Abstract
To determine whether MDR1 reversal by the addition of the P-glycoprotein (P-gp) inhibitor PSC-833 to standard induction chemotherapy would improve event-free survival (EFS), 419 untreated patients with acute myeloid leukemia (AML) aged 60 years and older were randomized to receive 2 induction cycles of daunorubicin and cytarabine with or without PSC-833. Patients in complete remission were then given 1 consolidation cycle without PSC-833. Neither complete response (CR) rate (54% versus 48%; P = .22), 5-year EFS (7% versus 8%; P = .53), disease-free survival (DFS; 13% versus 17%; P = .06) nor overall survival (OS; 10% in both arms; P = .52) were significantly improved in the PSC-833 arm. An integrated P-gp score (IPS) was determined based on P-gp function and P-gp expression in AML cells obtained prior to treatment. A higher IPS was associated with a significantly lower CR rate and worse EFS and OS. There was no significant interaction between IPS and treatment arm with respect to CR rate and survival, indicating also a lack of benefit of PSC-833 in P-gp–positive patients. The role of strategies aimed at inhibitory P-gp and other drug-resistance mechanisms continues to be defined in the treatment of patients with AML.
Introduction
The overall outcome of treatment of patients of older age with acute myeloid leukemia (AML) has remained highly unsatisfactory. In patients older than 60 years, complete response (CR) rates are 45% to 60% only, while median disease-free survival (DFS) values have been estimated at less than 12 months and the 4- to 5-year overall survival (OS) rates are approximately 10%.1-5
A potentially important biologic factor that may account for chemotherapy resistance of AML in patients of higher age is the high incidence of the intrinsic multidrug resistance (MDR) phenotype of leukemic blast cells.6 The MDR phenotype results from expression of the MDR1 gene7,8 and its 170-kDa protein product, P-glycoprotein (P-gp),9 also designated as adenosine triphosphate (ATP)–binding cassette (ABC) transporter B1 (ABCB1).10 P-gp is a transmembrane protein that acts as an energy-dependent drug efflux pump for chemotherapeutic drugs such as the anthracyclines and epipodophyllotoxins, commonly used in AML therapy.
Increased P-gp expression and enhanced drug efflux have been reported with increasing age: from 17% in patients under the age of 35, 27% at 35 to 50 years, and 39% in patients over 50 years11 to 71% in a group with median age 68 years (range, 56 to 88 years).6 In retrospective studies MDR1/P-gp expression was associated with lower CR rates and decreased OS and DFS in AML.12-16 Also P-gp positivity of AML is associated with other adverse prognostic factors such as CD34 expression, secondary leukemia, and unfavorable cytogenetics.6,13,14,17
Based on these studies a rationale was developed for MDR1 modulation as a therapeutic approach.18 A variety of noncytotoxic agents, such as verapamil, quinine, and cyclosporin A (CsA), inhibit the P-gp transporter through competition with other substrates for the binding sites of P-gp. These agents block P-gp–mediated efflux of drugs from the intracellular compartment and increase the intracellular accumulation of MDR-related drugs in MDR-positive cells.19,20 Many of these P-gp reversal agents also block the elimination of cytostatic drugs from the molecular pores in the hepatobiliary canaliculi. By doing so they reduce hepatic elimination of antileukemic drugs like anthracyclines, which results in a prolonged half-life, an increase of the plasma area under the curve (AUC), and potentially increased toxicity from these agents.21
Randomized phase 2 and phase 3 studies with first-generation P-gp inhibitors in AML were mostly nonconclusive because of poor therapeutic benefit or unexpected interactions with the pharmacokinetics of the cytostatic agent.22,23 Quinine and verapamil24 have a cardiotoxic risk profile that prohibits adequate dosing.
The second-generation P-gp inhibitor PSC-833 (Valspodar, Amdray; Novartis Pharma, Basel, Switzerland) is a cyclosporin analog that is 7- to 20-fold more potent than CsA in increasing daunorubicin (DNR) retention in MDR cells, while lacking the immunosuppression and nephrotoxicity. The dose-limiting toxicity is cerebellar ataxia, which, however, is transient and fully reversible.25-27 Phase 1 and phase 2 studies suggest that substantial inhibition of P-gp can be achieved in vivo at clinically tolerable doses of both PSC-833 and DNR.28,29
Here we report the results of an international, multicenter, open-label, randomized phase 3 trial of PSC-833 plus standard chemotherapy in 419 previously untreated elderly patients with AML, performed under the auspices of the Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON) and the United Kingdom Medical Research Council (UK MRC). Two remission induction regimens of DNR/cytarabine (Ara-C) and DNR/Ara-C/PSC-833 were compared for their effect on CR rate, event-free survival (EFS), DFS, and OS. The effect of PSC-833 plus chemotherapy on these outcome parameters in relation to the P-gp status at diagnosis was also investigated.
Patients, materials, and methods
Patients
Patients 60 years or older with previously untreated primary or secondary AML (M0 to M2 and M4 to M7, French-American-British [FAB] classification adapted from Cheson et al30 ) and World Health Organization (WHO) performance status 2 or below were eligible for this study. Patients with secondary AML progressing from antecedent myelodysplastic syndrome (MDS) were eligible if they had not been given chemotherapy previously. Antecedent MDS was defined by a duration of at least 4 months. Patients with promyelocytic leukemia (M3), blast crisis of chronic myeloid leukemia, previous polycythemia rubra vera, or primary myelofibrosis were not eligible. Other exclusion criteria were cytopathologically confirmed central nervous system (CNS) infiltration, neurosensory toxicity grade 2 or above, neurocerebellar toxicity grade 1 or above (National Cancer Institute of Canada [NCIC] Expanded Common Toxicity Criteria [CTC]), known positivity for HIV, impaired hepatic or renal function (alanine aminotransferase [ALT] and/or aspartate aminotransferase [AST] 2.5 or more times the institutional upper limit of normal [IULN], alkaline phosphatase [AP] 2.5 or more times the IULN, serum total bilirubin 1.5 or more times the IULN, and serum creatinine 1.5 or more times the IULN after adequate hydration), those receiving treatment interacting with CsA, previous surgery within 2 weeks or investigational therapy or radiotherapy within 4 weeks of study entry, other primary malignancy except basal cell carcinoma of the skin or stage 1 cervical carcinoma within the last 5 years, concurrent severe and/or uncontrolled medical condition, psychological, intellectual, or sensory dysfunction that was likely to impede ability to understand and comply with study requirements, or severe cardiac or pulmonary disease.
