Abstract
Hematopoietic stem cell (HSC) gene transfer has been attempted almost entirely ex vivo and has been limited by cytokine-induced loss of self-renewal capacity and transplantation-related defects in homing and engraftment. Here, we attempted to circumvent such limitations by injecting vectors directly into the bone marrow (BM) to transduce HSCs in their native environment. Simian virus 40 (SV40)–derived gene delivery vectors were used because they transduce resting CD34+ cells very efficiently. Rats received SV-(Nef-FLAG), carrying FLAG marker epitope—or a control recombinant SV40 (rSV40)—directly into both femoral marrow cavities. Intracellular transgene expression by peripheral blood (PB) or BM cells was detected by cytofluorimetry. An average of 5.3% PB leukocytes expressed FLAG for the entire study—56 weeks. Transgene expression was sustained in multiple cell lineages, including granulocytes (average, 3.3% of leukocytes, 20.4% of granulocytes), CD3+ T lymphocytes (average, 0.53% of leukocytes, 1% of total T cells), and CD45R+ B lymphocytes, indicating gene transfer to long-lived progenitor cells with multilineage capacity. An average of 15% of femoral marrow cells expressed FLAG up to 16.5 months after transduction. Thus, direct intramarrow administration of rSV40s yields efficient gene transfer to rat BM progenitor cells and may be worthy of further investigation.
Introduction
Hematopoietic stem cells (HSCs) have been among the most alluring targets for gene transfer because of their unique lifelong ability to proliferate and differentiate into all of the cell lineages in peripheral blood (PB). It has been proposed that gene delivery to HSCs could potentially ameliorate selected genetic diseases, as well as be applicable to treatment of such acquired diseases as cancer, AIDS, and autoimmune and neurodegenerative disorders. Recent reports of hematopoietic stem cell plasticity, that is, the ability to develop into cells of nonhematopoietic lineages, may open new vistas for hematopoietic stem cell gene delivery.
Human HSC gene therapy clinical trials generally have yielded disappointing results, the main exception being successful treatment of severe combined immune deficiency (SCID-X1 and adenosine deaminase [ADA]–SCID).1-3 Successful gene transfer has been hampered by low transduction efficiency of human HSCs because oncoretroviruses, the viral vectors usually used for this purpose, require cell division in order to integrate into host genome.4 Primitive HSCs, by contrast, are naturally quiescent.5,6
To overcome this obstacle, strategies have been developed using different cytokines in various combinations that aim to elicit cell division while preserving self-renewal and differentiation potential. Achieving this aim has proven difficult. Cytokine treatment often leads to HSC differentiation, limiting pluripotency and compromising the capacity to replenish the stem cell pool. Such ex vivo treatment also interferes with bone marrow cell homing and engraftment, which are obligatory because the cultured transduced cells must be reimplanted in vivo.7-13
Lentiviral vectors have recently evoked interest because they can transduce nondividing cells, including HSCs.14 However, fully quiescent G0 cells are poorly transduced by these vectors,15,16 so that effective lentiviral gene transfer to HSCs still requires ex vivo cytokine stimulation.17 The requisite short-term culture also may interfere with HSC engraftment upon transplantation. Nonetheless, lentiviral vectors have proven more effective than their oncoretroviral cousins in delivering genes to primitive human HSCs, including human SCID-repopulating cells (SRCs) in NOD/SCID mice.18-20
However, these encouraging data from mice have not translated to higher animals. HIV-based lentiviral vector gene transfer to primitive HSCs in nonhuman primates has yielded disappointing results.21-25 For these and other reasons, clinical trials of lentiviral HSC gene therapy have been long in coming. Other retroviral gene delivery systems offer promise along these lines but await further development.26,27
It may thus be useful to approach the challenge of HSC gene delivery using different paradigms, for example, applying both novel vector systems and direct in situ gene delivery to HSCs. Recombinant simian virus 40–derived viral vectors (rSV40s) transduce nondividing cells, including neurons and hematopoietic stem cells, efficiently and achieve long-term transgene expression in vitro and in vivo,28-31 although levels of protein production tend to be lower than with other vector systems. We and others have demonstrated efficient transduction of hematopoietic progenitor cells using SV40 viral vectors ex vivo.30,32 In this study, we report an in vivo gene transfer approach using percutaneous intrafemoral bone marrow (BM) injection to deliver rSV40s directly into the BM cavity. Our results applying this strategy in rodents suggest that intramarrow gene delivery, using these or other vectors, may hold promise.
