time point (4 and 16 hours) using TRIzol LS Reagent (Invitrogen Life Technologies, Carlsbad, CA). RNA was also extracted from the same type of cells cultured for 4 and 16 hours in the absence of IFN-γ. Preparation of cRNA, hybridization, and scanning of the microarrays was performed according to the manufacturer's protocol (Affymetrix, Santa Clara, CA). Labeled cRNA was hybridized to the Affymetrix GeneChip MG-U74Av2, which contained 12 488 probe sets. Probe level data were converted to expression values using the Bioconductor function (freely available at http://www.bioconductor.org) for robust multiarray (RMA) average procedure, in which perfect match values are background adjusted, normalized using quantile-quantile normalization, and log transformed.23 Probes whose standard deviation of the signal was less than 0.15 in all arrays were filtered out, leading to a data set described by 7545 probe sets and 8 samples. Discriminating genes were identified using a fixed-effects ANOVA model with factors accounting for time and treatment effect and their interaction. The ANOVA procedure was implemented using the MAANOVA package in Bioconductor, and probe significance was assessed through residual permutation and variance stabilized F-statistic (F2 model).24,25 No probes were significantly correlated to interaction between treatment and time factors, whereas 102 probes (Table S1, available on the Blood website; see the Supplemental Table link at the top of the online article) were significantly correlated with treatment factor (P < .05).
PCR and Western blot analyses
Real-time polymerase chain reaction (PCR) was run on a Chromo4 4-Color Real-Time Detector (Bio-Rad Laboratories, Mississauga, ON, Canada) using specific primers (Table 1). Gapdh was the normalizer gene. Reverse transcription-PCR (RT-PCR) analysis was performed (30 cycles; annealing temperature, 60°C) using sense and antisense primers also listed in Table 1. RT-PCR products were normalized to murine Gapdh using specific primers. DAP12 protein expression was analyzed by Western blot using DAP12-specific antibodies, purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Small interfering RNA synthesis and transfection
Small interfering RNA (siRNA) sequences (Table 1) were selected, synthesized, and annealed by the manufacturer (Ambion, Austin, TX). Transfection was performed as described.22,26,27 Control treatments consisted of DCs transfected with negative control siRNA (Ambion). After siRNA transfection, DC viability always exceeded 95%.
Kynurenine and skin test assays
IDO functional activity was measured in vitro in terms of DC ability to metabolize tryptophan to kynurenine, the concentrations of which were measured by high-performance liquid chromatography (HPLC), as previously described.14 A skin test was used for measuring class I-restricted, delayed-type hypersensitivity responses to synthetic peptides, as previously described.10-12,15 Results were expressed as the increase in footpad weight of peptide-injected footpads over that of vehicle-injected counterparts. Data are the mean ± SD for at least 6 mice per group. Statistical analysis was performed using Student paired t test by comparing the mean weights of experimental footpads with those of control counterparts. The data reported are representative of at least 3 independent experiments.
T-cell proliferation assay
Human T cells were purified from PBMCs by negative immunomagnetic selection, as described.17 For MLR, DCs were irradiated (200 cGy) and added in various ratios to 1 × 105 allogeneic T lymphocytes in 96-well plates. Cultures were pulsed with 1 μCi (0.037 MBq)/well of tritiated thymidine during the last 16 hours of a 4-day culture. Thymidine incorporation was quantified according to standard procedures, and results are mean ± SD of quadruplicate samples.
Results
IDO competence requires a specific gene-expression profile in combination with appropriate stimuli
Splenic mature CD8+ DCs mediate the tolerogenic presentation of NRP-A7, a synthetic peptide that acts as a mimotope for autoimmune diabetes. NRP-A7-loaded CD8+ DCs from DBA/2J mice initiate durable antigen-specific anergy on transfer to recipient hosts, and adding as few as 3% CD8+ DCs inhibits the induction of immunity to NRP-A7 by purified CD8- DCs in the same model system in vivo when antigen-specific skin test reactivity is measured 2 weeks after cell transfer.9,10,21 Table 2 summarizes the final in vivo outcomes—immunity or tolerance—elicited by CD8- or CD8+ DCs pulsed with NRP-A7 and administered as such or after in vitro treatment with different stimuli. In particular, IL-6 and CD28-Ig can similarly increase the default immunogenic ability of CD8- DCs to present NRP-A7 in vivo and can revert the default tolerogenic potential of CD8+ DCs. In contrast, though CTL0-4-Ig induces IDO and thus confers IDO competence and tolerogenic potential on CD8+ and CD8- DCs in an IFN-γ-dependent fashion,9,14,15 IFN-γ alone does not apparently modify the functional program of the CD8- DC subset. Because murine splenic CD8+ and CD8- DCs are characterized by a distinct basal gene-expression profile,28 our data indicate that the acquisition of IDO-dependent tolerogenic properties by a DC subset may rely on the use of appropriate stimuli and on a specific preexisting gene-expression program. Although the mechanisms for posttranslational control of IDO activity are unknown, they must depend on the specific gene-expression program of the DC subset naturally programmed to direct tolerance.