The study was approved by the ethics committees of the participating institutions and was conducted in accordance with the Declaration of Helsinki. All patients gave written informed consent.
Registration and randomization procedures
Patients were randomly assigned to 1 of 2 induction chemotherapy regimens without or with PSC-833, using a validated, voice-activated telephone system, and stratified according to age (60 to 65, 66 to 70, and 71 or above) and secondary AML (no or yes).
Treatment
Induction chemotherapy consisted of 2 cycles of 45 mg/m2 DNR, 15 minutes of infusion on days 1 through 3, and 200 mg/m2 Ara-C every 24 hours, continuous infusion on days 1 through 7 (arm A); or a similar regimen but with a lower dose of DNR (35 mg/m2) and with PSC-833, 2 mg/kg in a 2-hour loading dose followed by 10 mg/kg continuous intravenous infusion every 24 hours for 72 hours days 1 through 3 (arm B). The 22% dose reduction of DNR was based on the results of the pilot study,28 which had established 35 mg/m2 DNR as the maximum tolerated dose when administered concurrently with 10 mg/kg PSC-833 per day. Cycle 2 was given to all patients who achieved a normocellular marrow with less than 5% blasts, no Auer rods, and no evidence of extramedullary involvement with full peripheral blood (PB) recovery (absolute neutrophil count [ANC] above 1.0 × 109/L and platelets above 80 × 109/L) within 60 days of start of induction cycle 1. In patients with evidence of persistent AML, the second cycle was administered independent of PB recovery.
Patients in both arms who attained a CR were to receive 1 consolidation cycle without PSC-833 consisting of Ara-C 1 g/m2 6-hour infusion on days 1 to 4, mitoxantrone 6 mg/m2 by slow intravenous bolus on days 1 to 4, and etoposide 80 mg/m2 1-hour infusion on days 1 to 4.
Definition of end points
In this analysis, CR was defined as a normocellular bone marrow (BM) with less than 5% blasts, no Auer rods, and no evidence of extramedullary involvement. Data on PB recovery within 60 days after start of a cycle were not always available and were not considered as a criterion for CR. When the BM blast cell count remained between 5% and 25% but was reduced by at least 50% in comparison with the initial value, a patient was considered in partial remission (PR). Patients who relapsed or died within 28 days after CR were considered as not having achieved a CR. Patients who did not enter CR following induction therapy were classified as induction death if the patient died within 30 days after start of induction cycles 1 or 2 or as having refractory disease otherwise.
Early death was defined as death during the first 7 days of the first induction cycle.
EFS was determined from the date of randomization until no CR on induction therapy, relapse after CR, or death in CR, whichever came first. Patients who did not attain a CR were considered a failure at 1 day after randomization. DFS was determined for all patients who achieved CR and was calculated from the date of CR until relapse or death, whichever came first. OS was measured from randomization until death from any cause. Patients still alive at the date of last contact were censored.
Analysis of P-gp expression and function in AML samples by flow cytometry
A BM aspirate of 3 to 10 mL was collected in a tube containing 0.5 mL Hanks balanced salt solution (HBSS; Gibco, Paisley, United Kingdom) with 625 U/mL sodium heparin. These samples were transported at 4°C or cryopreserved at –160°C and then transported to the central laboratory in Rotterdam.
Mononuclear BM cells were collected from patient BM aspirates by centrifugation over Lymphoprep (Nycomed, Oslo, Norway). They were frozen in 10% dimethyl sulfoxide (DMSO) and 20% fetal calf serum (FCS) and stored in liquid nitrogen. At the day of the analysis BM cells were thawed, washed, and resuspended in Dulbecco modified Eagle medium (DMEM; Gibco) supplemented with 10% FCS, gentamicin at a concentration of 4 × 106 cells per milliliter.
Measurement of the expression of P-gp. For measurement of the expression of P-gp, cells were incubated (at room temperature) with monoclonal anti–P-gp antibody MRK 1631 (Kamiya Biomedical, Tukwila, WA) at a concentration of 10 μg/mL or with UIC2 monoclonal antibody32 (mAb) (Immunotech, Marseille, France) at a concentration of 12.5 μg/mL or an isotype-matched control antibody monoclonal immunoglobulin G2a (mIgG2a) (Sigma Chemical; St Louis, MO) at a concentration of 10 μg/mL. The concentrations of antibodies were based on our quality control studies33 and were also used in the pilot study.28 Cellbound antibodies were detected by fluorescein isothiocyanate (FITC)–labeled rabbit anti–mouse immunoglobulin antibodies (DAKO, Glostrup, Denmark).
To measure expression of P-gp in CD34+ cells, cells were labeled with phycoerythrin (PE)–labeled CD34 antibody or, as a control, PE-labeled mIgG1 antibody (Becton Dickinson, San Jose, CA). Cells were incubated with 0.1 μM TO-PRO-3 (Molecular Probes, Eugene, OR) to exclude nonviable cells. Fluorescence was measured using a FACScalibur (Becton Dickinson).