Materials and methods
Animals
Female Sprague-Dawley rats (125-150 g) were purchased from Charles River Laboratories (Wilmington, MA). Adult female BALB/c mice were purchased from Jackson Laboratories (Bar Harbor, ME).
Protocols for injecting, bleeding, and killing animals were approved by the Thomas Jefferson University Institutional Animal Care and Use Committee and are consistent with Assessment and Accreditation of Laboratory Animal Care (AAALAC) International standards.
Recombinant SV40 vectors (rSV40) used
We used 2 different rSV40 vectors, constructed as described previously.33 All rSV40 vectors are derived from large T-antigen–deleted SV40 genomes, remain free of Tag, and are thus replication incompetent. SV(Nef-FLAG) carries cDNA encoding HIV Nef protein with a C-terminal FLAG epitope tag. Expression of FLAG-fusion protein was driven by cytomegalovirus–immediate early promoter (CMV-IEP)34 and was tested by flow cytometry (see “Flow cytometry analysis”) or fluorescence microscopy (Olympus, Melville, NY) following intracellular immunostaining. SV(BUGT), a control vector used in this study, carries the cDNA for human bilirubinuridine 5′-diphosphate-glucuronysyl transferase (BUGT), driven by 2 tandem SV40 early promoters (SV40-EP).35
Production of rSV40 viral vectors
We have reported the general principles for making recombinant replication-defective SV40 viral vectors. Briefly, the cloned SV40 genome was excised from its carrier plasmid, gel-purified and recircularized, then transfected into COS-7 cells. These cells supply in trans the large T-antigen (Tag) and the SV40 capsid proteins, which are needed to produce recombinant replication-defective SV40 viral vectors.31 Crude virus stocks were prepared as cell lysates and were then band-purified by discontinuous sucrose density gradient ultracentrifugation and titered by in situ polymerase chain reaction (PCR).36 Infectious titers of such rSV40 viral vectors are generally 1011 to 1012 infectious units (IU)/mL.
Procedure of percutaneous intrafemoral BM injection
Sprague Dawley rats were anesthetized, and the region from the hip to the knee joint was shaved. The knee was flexed to 90°C, and a 27G-gauge needle attached to a 1-cc syringe was lodged between the condyles at the distal femur, and access to the BM cavity was gained using a drilling technique. 1 × 1011 IU of SV(Nef-FLAG) viral vector in 100 μL phosphate-buffered saline (PBS) was injected into the BM cavity of each femur. Control rats received SV(BUGT), administered identically. Rats were injected in staggered groups, with paired experimental and control animals.
Preparation of lineage-negative (Lin–) cells
Murine BM was prepared by flushing femurs and tibias from BALB/c mice with saline and collecting the resulting cells sterilely. Cells from several animals were pooled in order to have enough HSCs for further study. After lysis of erythrocytes (rbc), cells were treated with a mouse lineage antibody cocktail (BD Pharmingen, Mountain View, CA). The cells were separated using a magnetic bead separation (MACS) magnetic column according to manufacturer's instructions (Miltenyi Biotechnology, Aurora, CA), using magnetic beads. The effectiveness of this separation was confirmed using flow cytometry (see “Flow cytometry analysis”).