Gene-expression program of IDO+ DC has the profile of a mature, stimulatory APC
To identify genes whose products may contribute to IDO competence, we examined the results of hybridization of 2 independent isolations of RNA from CD8+ DCs incubated with medium alone or with IFN-γ for 4 and 16 hours (Gene Expression Omnibus [GEO] accession no. GSE3337). By using a fixed-effects ANOVA model, we found that IFN-γ treatment determined significant up-regulation of 64 transcripts, whereas 38 were repressed (Table S1). Indo did not appear in the list of transcripts significantly modulated by cytokine treatment because the signal of the microarray probe was too low to be statistically evaluated. Among the 102 transcripts significantly modulated by IFN-γ, we selected 25 genes with possible relevance to tryptophan metabolism, IFN-γ signaling, and DC functions (Table 3). In accordance with the literature,29 we detected the up-regulation of Wars, a gene encoding tryptophanyl-tRNA synthetase that protects IDO+ cells from tryptophan starvation and toxic catabolites. Stat1, Irf1, Icsbp1, and Socs1, each of which codes for IFN-γ signaling mediators, also resulted in up-regulation at the early and late time points. A few cytokine and cytokine receptor genes were significantly modulated (all shown in Table 3). In addition to Il1b, the modulation involved chemokine genes. As expected,30 genes coding for C-X-C chemokines were strongly up-regulated, whereas C-C transcripts showed an opposite pattern, thus suggesting a possible preferential recruitment of TH1 lymphocytes by IDO+ cells. Among the functional category of activating receptors, the up-regulation of Tnfrsf5, coding for CD40, and the down-regulation of Cd14 indicate increased stimulatory capacity associated with a higher degree of DC maturation. In contrast, Igsf7, coding for the DAP12-associated receptor MAIR-II, and Tyrobp, coding for DAP12, were down-regulated. Apart from evidence suggesting an inhibitory role for DAP12 in regulating inflammatory responses,31 the bulk of available data suggests that DAP12 represents an amplification signal in innate responses and contributes to autoimmunity.32,33 Regarding the antigen-presenting function, the expression profile resembled that of a mature DC, characterized by a reduced capacity to process and internalize exogenous antigens (Lyzs, Clecsf8, and Stab1) associated with a more efficient antigen-processing machinery (Tap1, Psmb9, Psmb10, and Ctsc). Therefore, with few exceptions (Il1b, Il18bp, Igsf7, Tyrobp, and Ctsl), the global gene-expression program of IDO+ CD8+ DCs is compatible with a mature and stimulatory APC, resistant to IDO effects.
Down-regulation of DAP12 correlates with IDO competence
To identify genes whose modulation could be relevant to the acquisition of IDO-dependent tolerogenic properties, we compared the expression of the genes listed in Table 3 using CD8+ and CD8- DCs either untreated or subjected to treatment with IFN-γ, IL-6, CTL0-4-Ig, or CD28-Ig. Real-time PCR analysis validated the microarray data for all tested genes in CD8+ DCs treated with IFN-γ (Figure 1A and data not shown). In addition, the analysis confirmed the up-regulation of Indo at 4 hours in response to IFN-γ and CTL0-4-Ig, but not to IL-6 and CD28-Ig, in both types of DCs (Figure 1A). However, in most cases, we could not detect a straightforward correlation between gene modulation and acquisition of IDO competence. As an example, Figure 1A shows that the up-regulation of Cxcl9 and the down-regulation of Cd14 could be observed in CD8+ and CD8- DCs treated with IFN-γ. In contrast, Tyrobp down-modulation strictly correlated with the acquisition of IDO competence, whereas the immunostimulants IL-6 and CD28-Ig had negligible effects. Down-regulation of Tyrobp correlated with a reduced expression of DAP12 protein in CD8+ DCs treated with IFN-γ or CTL0-4-Ig and in CD8- DCs treated with CTL0-4-Ig but not IFN-γ (Figure 1B). Therefore, these data suggest that the down-regulation of the adapter molecule DAP12 may represent a feature common to different subpopulations of IDO+ DCs.