Results are given as the ratio of the mean of cell-associated fluorescence of cells incubated with the anti–P-gp antibody divided by the mean of cell-associated fluorescence of cells incubated with the control mIgG2a antibody.
Measurement of the function of P-gp. For measurement of the function of P-gp,34,35 the fluorescent compound rhodamine 123 (Rho123; Sigma) was used as a P-gp substrate. Cells were incubated for 1 hour at 37°C at 5% CO2 in the absence and presence of 2 μM PSC-833. After this incubation, 200 ng/mL Rho123 was added to the cells. A sample was taken at t = 0 minutes to correct for background fluorescence and at t = 75 minutes to measure intracellular rhodamine accumulation.
To measure function of P-gp in CD34+ cells, cells were labeled with PE-cyanine 5 (Cy5)–labeled CD34 antibody or, as a control, PE-Cy5–labeled mIgG1 antibody (Immunotech).
Cells were incubated with 0.1 μM TO-PRO-3 to exclude nonviable cells. Fluorescence was measured using a FACScalibur.
Results are given as the ratio of the mean intracellular Rho123 fluorescence of cells exposed to PSC-833 divided by the mean intracellular Rho123 fluorescence of cells not exposed to PSC-833.
Interpretation. As controls in each analysis, the drug-sensitive human myeloma cell line 8226 S and the drug-resistant variant 8226 D6 cells36 were used. Taking all experiments together, the mean efflux ratio (Rho123 + PSC-833: Rho123) of the negative control cell line 8226 S was 0.92 ± 0.06 (mean ± SD; n = 88) and of the positive control cell line 8226 D6 was 6.12 ± 4.11.
Patient BM cells were considered positive for P-gp function if the efflux ratio was more than 1.05. This ratio of P-gp efflux is given for the whole population of blasts and also for the CD34+ cells. Only patients with more than 10% P-gp–positive cells in all experiments were considered positive.
The means of the MRK 16 expression ratio of the negative cell line 8226 S and of the positive cell line 8226 D6 were 1.28 ± 0.26 and 27.17 ± 6.37, respectively. The mean UIC2 expression ratios were 1.16 ± 0.19 and 25.97 ± 7.05, respectively. Patient BM cells were considered positive for the expression of P-gp if the expression ratio was more than 1.65 for either MRK 16 or for UIC2. This ratio of the expression was given for the whole population of blasts and also for the CD34+ cells together with the percentage of CD34+ cells, which could be the leukemic tumor cell population. Only patients with a subpopulation of more than 10% of positive cells were considered positive. Some patients had P-gp expression but no function. These patients were considered negative because it is possible that these patients express a nonfunctional P-gp. Some patients showed P-gp function but no expression. These patients were considered positive because of the possible clinical relevance of this phenomenon.
P-gp assessment. For patients with P-gp data available, an integrated P-gp score (IPS) was based on the P-gp function or, if not available, on the expression ratios.33 Patient BM samples were categorized as negative (efflux ratio between 0 and 1.05), low-positive (more than 1.05 to 1.40), intermediate-positive (more than 1.40 to 2.50), or high-positive (more than 2.50). Similarly, cut points for expression ratios were 1.65, 2.50, and 5.00. These cutoff values had been defined a priori and were chosen based on the efflux ratios observed with the doxorubicin-sensitive, P-gp–negative myeloma cell line RPMI 8226S and its doxorubicin-resistant cell lines 8226DOX1, 8226DOX6, and 8226DOX40, which exhibit increasing levels of P-gp function, P-gp expression, and cellular resistance (kindly provided by Dr W. S. Dalton, H. Lee Moffitt Center and Research Institute, Tampa, FL).
Cytogenetic analysis
Cytogenetic analysis of BM samples obtained at diagnosis was performed using standard cytogenetics techniques. All available cytogenetic reports were reviewed by 2 expert cytogeneticists. Chromosomal abnormalities were described according to the International System for Human Cytogenetics Nomenclature (ISCN).37 Favorable risk was defined as the presence of t(8;21), inv(16), or t(16;16). Unfavorable risk was defined by the presence of monosomies or deletions of chromosomes 5 or 7, abnormalities of the long arm of chromosome 3(q21;q26), t(6;9), abnormalities involving the long arm of chromosome 11 (11q23), or complex cytogenetic abnormalities (defined as at least 3 unrelated cytogenetic clones). Patients who did not meet the criteria for favorable or unfavorable risk were classified as being intermediate risk.
Statistical considerations
The primary objective of the study was to compare EFS between the 2 treatment arms on an intention-to-treat basis—that is, patients were analyzed according to assignment to treatment arm A (without PSC-833) or B (with PSC-833). To detect with a power of 80% an increase in 2-year EFS from 9.5% to 18% (2-sided significance level α= .05) and assuming an accrual of 18 months and a follow-up time of 12 months, 400 patients were required and 331 events had to be observed.
Secondary end points were CR rate, DFS, and OS between the 2 treatment arms, safety and tolerability of the 2 treatment regimens, and the association between IPS and outcome and the interaction with additional PSC-833.
Patient characteristics between the 2 treatment arms were compared using the Fisher exact test38 or the Pearson χ2 test in case of discrete variables or the Wilcoxon rank sum test39 in case of continuous variables.
The CR rate was compared between the 2 treatment arms using logistic regression.40 The odds ratio (OR) was calculated with a 95% confidence interval (CI).