Isolation and transduction of rat BM mononuclear cells (BMMNCs)
BM was obtained from the rats after killing. Low-density BMMNCs were selected after centrifugation over Ficoll-Hypaque (Amersham Biosciences, Uppsala, Sweden). BMMNCs were treated with SV(Nef-FLAG) viral vectors at a multiplicity of infection (MOI) of 100 for 2 hours at 37°C in 5% CO2 in Iscove modified Dulbecco medium (IMDM) medium supplemented with 1 mM glutamine, 10% fetal bovine serum (FBS, Hyclone Laboratories, Logan, UT), 100 U/mL penicillin, 100 μg/mL streptomycin (Gibco, Carlsbad, CA). After 2 hours, cells were cultured for 6 days in fresh IMDM medium containing stem cell factor (50 ng/mL), interleukin-6 (IL-6) (20 ng/mL), and IL-3 (20 ng/mL) (all from PeproTech, Rocky Hill, NJ).
Flow cytometry analysis
PB and BM were collected at various time points and were analyzed by flow cytometry for FLAG epitope expression or for cell-lineage markers. After red blood cell lysis, cells were incubated with 10% mouse serum (Sigma, St Louis, MO) then immunostained with phycoerythrin (PE)–conjugated mouse antibodies versus rat CD3, CD8, CD45R, granulocytes, or with allophycocyanin (APC)–conjugated mouse anti–rat CD4. In addition, cells were stained with fluorescein isothiocyanate (FITC)–conjugated mouse anti–FLAG IgG1 monoclonal antibody (mAb) (Sigma) after fixation and permeabilization of cells with BD Cytofix/cytoperm Kit (BD Pharmingen). Flow cytometry analyses were performed with a FACSCalibur (Becton Dickinson, Mountain View, CA). At least 10 000 events (forward scatter and side scatter-gated) were collected for each analysis. Analyses of flow cytometry data were performed using CELL Quest v3.3 (Becton Dickinson) using a Macintosh computer (Apple Computer, Cupertino, CA).
FLAG immunostaining
Immunostaining to detect intracellular expressed FLAG epitope on Nef was performed for detection using indirect immunofluorescence. We cultured SV(Nef-FLAG)–transduced BMMNCs on glass slides over night, fixed them with 4% paraformaldehyde (PFA) at room temperature for 20 minutes, and permeabilized with 0.1% NP-40 at room temperature for 10 minutes. We blocked cells with 10% Donkey serum (Sigma) overnight and incubated them with mouse anti–FLAG IgG1 mAb (Sigma), followed by Alexa Fluor 488–conjugated donkey anti–mouse IgG (Molecular Probes, Eugene, OR). We analyzed slides using an Olympus 1 × 70 inverted fluorescence photomicroscope with a 20×/1.0 numeric aperture objective, and digitalized images using Spot software and a Macintosh computer (Apple Computer). Photographic images were composited using Adobe Photoshop (Adobe Systems, San Jose, CA).
Results
Transduction of Lin– cells by rSV40 vectors
rSV40s gene transfer to human and primate HSCs has been reported.30 However, effective transduction by these vectors of the equivalent rodent cell populations (Lin– cells) has not been documented. Antibodies to rat BM hematopoietic lineage markers were unavailable, and the monoclonal antibodies against mouse BM populations were of rat origin, and so of undefined reactivity versus rat BM cells. Therefore, to evaluate rSV40 gene delivery to rodent Lin– cells, we used mouse BM cells as targets.
Mouse BM cells were sorted using MACS (see “Materials and methods”) to prepare Lin– cells, which were transduced with SV(Nef-FLAG) or SV(BUGT) (negative control), without stimulation. After 7 days in culture, with cytokines added after transduction to maintain cell viability, more than 98% of cells were FLAG+ by intracellular immunostaining for transgene expression using anti-FLAG antibody (Figure 1).
SV40 viral vectors efficiently transduce unstimulated rat BM mononuclear cells in vitro
Before attempting intramarrow administration, we first tested whether rSV40 vectors could deliver genes to rat BMMNCs in vitro. Low-density BMMNCs were selected from rat femurs and tibias using Ficoll-Hypaque.