Tyrobp silencing confers IDO competence and tolerogenic properties on murine DCs
To investigate the possible role played by DAP12 in IDO-dependent tolerogenesis, we silenced Tyrobp in CD8- DCs by means of siRNA technology. Figure 2A shows that Tyrobp transcripts selectively disappeared at 24 to 48 hours in CD8- DCs treated with Tyrobp siRNA. Tyrobp silencing was accompanied by significant kynurenine production by CD8- DCs treated with IFN-γ, comparable to that detected in CD8+ DCs treated with the same cytokine (Figure 2B). To evaluate the tolerogenic potential of Tyrobp siRNA-treated CD8- DCs, groups of mice were sensitized with NRP-A7-pulsed CD8- DCs, alone or in combination with 5% of the same type of cells pretreated with Tyrobp siRNA before stimulation with IFN-γ in the presence or absence of 1-MT, a competitive inhibitor of IDO. Figure 2C shows that the absence of Tyrobp conferred suppressive properties on IFN-γ-treated but not untreated (data not shown) CD8- DCs, yet the suppressive activity acquired by CD8- DCs was lost upon blockade of IDO activity. When mice sensitized with Tyrobp siRNA- and IFN-γ-treated CD8- DCs were further immunized after 2 weeks with untreated, immunogenic CD8- DCs, no significant skin test reactivity could be measured in response to NRP-A7 (data not shown), suggesting that unresponsiveness was the result of an actively induced tolerant state. These data demonstrate that the loss of DAP12 function ablates the refractoriness of CD8- DCs to the IDO-inducing tolerogenic effect of IFN-γ.
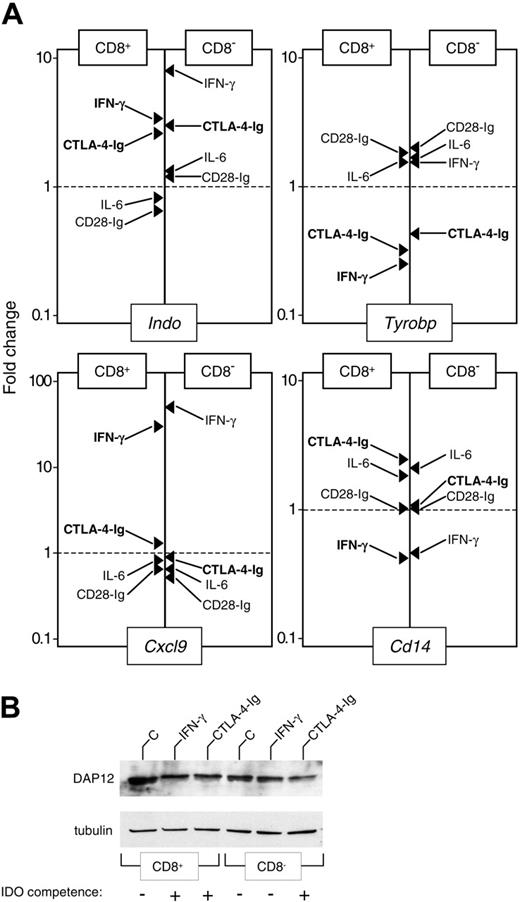
Down-regulation of DAP12 expression correlates with the acquisition of an IDO-competent phenotype by CD8+ and CD8- DCs. (A) Real-time PCR analysis of mRNA expression for IDO (Indo), DAP12 (Tyrobp), MIG (Cxcl9), and CD14 (Cd14). Purified CD8+ and CD8- DCs were incubated for 4 hours with medium alone or were treated with IFN-γ, IL-6, CTL0-4-Ig, or CD28-Ig, as specified in “Materials and methods.” mRNA levels were quantified by real-time PCR using Gapdh normalization. Data (means of duplicate samples in 1 of 2 experiments) are presented as normalized specific gene transcript expression in the samples relative to normalized transcript expression in the respective control cultures—that is, CD8+ or CD8- DCs maintained in control medium (fold change = 1; dotted line). Indicated in bold are treatments resulting in the acquisition of IDO competence in a specific DC subset. (B) Western blot analysis of DAP12 protein expression. Purified CD8+ and CD8- DCs were incubated overnight with medium alone (C), IFN-γ, or CTL0-4-Ig. DC lysates were resolved on SDS-PAGE. DAP12 expression was investigated with a specific antibody reagent. Loading controls consisted of samples reprobed with tubulin-specific antibody. Results are from 1 of 2 representative experiments.