EFS, DFS, and OS were estimated by the Kaplan-Meier method,41 and 95% CIs were constructed. Survival analysis was performed using Cox regression42 to see whether there was a difference in survival between the 2 treatment arms. The hazard ratios (HRs) and corresponding 95% CIs were determined for all 3 survival end points. Kaplan-Meier curves were generated to illustrate differences between the 2 treatment arms and compared using the log-rank test.43 Competing risk analysis was used to calculate cumulative competing risks of treatment failure among patients with a CR (either relapse after CR or death in first CR).
Safety was analyzed using descriptive statistics to summarize the incidence of adverse events (AEs) and laboratory findings. Toxicity of the 2 regimens was assessed by laboratory evaluation, physical examination, vital signs, and AE assessments. AEs were scored using the NCIC Expanded CTC.
All reported P values are 2 sided, and a significance level α= .05 was used.
Results
Patient characteristics
Between May 1997 and February 1999, 428 patients from 99 centers in 15 countries were randomized for study treatment. Eight patients were not eligible because of previous treatment (n = 2), impaired hepatic or renal function (n = 2), or other (n = 4). One patient refused treatment after randomization and has been lost to follow-up since. One patient aged 58 years was accidentally randomized, but this was considered a minor protocol violation and he has been included in the analysis. Of 419 remaining patients, 211 were randomized to arm A (control arm) and 208 were randomized to arm B (induction therapy with PSC-833). Median age was 67 years (range, 58 to 85 years). Patient baseline characteristics were comparable between the 2 arms (Table 1).
Cytogenetics
Successful cytogenetic data were available for 293 (70%) of the patients (Table 1). Five patients (2%) were classified as favorable risk and 66 patients (23%) as unfavorable risk, while the remaining 222 patients (76%) were classified as intermediate risk, equally distributed over the 2 treatment arms (P = .70). A total of 158 of 222 of intermediate-risk patients (71%) presented with a normal karyotype.
Response to chemotherapy
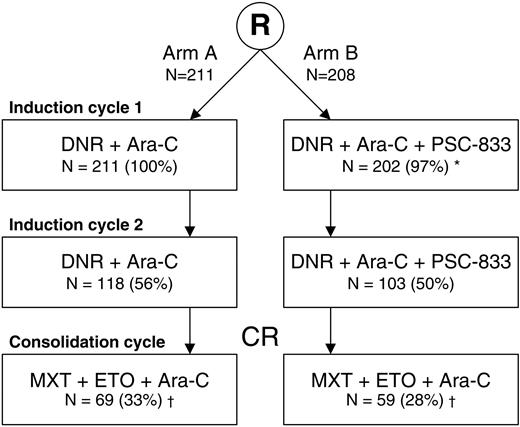
Of 419 patients, 285 patients (68%) received induction cycles only and 128 patients (31%) received induction and consolidation treatment. Six of 419 patients (1%) did not receive any protocol treatment (Figure 1). Three of them received alternative induction therapy, resulting in a CR in 2 patients: one patient withdrew consent before cycle 1 and was treated with 6-mercaptopurine instead, and one patient had an AE before the start of treatment and was then treated with idarubicin instead of daunorubicin. Both patients were considered as CR patients according to the intention to treat. The overall CR rate was 51%. CR was achieved in 101 of 211 patients (48%) in the control arm as compared with 112 of 208 patients (54%) in the PSC-833 arm (P = .22). A total of 80 patients in arm A and 87 patients in arm B achieved a CR after the first induction cycle. Table 2 shows the CR rates by stratification factor. Induction failures were classified as refractory disease or as induction death in 79 (37%) and 31 (15%) patients, respectively, in arm A, versus 63 (30%) and 33 (16%) patients, respectively, in arm B. Early death rates were similar in both arms: 4 (2%) and 5 (2%) patients, respectively.
Flow diagram of 419 elderly patients with AML by treatment arm. Per treatment arm, the number and percentage of patients who received a specific induction or consolidation cycle are shown. R indicates randomization; MXT, mitoxantrone; and ETO, etoposide.
*Six patients in arm B did not receive any protocol treatment.
†Two patients, one in each treatment arm, received only induction cycle 1 and one consolidation cycle.
Flow diagram of 419 elderly patients with AML by treatment arm. Per treatment arm, the number and percentage of patients who received a specific induction or consolidation cycle are shown. R indicates randomization; MXT, mitoxantrone; and ETO, etoposide.
*Six patients in arm B did not receive any protocol treatment.
†Two patients, one in each treatment arm, received only induction cycle 1 and one consolidation cycle.
Event-free survival, disease-free survival, and overall survival
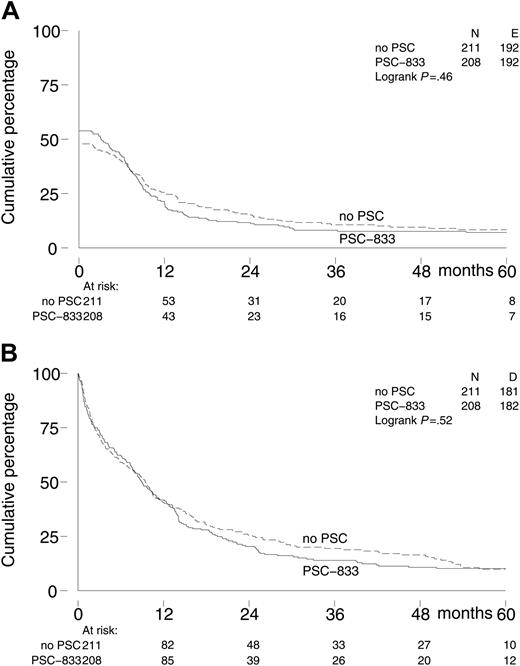
The survival end points are based on follow-up data available as of June 2004. The median follow-up of 56 patients still alive is 56 months; 35 of these patients were still in continuous first CR at last contact, including 19 in the control arm and 16 in the PSC-833 arm. Long-term EFS, DFS, and OS were similar for both treatment groups (Table 3; Figure 2). Five-year EFS was 7% (95% CI, 4%-11%) for the PSC-833 group as compared with 8% (95% CI, 5%-13%) for the control group (P = .53). Most patients who reached a CR relapsed afterward. Ten versus 14 patients died in CR, resulting in 5-year DFS of 17% (95% CI, 11%-26%) in the PSC-833 arm and 13% (95% CI, 8%-20%) in the control arm; P = .06. The 5-year OS was 10% (95% CI, 6%-15%) in both treatment arms; P = .52.