The freshly isolated rat BMMNCs were transduced with SV40 (Nef-FLAG) at a virus-to-cell ratio (MOI) of 100 for 2 hours without cytokine stimulation. After transduction, cells were cultured for 3 weeks in the presence of cytokines (IL-3, IL-6, and stem cell factor [SCF] (see “Materials and methods”). Control cells were mock-transduced and cultured under the same conditions. On days 7 and 21 after transduction, the cultured cells were analyzed for FLAG expression using immunostaining as described in “Materials and methods.” As shown in Figure 2, FLAG expression was detected in almost all SV(Nef-FLAG)–transduced BMMNCs 7 (Figure 2A) and 21 (Figure 2B) days after transduction. Control cells were negative. Percentages of FLAG-expressing cells were identical in transduced cultures assayed at 7 days and 21 days, indicating that transgene expression delivered by the rSV40 viral vector persisted. Taken together, our data demonstrate that SV40 viral vectors transduce rat BMMNCs with very high efficiency and that they deliver sustained transgene expression.
Efficient in vivo transduction of rat BM cells using SV40 viral vectors
To test the transduction efficiency of BM cells in vivo, 4 animals were injected with SV40 viral vectors at a dose of 1 × 1011 IU/femur. Two rats were killed each on day 7 and day 42 after injection. Bone marrow cells were assayed for FLAG expression by flow cytometry after intracellular immunostaining. Figure 3 shows representative FACS scattergrams for FLAG expression in both BM leukocytes (Figure 3A) and subpopulations (Figure 3B) examined on day 7. Data from 6 weeks were virtually identical (not shown). Percentages of BM cells expressing FLAG ranged from 20% to 30%. The intensity of FLAG immunopositivity also was high. In these analyses, gating for granulocytes and nongranulocytes was based on forward scatter (FSC) and side scatter (SSC).
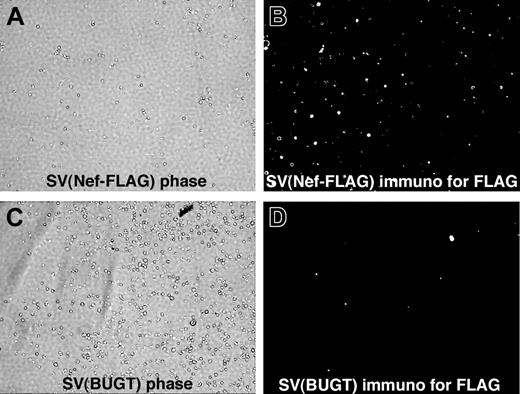
FLAG expression was detected in mouse BM lineage-negative (Lin–) cells transduced with SV(Nef-FLAG) in vitro. Mouse Lin– BM cells were prepared and transduced in the absence of cytokines, with SV(Nef-FLAG) (A-B) or SV(BUGT) (negative control; C-D) as described in “Materials and methods.” They were cultured for 7 or 42 days, then subjected to intracellular immunostaining to detect FLAG expression. Results are shown here for the 7-day time point. Immunofluorescence photomicrographs (original magnification, × 200) are shown next to phase contrast photomicrographs of the same fields. Sporadic background autofluorescence is seen in the control cultures (D). Results for the 6-week assay point were comparable (data not shown).
FLAG expression was detected in mouse BM lineage-negative (Lin–) cells transduced with SV(Nef-FLAG) in vitro. Mouse Lin– BM cells were prepared and transduced in the absence of cytokines, with SV(Nef-FLAG) (A-B) or SV(BUGT) (negative control; C-D) as described in “Materials and methods.” They were cultured for 7 or 42 days, then subjected to intracellular immunostaining to detect FLAG expression. Results are shown here for the 7-day time point. Immunofluorescence photomicrographs (original magnification, × 200) are shown next to phase contrast photomicrographs of the same fields. Sporadic background autofluorescence is seen in the control cultures (D). Results for the 6-week assay point were comparable (data not shown).
Immunostaining demonstrates FLAG expression in rat BMMNCs transduced with SV(Nef-FLAG) in vitro. Rat BMMNCs were selected by centrifugation over Ficoll-Hypaque and transduced with SV(Nef-FLAG) or SV(BUGT) (negative control) as described in “Materials and methods.” FLAG expression in transduced rat BMMNCs was analyzed by intracellular immunostaining. (A) Seven days after transduction; (B) 21 days after transduction. Original magnification, × 200.