Down-regulation of DAP12 expression correlates with the acquisition of an IDO-competent phenotype by CD8+ and CD8- DCs. (A) Real-time PCR analysis of mRNA expression for IDO (Indo), DAP12 (Tyrobp), MIG (Cxcl9), and CD14 (Cd14). Purified CD8+ and CD8- DCs were incubated for 4 hours with medium alone or were treated with IFN-γ, IL-6, CTL0-4-Ig, or CD28-Ig, as specified in “Materials and methods.” mRNA levels were quantified by real-time PCR using Gapdh normalization. Data (means of duplicate samples in 1 of 2 experiments) are presented as normalized specific gene transcript expression in the samples relative to normalized transcript expression in the respective control cultures—that is, CD8+ or CD8- DCs maintained in control medium (fold change = 1; dotted line). Indicated in bold are treatments resulting in the acquisition of IDO competence in a specific DC subset. (B) Western blot analysis of DAP12 protein expression. Purified CD8+ and CD8- DCs were incubated overnight with medium alone (C), IFN-γ, or CTL0-4-Ig. DC lysates were resolved on SDS-PAGE. DAP12 expression was investigated with a specific antibody reagent. Loading controls consisted of samples reprobed with tubulin-specific antibody. Results are from 1 of 2 representative experiments.
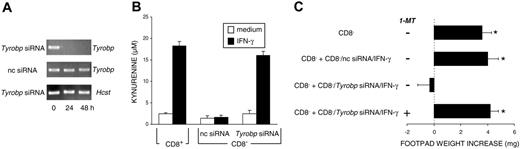
Silencing of the gene coding for DAP12 induces the appearance of an IDO-dependent, tolerogenic phenotype in CD8- DCs in response to IFN-γ. (A) RT-PCR analysis of Tyrobp expression in CD8- DCs treated with Tyrobp-specific siRNA. Control cells were treated with negative control (nc) siRNA. The expression of the Hcst gene, coding for DAP10, was also assayed as a specificity control. (B) Functional IDO activity was measured in terms of kynurenine levels by means of HPLC in supernatants of CD8+ or CD8- DCs treated overnight with IFN-γ. The CD8- DC fraction was used after Tyrobp gene silencing with specific siRNA or treatment with nc siRNA. Results (mean ± SD) are from 1 of 3 representative experiments. (C) Untreated CD8- DCs alone or in combination with 5% treated CD8- DCs (treatment consisting of nc or Tyrobp siRNA for 24 hours followed by IFN-γ for an additional 18 hours) were pulsed with NRP-A7 and transferred to recipient mice to be assayed for skin test reactivity to the eliciting peptide. Experimental groups included the use of cells treated with 2 μM 1-MT. *P < .001, experimental versus control footpads. Results are from 1 of 3 representative experiments.
Silencing of the gene coding for DAP12 induces the appearance of an IDO-dependent, tolerogenic phenotype in CD8- DCs in response to IFN-γ. (A) RT-PCR analysis of Tyrobp expression in CD8- DCs treated with Tyrobp-specific siRNA. Control cells were treated with negative control (nc) siRNA. The expression of the Hcst gene, coding for DAP10, was also assayed as a specificity control. (B) Functional IDO activity was measured in terms of kynurenine levels by means of HPLC in supernatants of CD8+ or CD8- DCs treated overnight with IFN-γ. The CD8- DC fraction was used after Tyrobp gene silencing with specific siRNA or treatment with nc siRNA. Results (mean ± SD) are from 1 of 3 representative experiments. (C) Untreated CD8- DCs alone or in combination with 5% treated CD8- DCs (treatment consisting of nc or Tyrobp siRNA for 24 hours followed by IFN-γ for an additional 18 hours) were pulsed with NRP-A7 and transferred to recipient mice to be assayed for skin test reactivity to the eliciting peptide. Experimental groups included the use of cells treated with 2 μM 1-MT. *P < .001, experimental versus control footpads. Results are from 1 of 3 representative experiments.