P-gp assessment
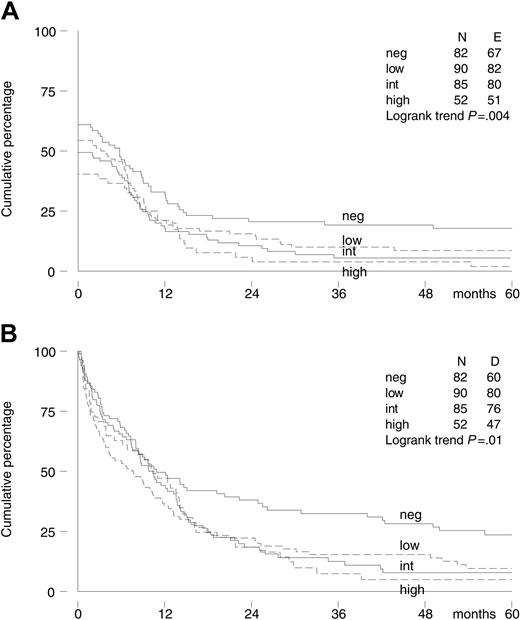
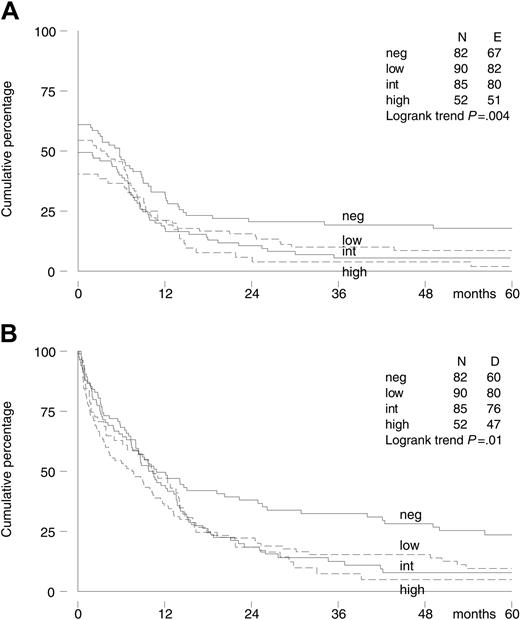
Viable BM samples to assess P-gp status at diagnosis were available in 309 of 419 patients (74%). Most samples were transported at 4°C. Only samples from the United States, Canada, and Australia were cryopreserved before transportation. Ultimately, we had P-gp data of 35 patients with cryopreserved samples, which is 11% of all patients with P-gp data available. P-gp functional data were available in 282 patients, and P-gp expression data were available in 293 patients. These data were highly correlated. The Spearman rank correlation coefficient between the efflux ratio and MRK 16 expression ratio was 0.64 (P < .001), while between the MRK 16 and UIC2 expression ratio it was 0.81 (P < .001). Of the 265 patients with both P-gp functional and expression data available, 11 patients had positive function and negative expression, while in 30 function was negative and expression positive. The 309 patients were classified as IPS negative (27%), low-positive (29%), intermediate-positive (28%), or high-positive (17%), with no difference between the 2 treatment arms (P = .91; Table 1). An increased IPS was associated with a lower CR rate; the CR rate decreased from 61% (95% CI, 50%-72%) and 54% (95% CI, 44%-65%) in the IPS-negative and low-positive patients to 49% (95% CI, 38%-60%) and 40% (95% CI, 27%-55%) in the IPS-positive and high-positive patients, respectively (P = .02, test for trend). A higher IPS was also associated with significantly worse EFS and OS (Figure 3), and a trend for decreased DFS was observed.
We also evaluated the mutual effect of PSC-833 and IPS on CR rate and survival. A multivariate logistic regression analysis of CR rate was performed with treatment arm, IPS, and a treatment arm by IPS interaction term as covariates. EFS, DFS, and OS were also evaluated, using multivariate Cox regression analyses. Similar results as in the univariate analyses were obtained. No benefit of PSC-833 was seen, while a higher IPS suggested a lower CR rate (OR = 0.75; 95% CI, 0.54-1.03; P = .07), a significantly lower EFS (HR = 1.19; 95% CI, 1.01-1.40; P = .04), and a trend for worse DFS (HR = 1.22; 95% CI, 0.95-1.57; P = .12) and OS (HR = 1.12; 95% CI, 0.95-1.32; P = .17). Detailed results for CR rate, EFS, and OS are shown in Table 4. However, none of the 4 analyses suggested an interaction between IPS and treatment arm. Especially no greater benefit of PSC-833 was apparent with increasing IPS. This was illustrated by a subgroup analysis of the 227 patients with low-, intermediate-, or high-positive IPS, who were expected to benefit most from PSC-833. Although the CR rate was somewhat higher in the PSC-833 arm (55% versus 44%; P = .07), survival was not better. HRs for PSC-833 were 1.02 (95% CI, 0.78-1.33; P = .91) for EFS, 1.34 (95% CI, 0.90-2.01; P = .15) for DFS, and 1.03 (95% CI, 0.78-1.35; P = .84) for OS.