Immunostaining demonstrates FLAG expression in rat BMMNCs transduced with SV(Nef-FLAG) in vitro. Rat BMMNCs were selected by centrifugation over Ficoll-Hypaque and transduced with SV(Nef-FLAG) or SV(BUGT) (negative control) as described in “Materials and methods.” FLAG expression in transduced rat BMMNCs was analyzed by intracellular immunostaining. (A) Seven days after transduction; (B) 21 days after transduction. Original magnification, × 200.
High levels of in vivo transgene expression in rat PB cells after rSV40 gene delivery by percutaneous intramarrow injection
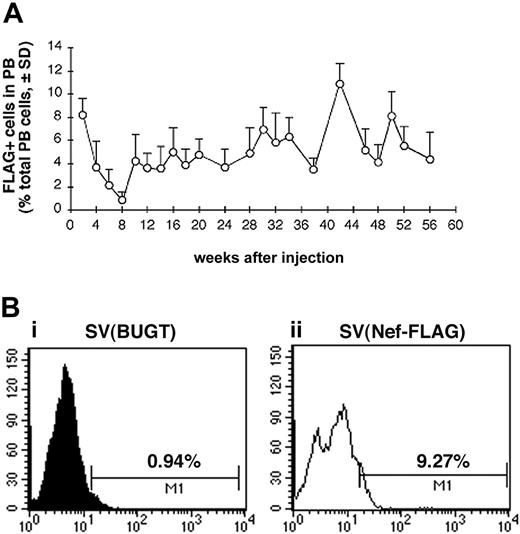
Having therefore established that intramarrow administration allowed for gene delivery to BM hematopoietic cells, we tested whether SV40 viral vectors given this way could transduce BM progenitor cells in vivo. We used this percutaneous BM injection technique in a rat model to deliver SV40 viral vectors directly into BM cavity of both femurs and then followed peripheral blood cells for FLAG expression. Test rats received percutaneous intrafemoral BM injections of SV(Nef-FLAG) at 2 × 1011 IU/animal, as described in “Materials and methods.” Control rats received SV(BUGT), carrying human BUGT cDNA under identical conditions. All rats were followed by PB sampling every 2 to 4 weeks up to 56 weeks for FLAG expression in nucleated cells using flow cytometry following intracellular immunostaining (“Materials and methods”). Figure 4A shows the percentage of FLAG-expressing cells among PB leukocytes of SV(Nef-FLAG)–injected animals for the duration of the study averaged for all rats for each time point. Within the first 2 weeks of injection, FLAG was expressed in between 7% and 10% of PB leukocytes, averaging approximately 9%. Interestingly, in all animals, beginning 2 weeks thence, the percentage of FLAG-expressing cells in PB leukocytes decreased gradually to its nadir of between 0.5% to 1% at week 8 after injection. These percentages then rose again at week 10, at which point they ranged from 2.8% to 7%, averaging 4.3%. After week 12, percentages of FLAG+ cells among PB nucleated cells were relatively stable, ranging between 3.5% and 10% and averaging 6.04% throughout the 13-month follow-up period. The sole exception was rat no. 4, in which FLAG expression decreased after 10 weeks and became undetectable by week 22. Figure 4B shows representative histogram analyses for FLAG-expressing cells in PB of injected rats.
FLAG expression is detected by cytofluorimetry in BM cells of rats injected with SV(Nef-FLAG). Rats received SV(Nef-FLAG) or SV(BUGT) as a negative control: 2 × 1011 IU/rat (1 × 1011 IU/femur). They were killed 1 and 6 weeks later. Bone marrow cells were obtained by flushing the marrow cavities of the femurs with PBS, lysing with ammonium chloride, permeabilizing then immunostaining with anti-FLAG antibody, and analyzing by FACS. (A) FACS scattergram for FLAG expression in BM cells of a representative control rat (SV(BUGT) recipient) 7 days after transduction; (B) of a representative SV(Nef-FLAG) recipient (rat no. 12), 7 days after transduction. Comparable results were obtained at 6 weeks after transduction (not shown). (C) Double immunostaining of BM cells from these rats for granulocyte lineage marker (CD11b) + FLAG, comparing SV(BUGT) recipient (dashed line) and SV(Nef-FLAG) recipient (solid gray line). Insufficient CD3+ cells were present in the marrow to allow analysis of FLAG expression in resident BM lymphocytes (data not shown).