ICSBP/IRF-8 mediates down-regulation of DAP12 and hence acquisition of IDO competence
DAP12 is expressed primarily on NK cells and myeloid-cell types, including macrophages, neutrophils, and DCs.33 Although increased expression of this protein has been observed after LPS-induced differentiation of a mouse myeloid leukemic cell line34 and in lung tissue during mycobacterial infection,35 no evidence exists for the occurrence of negative modulatory effects under any in vivo or in vitro conditions. IFN-γ, in addition to its well-known capacity to up-regulate the transcription of several genes, can also induce gene repression through members of the IFN regulatory factor (IRF) family.36 Although most IRFs act as potent activators of IFN-stimulated response element (ISRE)-dependent transcription, IRF-2 is a prototypic ISRE-dependent transcriptional repressor. IRF-8, also known as ICSBP, can act as an activator or as a repressor. In our microarray analysis, we found a significant expression level in CD8+ DCs for Irf1, Irf5, Irf7, and Icsbp1/Irf8, the first and the last being the only IRF genes significantly up-regulated by IFN-γ (Table 3 and GEO GSE3337). We therefore investigated whether Icsbp1/Irf8 could be involved in the down-regulation of Tyrobp. Using RT-PCR, we measured Tyrobp and Indo message expression in CD8+ murine DCs transfected with Icsbp1 siRNA before incubation with IFN-γ or medium alone for 4 hours. Icsbp1 expression was also assayed as a control for gene silencing. Figure 3A shows that, as expected, IFN-γ induced the up-regulation of Icsbp1 and Indo and the down-regulation of Tyrobp in mock-transfected cells, but no Icsbp1 message was found in Icsbp1 siRNA-transfected DCs. In contrast, no significant modulation of Tyrobp was observed in Icsbp1 siRNA-transfected DCs after treatment with IFN-γ. Interestingly, Icsbp1 silencing completely ablated Indo expression in untreated and IFN-γ-treated CD8+ DCs. Undetectable Indo expression in silenced cells was associated with loss of enzymatic conversion of tryptophan to kynurenine (Figure 3B). These data suggest that the transcription factor ICSBP/IRF-8 may act as a repressor of Tyrobp in CD8+ DCs exposed to IFN-γ, thus releasing IDO from a negative control by DAP12. Moreover, these data suggest that constitutive ICSBP/IRF-8 may be involved in the transcriptional activation of Indo under basal conditions.
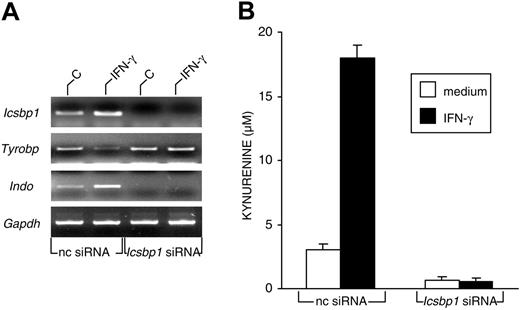
Expression of ICSBP/IRF-8 is required for the acquisition of IDO competence in murine CD8+ DCs. (A) RT-PCR analysis of Icsbp1, Tyrobp, and Indo gene expression in CD8+ DCs first treated with negative control (nc) or Icsbp1 siRNA for 24 hours and then incubated with medium alone (C) or IFN-γ for 4 hours. cDNA levels were normalized against the Gapdh gene. Results are from 1 of 3 representative experiments. (B) Kynurenine levels in supernatants of CD8+ DCs first subjected to siRNA treatment as in panel A and then incubated overnight with medium alone or IFN-γ. Results (mean ± SD) are from 1 of 3 representative experiments.
Expression of ICSBP/IRF-8 is required for the acquisition of IDO competence in murine CD8+ DCs. (A) RT-PCR analysis of Icsbp1, Tyrobp, and Indo gene expression in CD8+ DCs first treated with negative control (nc) or Icsbp1 siRNA for 24 hours and then incubated with medium alone (C) or IFN-γ for 4 hours. cDNA levels were normalized against the Gapdh gene. Results are from 1 of 3 representative experiments. (B) Kynurenine levels in supernatants of CD8+ DCs first subjected to siRNA treatment as in panel A and then incubated overnight with medium alone or IFN-γ. Results (mean ± SD) are from 1 of 3 representative experiments.