Adverse events
AEs affecting the central and peripheral nervous system and liver and biliary disorders were more frequently reported in the patients treated with PSC-833 and DNR than in those receiving DNR alone. More AEs related to the nervous system were reported in patients treated with PSC-833 and DNR, such as paresthesia (16.3% versus 1.9%), ataxia (13.9% versus 1.4%), or dizziness (26.2% versus 11.3%), whereas excess liver AEs among the PSC-833 treatment group reflected more frequent reports of bilirubinemia (18.8% versus 7.1%). The patterns of other reported AEs were similar for the 2 treatment arms (Table 5).
Premature discontinuation of chemotherapy due to nonfatal AEs was more frequent in the group treated with PSC-833 and DNR than in the group treated with DNR alone (15.4% versus 9.5%). In both groups, the most frequently reported reasons for premature discontinuation were infections, while discontinuations due to ataxia, cerebellar toxicity, or peripheral neuropathy were only reported in patients treated with PSC-833 and DNR.
Kaplan-Meier survival curves of 419 elderly patients with AML by treatment arm. (A) Event-free survival. (B) Overall survival. No PSC indicates patients in arm A.
Kaplan-Meier survival curves of 419 elderly patients with AML by treatment arm. (A) Event-free survival. (B) Overall survival. No PSC indicates patients in arm A.
Deaths resulted from common complications of chemotherapy or from progression of AML. PSC-833, which is known to increase exposure to DNR by decreasing the clearance of the drug, did not lead to increased incidence or severity of chemotherapy-related AEs.
Discussion
Expression of the MDR1 gene has been associated with lower CR rates and worse OS and DFS in AML.
This randomized phase 3 study of the P-gp inhibitor PSC-833 aimed at overcoming classic MDR in patients with AML who were 60 years and older. It was designed to investigate whether the response of AML would improve by adding PSC-833 to standard induction treatment. This large study shows that PSC-833 does not improve response rate or survival in patients with AML 60 years of age and older. The overall CR rate was 51% and 5-year survival was 10% in both treatment arms, which is comparable with other published trials in this age group.1-5 To establish the independent prognostic value of P-gp, an integrated P-gp score (IPS) of AML blasts was prospectively determined at diagnosis. In this laboratory analysis, 73% of evaluable patients were classified as P-gp positive based on demonstrated P-gp reversal in vitro, which is in accordance with previously published data in this patient group.6 We confirmed that IPS is an independent adverse prognostic factor in older patients with AML, because CR rate and EFS, DFS, and OS decreased with increasing IPS. There was, however, no significant interaction between IPS and PSC-833 with regard to CR rate and survival end points.
Therapeutic P-gp reversal has been examined in several randomized phase 2 and phase 3 trials using first-generation P-gp modulators such as quinine and CsA.
Quinine did not show an improvement in CR rate and OS in 2 consecutive trials by the French Groupe Ouest Est Leucemies Aigues Myeloblastiques (GOELAM) mainly in patients with AML aged 15 to 65 years,22,44 while toxicity was significantly increased.22 In the subgroup of patients who were tested for P-gp, a higher CR rate was observed in the 29 P-gp–positive patients in the quinine arm (83% versus 48%; P = .01).44
The effect of CsA has been assessed in several trials in refractory and relapsed AML. In the UK MRC Randomised Trial for Patients with Refractory or Relapsed Acute Myeloid Leukaemia in Adults (AML-R), OS and DFS did not differ between the CsA and the standard arm, while a lower CR rate, due to increased induction deaths and resistant disease, and worse OS were observed in the subgroup of patients 60 years of age and older treated with CsA.45 In the Southwest Oncology Group (SWOG) 9126 trial, the incidence of refractory AML was lower in CsA-treated patients, while OS and relapse-free survival (RFS) were better. The positive effect of CsA was greatest in the subgroup of patients with moderate or bright P-gp expression.46 A phase 2 trial by HOVON in patients with relapsed/primary refractory AML failed to show improved treatment outcome in the CsA-arm.23
Kaplan-Meier survival curves of 309 elderly patients with AML by P-gp assessment (IPS). (A) Event-free survival. (B) Overall survival.
Kaplan-Meier survival curves of 309 elderly patients with AML by P-gp assessment (IPS). (A) Event-free survival. (B) Overall survival.
PSC-833 is a second-generation P-gp reversal agent that lacks immunosuppressive activity. Several phase 1 and phase 2 trials of PSC-833 with natural product–based (re)induction chemotherapy for AML were performed in refractory/relapse patients29,47-50 as well as in untreated elderly patients.28,51,52 PSC-833 significantly inhibits the hepatobiliary metabolism and excretion of cytotoxic agents, which results in increased plasma exposure and slower terminal elimination of anthracyclines. Therefore, the dosage of agents that are substrates for P-gp (DNR, mitoxantrone, etoposide) was reduced by 22% to 66% when applied concomitantly with PSC-833 to accomplish equitoxicity with the control chemotherapy regimen.28,48,51,53 In our trial the dosage of DNR was reduced by 22% in patients treated with PSC-833. While reduction of DNR dose in PSC-833 patients may have contributed to an equitoxic plasma exposure, it was expected that the inhibition of P-gp–mediated drug efflux would compensate for that and would overcome the P-gp–mediated drug efflux in leukemic cells. While such an effect was indeed observed in the in vitro analysis of these patient samples, PSC-833 did not confer a better therapeutic effect. Other trials like the randomized phase 3 Cancer and Leukemia Group B (CALGB) 9720 trial in elderly patients with untreated AML54 and the Eastern Cooperative Oncology Group (ECOG) 2995 trial in relapsed/refractory AML and high-risk MDS55 also failed to show a benefit of PSC-833. The first trial was prematurely closed because of excessive early mortality in the PSC-833 arm, while accrual to the second trial was discontinued early due to lack of superiority in achieving CR in patients treated with PSC-833. In these trials, there was no apparent difference in OS and DFS between the 2 arms, but the power to detect moderate differences was low due to the small patient numbers. The CALGB 19808 trial in patients with AML younger than 60 years was halted in August 2003 because PSC-833 was no longer available,56 and results are awaited. Our study in significantly greater numbers of patients, with long follow-up and with elaborate P-gp analysis, failed to reveal a benefit for the use of PSC-833, while the independent prognostic value of P-gp at diagnosis was established.