FLAG expression is detected by cytofluorimetry in BM cells of rats injected with SV(Nef-FLAG). Rats received SV(Nef-FLAG) or SV(BUGT) as a negative control: 2 × 1011 IU/rat (1 × 1011 IU/femur). They were killed 1 and 6 weeks later. Bone marrow cells were obtained by flushing the marrow cavities of the femurs with PBS, lysing with ammonium chloride, permeabilizing then immunostaining with anti-FLAG antibody, and analyzing by FACS. (A) FACS scattergram for FLAG expression in BM cells of a representative control rat (SV(BUGT) recipient) 7 days after transduction; (B) of a representative SV(Nef-FLAG) recipient (rat no. 12), 7 days after transduction. Comparable results were obtained at 6 weeks after transduction (not shown). (C) Double immunostaining of BM cells from these rats for granulocyte lineage marker (CD11b) + FLAG, comparing SV(BUGT) recipient (dashed line) and SV(Nef-FLAG) recipient (solid gray line). Insufficient CD3+ cells were present in the marrow to allow analysis of FLAG expression in resident BM lymphocytes (data not shown).
Statistical analysis comparing all test animals versus their paired control (SV[BUGT]-injected) counterparts for each assay point showed that the percentages of FLAG+ cells in the blood were significantly above background levels at each biweekly time point. Although levels of statistical significance varied from week to week, the significance was always at least a P value of less than .05, and at most time points, a P value of less than .01 by Wilcoxon signed rank test. Taken together, our results demonstrate that BM injection with rSV40 vectors provides efficient in vivo gene transfer to primitive BM progenitor cells with long-term hematopoietic capacity.
FLAG expression is detected for more than 13 months in the peripheral blood (PB) of SV(Nef-FLAG) recipients. Rats were injected intrafemorally with SV(Nef-FLAG) or SV(BUGT), as a control, at doses of 2 × 1011 IU/rat. PB obtained at 2- to 4-week intervals after injection was treated with NH4Cl to lyse red blood cells, then stained with anti-FLAG antibody. FLAG expression was detected by flow cytometry, and data were analyzed as described in “Materials and methods.” (A) Time course of FLAG expression in PB leukocytes of all rats injected with SV(Nef-FLAG). Background positivity (ie, that seen in control rats' blood) was subtracted. (B) FACS histogram analyses for FLAG expression level in PB leukocytes from representative control and test rats 2 weeks after injection: (i), control rat; (ii), SV(Nef-FLAG) recipient rat no. 1.
FLAG expression is detected for more than 13 months in the peripheral blood (PB) of SV(Nef-FLAG) recipients. Rats were injected intrafemorally with SV(Nef-FLAG) or SV(BUGT), as a control, at doses of 2 × 1011 IU/rat. PB obtained at 2- to 4-week intervals after injection was treated with NH4Cl to lyse red blood cells, then stained with anti-FLAG antibody. FLAG expression was detected by flow cytometry, and data were analyzed as described in “Materials and methods.” (A) Time course of FLAG expression in PB leukocytes of all rats injected with SV(Nef-FLAG). Background positivity (ie, that seen in control rats' blood) was subtracted. (B) FACS histogram analyses for FLAG expression level in PB leukocytes from representative control and test rats 2 weeks after injection: (i), control rat; (ii), SV(Nef-FLAG) recipient rat no. 1.