DAP12 and IRF-8 regulate IDO competence in human DCs
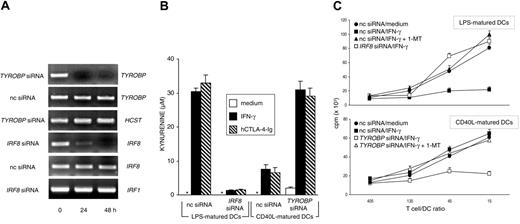
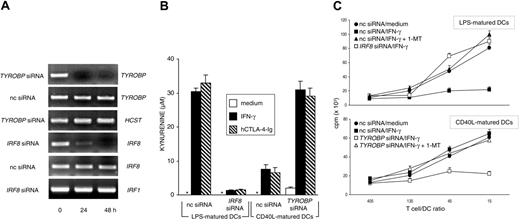
Monocyte-derived human DCs become IDO competent on maturation with LPS followed by stimulation with hCTL0-4-Ig, whereas the same stimulation is ineffective when the DCs are matured by soluble CD40L.17 To investigate the possible role of DAP12 and IRF-8 in the regulation of IDO competence in human DCs, we transfected CD40L-matured human DCs with TYROBP siRNA and LPS-matured DCs with IRF8 siRNA. Figure 4A shows that both siRNA treatments resulted in the complete and specific disappearance of relevant genes at 24 to 48 hours. Although silencing of IRF8 completely abrogated the capacity of LPS-matured DCs to convert tryptophan to kynurenine in response to either hIFN-γ or hCTL0-4-Ig, TYROBP silencing conferred IDO activity on CD40L-matured DCs in response to both stimuli (Figure 4B). The effects of gene silencing were further investigated in an MLR with irradiated LPS- or CD40L-matured DCs as stimulator cells obtained from individual donors. T lymphocytes purified from PBMCs pooled from different donors were cultured with different types of DCs in the presence or absence of 1-MT (Figure 4C). The results showed that, in line with hCTL0-4-Ig effects,17 IFN-γ treatment of LPS-matured, but not CD40L-matured, DCs led to significant inhibition of the proliferation of allogeneic cells in the absence, but not the presence, of 1-MT. However, though silencing of IRF8 in LPS-matured DCs prevented the acquisition of suppressive properties in response to IFN-γ, silencing of TYROBP in CD40L-matured DCs induced the suppression of T-cell proliferation in the absence, but not the presence, of 1-MT. Therefore, DAP12 and ICSBP/IRF-8 may act as negative and positive regulators, respectively, of IDO-dependent suppression not only in murine but also in human DCs.
Expression of IRF8 and TYROBP regulate the acquisition and loss of IDO competence in human DCs. (A) RT-PCR analysis of IRF8 and TYROBP gene expression in human DCs matured with LPS (IRF8 analysis) or CD40L (TYROBP), both treated with gene-specific siRNA for different times. Control cells were treated with negative control siRNA (nc siRNA). HCST (coding for human DAP10) and IRF1 gene expression was assayed as specificity control for TYROBP and IRF8 siRNA, respectively. (B) Kynurenine levels in supernatants of human DCs subjected to treatment with nc or gene-specific (indicated) siRNA for 24 hours, followed by overnight incubation with medium alone, IFN-γ, or hCTL0-4-Ig. LPS-matured DCs (IDO+ DCs) were subjected to IRF8 gene silencing, whereas CD40L-matured DCs (IDO- DCs) were subjected to TYROBP gene silencing. Results (mean ± SD) are from 1 of 2 representative experiments. (C) Proliferative response of human CD4+ T lymphocytes in response to allogeneic DCs at 4 days. MLR was established with T lymphocytes purified from allogeneic donors and DCs subjected to siRNA treatment as in panel B, followed by overnight incubation with medium alone or hIFN-γ. Cocultures were also established in the presence of 20 μM 1-MT. Results are expressed as mean cpm ± SD in 1 of 3 representative experiments. Radiolabel incorporation by DCs or T cells cultured alone was less than 1000 and less than 3000 cpm, respectively, and these values were not affected by the addition of 1-MT.
Expression of IRF8 and TYROBP regulate the acquisition and loss of IDO competence in human DCs. (A) RT-PCR analysis of IRF8 and TYROBP gene expression in human DCs matured with LPS (IRF8 analysis) or CD40L (TYROBP), both treated with gene-specific siRNA for different times. Control cells were treated with negative control siRNA (nc siRNA). HCST (coding for human DAP10) and IRF1 gene expression was assayed as specificity control for TYROBP and IRF8 siRNA, respectively. (B) Kynurenine levels in supernatants of human DCs subjected to treatment with nc or gene-specific (indicated) siRNA for 24 hours, followed by overnight incubation with medium alone, IFN-γ, or hCTL0-4-Ig. LPS-matured DCs (IDO+ DCs) were subjected to IRF8 gene silencing, whereas CD40L-matured DCs (IDO- DCs) were subjected to TYROBP gene silencing. Results (mean ± SD) are from 1 of 2 representative experiments. (C) Proliferative response of human CD4+ T lymphocytes in response to allogeneic DCs at 4 days. MLR was established with T lymphocytes purified from allogeneic donors and DCs subjected to siRNA treatment as in panel B, followed by overnight incubation with medium alone or hIFN-γ. Cocultures were also established in the presence of 20 μM 1-MT. Results are expressed as mean cpm ± SD in 1 of 3 representative experiments. Radiolabel incorporation by DCs or T cells cultured alone was less than 1000 and less than 3000 cpm, respectively, and these values were not affected by the addition of 1-MT.