Various reasons might be considered to explain why PSC-833 failed to overcome clinical refractory disease in older patients with AML. First, dose reduction of DNR in the presence of PSC-833 from 45 mg/m2 to 35 mg/m2 in the experimental arm to achieve equitoxicity may have inflicted reduced DNR exposure to AML cells. This may have caused failure of PSC-833 to achieve durable high intracellular levels of DNR in AML blasts. This issue may be clarified by the trial conducted by the MRC group (the AML 14 Trial, which will shortly close) where an additional comparison between a DNR dose of 35 mg/m2 and 50 mg/m2 is included.57 Second, the clinical benefit of PSC-833 may also have been masked by the confounding contributing effect of Ara-C, which is not a P-gp substrate. In fact, Ara-C is one of the most potent antileukemic drugs available today.58 Third, several alternative drug transport mechanisms may contribute to clinical resistance, including intratumoral transmembrane transport proteins such as members of the ABC superfamily of transport proteins including MDR1, the multidrug resistance–related protein (MRP1/ABCC1), and the breast cancer resistance protein (BCRP/ABCG2), or of the lung resistance related protein (LRP), which extrude a variety of cytotoxic drugs.21,59-61
The study presented here shows that addition of the P-gp inhibition agent PSC-833 to standard induction chemotherapy does not improve CR rate, EFS, DFS, and OS in patients aged 60 years and older. In addition, MDR1 status at diagnosis remains an independent adverse prognostic factor, indicating the need for other strategies to overcome MDR1-mediated resistance.
Appendix
The following people and institutions participated in this trial.
Principal investigators: S. Durrant, Royal Brisbane Hospital, Australia; H. Januszewicz, Peter MacCallum Cancer Institute, East Melbourne, Australia; A. Grigg, Royal Melbourne Hospital,Australia; R. Herrmann, Royal Perth Hospital, Australia; C. Arthur, Royal North Shore Hospital, St Leonards, Australia; A. Enno, Newcastle Mater Misercordiae Hospital, Waratah, Australia; D. Selleslag, St Jan's Hospital, Brugge, Belgium; R. Schots, Academic Hospital Free University Brussels, Belgium; A. Ferrant, Cliniques Universitaires St Luc, Brussels, Belgium; D. Bron, Institut Jules Bordet, Brussels, Belgium; L. Noens, University Hospital Ghent, Belgium; G. E. G. Verhoef, University Hospital Gasthuisberg, Leuven, Belgium; A. Bosly, Cliniques Universitaires UCL de Mont-Godinne, Yvoir, Belgium; S. Robinson, Queen Elizabeth II Health Sciences Centre, Halifax, Canada; M. J. Kovacs, London Health Sciences Centre, Canada; W. Brien, St Joseph's Health Centre, London, Canada; C. Shustik, Royal Victoria Hospital, Montreal, Canada; M. Crump, Princess Margaret Hospital, Toronto, Canada; J. Shepherd, Vancouver Hospital, Canada; P. Fenaux, CHU, Lille, France; J.-L. Harousseau, CHU Hotel Dieu, Nantes, France; G. Schlimok, Zentralklinikum, Augsburg, Germany; D. Huhn, Charite Campus VirchowKlinikum, Berlin, Germany; W. U. Knauf, Universitätsklinikum Benjamin Franklin, Berlin, Germany; H. Fricke, Universitätsklinikum Jena, Germany; H. G. Höffkes, Universitätskliniken, Magdeburg, Germany; M. Theobald, M. Schuler, Johannes Gutenberg-Universität, Mainz, Germany; C. Peschel, Technische Universität München, Munich, Germany; V. Nüssler, Klinikum Grosshadern, Munich, Germany; W. E. Aulitzky, Robert-Bosch Krankenhaus, Stuttgart, Germany; L. Kanz, Universitätsklinikum Tübingen, Germany; M. Schmid, Universitätsklinikum, Ulm, Germany; S. Fekete, Saint Laszlo Hospital, Budapest, Hungary; S. Nahajevszky, National Medical Center, Budapest, Hungary; H. Losonczy, University of Pecs Medical Faculty, Pecs, Hungary; Z. Borbenyi, Albert Szent-Gyorgyi Medical Center, University of Szeged, Hungary; P. Leoni, Torrette University Hospital, Ancona, Italy; V. Liso, University of Bari Medical School, Bari, Italy; P. P. Piccaluga, S. Tura, S. Orsola-Malpighi Hospital, University of Bologna, Italy; F. Ferrara, Cardarelli Hospital, Naples, Italy; P. Bernasconi, IRCCS Policlinico San Matteo, University of Pavia, Italy; M. Martelli, Policlinico Monteluce, University of Perugia, Italy; M. Petrini, University of Pisa, Ospedale S. Chiara, Italy; F. Nobile, Azienda Ospedaliera Bianchi-Melacrino-Morelli, Reggio Calabria, Italy; S. Wittebol, Meander Medical Center, Amersfoort, The Netherlands; J. van der Lelie, Academic Medical Center, Amsterdam, The Netherlands; G. J. Ossenkoppele, P. C. Huijgens, VU University Medical Center, Amsterdam, The Netherlands; M. R. Schaafsma, Medical Spectrum Twente, Enschede, The Netherlands; E. Vellenga, University Medical Center Groningen, The