Long-term gene expression in PB multiple hematopoietic lineages
To see whether BM injection using rSV40 viral vectors transduced progenitor cells that differentiated into multiple lineages, we followed FLAG expression in granulocytes, and T and B lymphocytes as hematopoietic subpopulations in PB of injected rats over time by flow cytometry (see “Materials and methods”). Figure 5A demonstrates sustained FLAG expression levels in different hematopoietic lineages in all injected rats (except animal no. 4, as indicated in “High levels of in vivo transgene expression in rat PB cells after rSV40 gene delivery by percutaneous intramarrow injection”). FLAG expression in granulocytes was between 1.2% and 8.85% of total PB nucleated cells, and between 7.5% and 55.4% of PB granulocytes. Cells positive for FLAG and either CD3 or CD45R (B lymphocytes, not shown) comprised lower percentages, both when figured as percentages of total PB leukocytes and when calculated as percentages of respective PB lymphocytes: between 0.13% and 1.89% of PB leukocytes, and between 0.24% and 3.5% of PB lymphocyte populations. Figure 5B shows representative FACS scattergrams for FLAG expression in PB granulocytes and CD8+ lymphocytes. Comparable scattergram patterns were seen in samples double-immunostained for FLAG and other lymphocyte markers (not shown). Thus, in vivo BM injection of rSV40 vectors transduced a BM progenitor cell population(s) with long-term multilineage potential very effectively.
Long-term gene expression in rat BM cells
To determine levels of FLAG expression among BM cells of injected rats, animals were killed from 13 to 16.5 months after intramarrow injection, and femoral BM cells were analyzed for FLAG expression by flow cytometry. Among the different animals, percentages of FLAG-expressing cells were very variable, ranging from 2% to 40% of all nucleated cells. The mean was 15.9%. Consistent with the data from rat PB, the percentages of FLAG expressing femoral BM leukocytes were high, averaging approximately 15% of all nucleated cells, but the FLAG+ population averaged 49% of all granulocyte marker+ cells in the BM. (Analysis of FLAG expression specifically in nucleated cells of the other hematopoietic lineages—for example, erythroid, megakaryocytic—was not performed. Similarly, 2-color FACS analyses of nonhematopoietic BM populations were not done.) Much higher percentages of FLAG expression were seen in cells of the granulocyte series than in cells bearing T-lymphocyte cell membrane markers, such as CD3 or B lymphocytes (CD45R+). This disparity may reflect the fact that mature lymphocytes are a very small percentage of BM cells. Figure 6 shows representative FACS scattergram analyses for FLAG expression in total BM cells (Figure 6A) and granulocytes (Figure 6B) of these animals.
FLAG expression is detected by FACS in both PB granulocytes and lymphocytes of rats receiving SV(Nef-FLAG). Blood from all rats that were injected as in Figure 4 legend was double-immunostained (after erythrocyte lysis) for FLAG and mature leukocyte lineage markers. FLAG and lineage marker expression among the several hematopoietic subpopulations was tested by flow cytometry beginning at 14 weeks after injection. Data were analyzed using Cell Quest software. (A) Time course of FLAG expression in PB granulocytes and CD3+ lymphocytes of rats injected with SV(Nef-FLAG). Background immunostaining for FLAG in control vector recipients was subtracted. (B) Representative FACS scattergrams for FLAG and lineage marker expression, shown here at 32 weeks after injection for PB granulocytes (top panels) and CD8+ T lymphocytes (bottom panels). Percentages shown are FLAG+ and lineage+ cells as a percentage of total lineage+ cells for that lineage.
FLAG expression is detected by FACS in both PB granulocytes and lymphocytes of rats receiving SV(Nef-FLAG). Blood from all rats that were injected as in Figure 4 legend was double-immunostained (after erythrocyte lysis) for FLAG and mature leukocyte lineage markers. FLAG and lineage marker expression among the several hematopoietic subpopulations was tested by flow cytometry beginning at 14 weeks after injection. Data were analyzed using Cell Quest software. (A) Time course of FLAG expression in PB granulocytes and CD3+ lymphocytes of rats injected with SV(Nef-FLAG). Background immunostaining for FLAG in control vector recipients was subtracted. (B) Representative FACS scattergrams for FLAG and lineage marker expression, shown here at 32 weeks after injection for PB granulocytes (top panels) and CD8+ T lymphocytes (bottom panels). Percentages shown are FLAG+ and lineage+ cells as a percentage of total lineage+ cells for that lineage.