Discussion
Indo, a gene found early in evolution, codes for IDO, a cytoplasmic and monomeric enzyme that catalyzes the oxidative cleavage of the indole nucleus of tryptophan.7,37 IDO transforms tryptophan to N-formylkynurenine, a product that is rapidly converted by formamidase to kynurenine, which in turn can either enter the bloodstream or undergo further metabolism to a series of molecules, collectively known as kynurenines. Transcription of Indo is stringently controlled and confined to a limited range of cell types.6 The highest levels of expression are found in APCs, including macrophages and DCs, particularly in response to the proinflammatory cytokine IFN-γ. The IFN-γ-inducible expression of Indo is dependent on a series of upstream elements, including IFN-γ activation sites (GAS) and 2 ISREs. STAT1 acts directly through the binding of GAS and indirectly through the induction of IRF-1 that binds the ISREs. NF-κB also contributes to this effect by binding to GAS/κB, which combines a GAS element with a nonconsensus site for NF-κB.38 In contrast, Bin1, a BAR-adapter-encoding gene, plays a major role in the negative regulation of Indo; in fact, the loss of Bin1 elevates the STAT1- and NF-κB-dependent expression of Indo.39 IDO protein can be expressed without functional enzyme activity.5,6 Therefore, a 2-step regulation, first transcriptional and then posttranslational, seems to be mandatory for the acquisition of full IDO competence by the cell.40 Despite the lack of mechanistic understanding of the posttranslational regulation, in most instances IFN-γ is apparently capable of providing all signals necessary to activate both steps.
By using microarray analysis, we found that IFN-γ up-regulates Stat1 and Irf1 in CD8+ DCs, whereas Bin1 is unaffected (data not shown). In RT-PCR experiments not shown here, we also found that a similar cytokine-induced pattern of Stat1, Irf1, and Bin1 expression is present in CD8- DCs, which do express IDO, but in an inactive form, in response to IFN-γ. To identify genes possibly involved in the posttranslational regulation of IDO, we compared the expression of a series of genes, selected from microarray analysis and with possible relevance to DC activity, using DCs competent or not for IDO function. We found that the down-modulation of a single gene, Tyrobp, was strictly associated with the acquisition of full IDO competence.
Tyrobp codes for DAP12 (also called KARAP), a transmembrane signaling adapter that associates with a family of activating receptors, including SIRPβ1, TREM-1, TREM-2, MAIR-II, PILRβ, and CD200R-like receptors in DCs.33,41 DAP12+ cells also express inhibitory receptors with extracellular domains highly homologous to their activating counterparts. Although the inhibitory receptors are characterized by immunoreceptor tyrosine-based inhibitory motifs (ITIM) in their cytoplasmic domains, the activating receptors are unable to transduce the signal on their own but do use the associating DAP12 molecule, which harbors an intracytoplasmic immunoreceptor tyrosine-based activation motif (ITAM).41 The physiologic functions of DAP12 in DC activation are suggested by the reported phenotypes of knock-in mice bearing a nonfunctional DAP12 and of DAP12-deficient mice.42,43 The former animals show an accumulation of DCs in mucocutaneous epithelia associated with impaired hapten-specific contact sensitivity, and the latter show an increased resistance to experimental autoimmune encephalomyelitis. Because the same ligand may trigger ITIM- and ITAM-associated receptor counterparts in at least some instances,41 the expression level of DAP12 may be critical in shifting the balance of inhibitory and activating signals. Interestingly, we found that the gene coding for MAIR-II was also down-regulated. However, this did not strictly correlate with the acquisition of full IDO competence because a similar effect could also be observed in noncompetent, IFN-γ-treated CD8- DCs (data not shown). Therefore, MAIR-II may not be relevant to the control of IDO or, alternatively, Tyrobp modulation may be more effective in this regard because activating receptors seem to be expressed on the cell surface only in the presence of DAP12.44 In accordance with this hypothesis, we found that forced down-regulation of Tyrobp by means of siRNA in CD8- DCs conferred IDO functional activity in vitro and, hence, tolerogenic properties in vivo on these cells in response to IFN-γ. Although the precise mechanisms of the unresponsiveness induced by those cells through Tyrobp silencing in combination with IFN-γ are unknown, it should be noted that the phenomenon is still observable in recipient hosts up to at least 90 days after transfer, thus suggesting the occurrence of deletional events (data not shown).