Netherlands; H. C. Schouten, University Hospital Maastricht, The Netherlands; G. E. de Greef, Erasmus MC–Daniel den Hoed Cancer Center, Rotterdam, The Netherlands; P. Sonneveld, B. Löwenberg, Erasmus MC, Rotterdam, The Netherlands; P. W. Wijermans, Leyenburg Hospital, The Hague, The Netherlands;A. W. Dekker, University Medical Center Utrecht, The Netherlands; M. van Marwijk Kooy, Isala Clinics, Zwolle, The Netherlands; I. Nesthus, Haukeland Hospital, Bergen, Norway; J. Hammerstrom, University Hospital Trondheim, Norway; P. Cernelc, University Clinical Center, Ljubljana, Slovenia; M. Jurado, Hospital Universitario Virgen de las Nieves, Granada, Spain; F. J. Batlle Fonrodona, Hospital Juan Canalejo, La Coruña, Spain; J. Besalduch Vidal, Hospital Universitario Son Dureta, Palma de Mallorca, Spain; A. Gratwohl, Kantonsspital Basel, Switzerland; M. F. Fey, Inselspital, Bern, Switzerland; M. Starobinski, Hopital Cantonal Universitaire, Geneva, Switzerland; T. Kovacsovics, CHUV, Lausanne, Switzerland; E. Jacky, Universitätsspital Zürich, Switzerland; M. Beksac, Ankara Medical School, Turkey; B. Ferhanoglu, Cerrahpasa Medical School, Istanbul, Turkey; Y. Pekcelen, Istanbul Medical School, Turkey; D. Milligan, Birmingham Heartlands Hospital, Birmingham, United Kingdom; P. R. Kelsey, Blackpool Victoria Hospital, United Kingdom; A. K. Burnett, Wales School of Medicine, Cardiff University, United Kingdom; J. A. Child, The General Infirmary at Leeds, United Kingdom; R. M. Hutchinson, Leicester Royal Infirmary, United Kingdom; R. E. Clark, The Royal Liverpool University Hospital, United Kingdom; A. Newland, The Royal London Hospital, United Kingdom; G. Mufti, King's College Hospital, London, United Kingdom; J. A. L. Yin, Manchester Royal Infirmary, United Kingdom; A. Prentice, Derriford Hospital, Plymouth, United Kingdom; A. Smith, Southampton General Hospital, United Kingdom; J. Mills, Southend Hospital, Westcliff-on-Sea, United Kingdom; C. Miller, Johns Hopkins Oncology Center, Baltimore, MD; G. I. Cohen, Greater Baltimore Medical Center Cancer Center, MD; K. B. Miller, Tufts New England Medical Center, Boston, MA; H. M. Lazarus, Ireland Cancer Center, Cleveland, OH; A. Makary, Geisinger Medical Center, Danville, PA; W. C. Ehmann, Penn State School of Medicine, Hershey, PA; R. Glasser, Memorial Regional Hospital, Hollywood, FL; L. Rice, Baylor College of Medicine, Houston, TX; M. Cooper, Community Cancer Care, Indianapolis, IN; D. Clausen, SUNY–Stony Brook Medical Oncology Health Science Center, Long Island, NY; W. L. Longo, University of Wisconsin Medical School, Madison; F. Yunus, Boston Cancer Group, Memphis, TN; S. G. Ericson, Mary Babb Randolph Cancer Center, Morgantown, WV; J. P. Greer, Vanderbilt University Medical Center, Nashville, TN; B. G. Raphael, NYU Medical Center, New York, NY; S. Luger, University of Pennsylvania Hospital, Philadelphia; C. N. Abboud, University of Rochester Medical School, NY; W. R. Friedenberg, Guthrie Clinic, Sayre, PA.
Data Monitoring Board for safety and efficacy interim analyses: B. Wörmann, Germany; D. Provan, United Kingdom; E. Solary, France; C. Schiffer, United States; V. Torri, Italy.
Data Monitoring Board for final response analyses: C. Schiffer, United States; J. Kolitz, United States.
Statisticians involved in the design of the trial: W. L. J. van Putten, The Netherlands; K. Wheatley, United Kingdom; and G. Rosenkranz, Switzerland.
Prepublished online as Blood First Edition Paper, June 30, 2005; DOI 10.1182/blood-2005-04-1395.
A complete list of the participating investigators and their institutions appears in “Appendix.”
Supported by Novartis Pharmaceuticals.
One of the authors (M.D.) is employed by a company (Novartis Pharmaceuticals Corp, East Hanover, NJ) whose product PSC-833 was studied in the present work.
B.v.d.H. analyzed the data and wrote the paper; B.L. designed and performed research and wrote the paper; A.K.B. designed and performed research; W.U.K., J.S., P.P.P., G.J.O., G.E.G.V., A.F., M.C., D.S., M.T., M.F.F., and E.V. performed research; M.D. designed research; and P.S. designed and performed research and wrote the paper. Data acquisition and the final analysis were independently performed by the HOVON Data Center (B.v.d.H., B.L., P.S.). All authors read the final version of the manuscript and agreed with its contents.
Presented in part at the 46th annual meeting of the American Society of Hematology, San Diego, CA, December 4-7, 2004.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank P. J. M. Vossebeld and A. Prins for laboratory experiments, E. van Stein for cytogenetics data management, and H. B. Beverloo and E. van den Berg-de Ruiter for cytogenetics review.