Our data suggest that DAP12 may represent a primary factor involved in the posttranslational modification(s) necessary for full IDO activity. Engagement of DAP12-associated receptors induces tyrosine phosphorylation of ITAM by Src kinases. The phosphorylated ITAM recruits Syk and ZAP70, triggering phosphorylation of multiple downstream mediators—including PI3K, PLC-γ, c-Cbl, Grb-2, and Vav—that ultimately lead to cellular activation.32,33,45 Although the pathway triggered by DAP12 leading to IDO control is still unknown, our previous data with CD8+ DCs from nonobese diabetic (NOD) mice demonstrate that IDO expression is opposed by the PI3K signaling pathway.26 CTL0-4-Ig, by down-regulating Tyrobp and contrasting PI3K-dependent DAP12 signaling,26 may overcome differences in DC-specific gene programs, thus leading to effective IDO-dependent immunosuppression. That DAP12 may be involved not only in posttranslational but also in transcriptional events linked to IDO is also suggested by our very recent data,27 which demonstrate that CD8+ DCs from mice bearing nonfunctional DAP12 express high levels of the active form of IDO constitutively and are highly tolerogenic in vivo. In contrast, CD8- DCs from the same mice have a reduced capacity to present antigen in vivo, yet they do not express IDO and are not suppressive. Our current data, therefore, further underscore the importance of DAP12 in IDO regulation, though the specific gene-expression program of the cell and the nature of the stimulus may be critical for fine-tuning the multiplicity of signals emanating from this adapter molecule.
Down-regulation of DAP12 expression has not been reported thus far. Therefore, we sought a possible mechanism involved in this effect. IRF-8 is one of the IFN-γ-inducible factors constituting the backbone of the molecular program regulating DC subset development and functional diversity.46,47 In particular, it is preferentially expressed by CD8+ DCs and is critically required for their in vivo differentiation and functional maturation. IRF-8 acts as an activator or a repressor, depending on interacting factors and target DNA elements.48 However, target genes modulated by IRF-8 may extend beyond genes carrying a classic ISRE. In cooperation with proteins of the Ets family, IRF-8 can also bind Ets/IRF response (EIRE) and composite (EICE) elements, in addition to the recently identified IRF/Ets composite sequence (IECS).49 Although we could not find a classic ISRE within the Tyrobp promoter, we did identify a canonic IECS at position -1213 bp relative to the ATG start codon. Silencing of Icsbp1 in CD8+ DCs impaired the ability of IFN-γ to down-regulate Tyrobp, thus indicating that IRF-8 may be implicated in the negative transcriptional regulation of DAP12. Unexpectedly, Indo transcripts could not be detected in Icsbp1-silenced cells either in basal conditions or after treatment with IFN-γ. Although additional experiments are required to directly demonstrate the possible role of IRF-8, alone or in combination with IRF-1,50 in the transcriptional regulation of Indo in DCs, murine plasmacytoid DCs that also depend on IRF-8 for their differentiation46 become IDO competent after treatment with IFN-γ (F.F., unpublished data, July 15, 2005).
In the human system, it appears that IDO competence in DCs may be regulated differently than in murine DCs.16 Our data confirm that different experimental conditions greatly influence the intrinsic capacity of human DCs to acquire IDO expression and function. However, as in the mouse system, silencing of the gene coding for DAP12 in human DCs confers full IDO competence and suppressive properties. Moreover, our current data suggest that, as in murine DCs, IRF-8 may represent an absolute requirement for human IDO expression (data not shown) and function, providing the first evidence for a possible role of this transcription factor in human DCs (Figure 5).
Schematic representation of how IFN-γ may affect IDO expression through modulation of IRF-8 and DAP12. In murine and human DCs, IFN-γ will act on IRF-8 to enhance IDO and to decrease DAP12, which basally opposes IDO expression. Also depicted are the activating receptors typically associated with DAP12 in DCs.
Schematic representation of how IFN-γ may affect IDO expression through modulation of IRF-8 and DAP12. In murine and human DCs, IFN-γ will act on IRF-8 to enhance IDO and to decrease DAP12, which basally opposes IDO expression. Also depicted are the activating receptors typically associated with DAP12 in DCs.
The concept that cells expressing IDO can suppress T-cell responses and promote tolerance is a relatively new paradigm in immunology, yet accumulating evidence suggests that the IDO mechanism is a general means of regulating immunity.5,6 This study indicates that tolerogenic DCs may use a specific code to control the expression of IDO activity. Although the DC universe is complex, this code seems to be simple and well conserved among different species. Besides underscoring the importance of “metabolic” enzymes in controlling immune responsiveness,51 our studies suggest that targeted expression and conditional deletion of IDO-related genes in immune cells may be important to the development of novel immunomodulatory strategies in humans.52
Prepublished online as Blood First Edition Paper, December 8, 2005; DOI 10.1182/blood-2005-10-4077.
Supported by Fondo per gli Investimenti della Ricerca di Base (FIRB) contract no. RBAU01935A.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Laura Santambrogio for providing expert advice in the choice of DC-relevant genes.