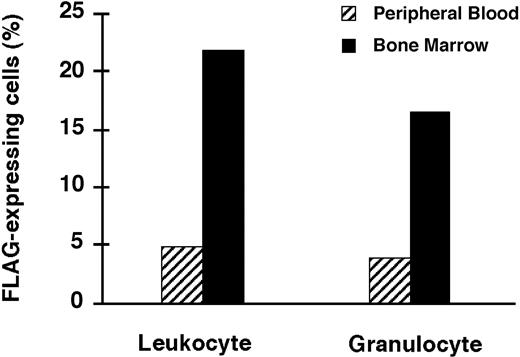
As expected, percentages of FLAG-expressing cells in femoral BM, the site of gene delivery, were much higher than in the PB for those animals for which both determinations were done at the time of killing (Figure 7).
In some animals, BM cells from the tibia also were assayed for FLAG expression. Interestingly, when assayed at 16 months, there was no difference in the percentages of FLAG-expressing cells in the BM from femur and tibia (data not shown), suggesting that BM progenitor cells may not be stationary within the hematopoietic system but may migrate and redistribute among the various blood-forming areas of the BM over time.
In addition to the data from peripheral blood cell marking over the study period, the continued strong positivity of a sizeable percentage of the BM pool of nucleated cells throughout this time underscores the effectiveness of this approach to gene delivery. It also should be noted that the intensity of FLAG expression in cells still in the BM was stronger than in peripheral blood cells. The significance of the gradient in transgene expression between BM cells, which are largely immature precursors, and their more mature peripheral blood derivatives is unclear, but may have significant implications for BM–directed gene transfer studies using this and other vectors.
Long-term FLAG expression is seen in BM (BM) cells of rats injected with SV(Nef-FLAG). Rats given SV(Nef-FLAG) or SV(BUGT), as a control, intrafemorally, at 2 × 1011 IU/rat. They were killed at the end of the 16.5-month follow-up period. BM cells obtained by flushing both femurs were treated to lyse red blood cells, after which the nucleated cells were immunostained for either FLAG or FLAG + granulocyte markers. Transgene expression was detected by FACS and analyzed as described in “Materials and methods.” (A) Representative dot plots for FLAG expression in BM cells, here from rats no. 5 and no. 6, as well as a contemporaneous control rat (CT). FSC = forward scatter. The proportions of FLAG+ cells as a percentage of total nucleated cells are shown. (B) Representative FACS dot plots for FLAG expression in BM granulocytes in the same rats. Percentages of FLAG+ granulocytes shown are relative to total granulocyte+ cells.
Long-term FLAG expression is seen in BM (BM) cells of rats injected with SV(Nef-FLAG). Rats given SV(Nef-FLAG) or SV(BUGT), as a control, intrafemorally, at 2 × 1011 IU/rat. They were killed at the end of the 16.5-month follow-up period. BM cells obtained by flushing both femurs were treated to lyse red blood cells, after which the nucleated cells were immunostained for either FLAG or FLAG + granulocyte markers. Transgene expression was detected by FACS and analyzed as described in “Materials and methods.” (A) Representative dot plots for FLAG expression in BM cells, here from rats no. 5 and no. 6, as well as a contemporaneous control rat (CT). FSC = forward scatter. The proportions of FLAG+ cells as a percentage of total nucleated cells are shown. (B) Representative FACS dot plots for FLAG expression in BM granulocytes in the same rats. Percentages of FLAG+ granulocytes shown are relative to total granulocyte+ cells.
Discussion
Over the past years, the effectiveness of HSC transduction using oncoretroviral vectors has significantly improved.1,37-39 However, extensive ex vivo cytokine stimulation to induce cell division is still needed for oncoretroviral gene delivery to HSCs, which, in the BM, are mostly in G0 phase. Because the consequences of combined cytokine stimulation and reimplantation are problematic, we and others have begun to test in vivo gene transfer to HSCs as a potential alternative to conventional ex vivo gene delivery. Consequently, viral vectors that transduce nondividing cells permanently should be considered as potential vehicles for HSC-directed gene transfer.
FACS analyses for FLAG expression in BM and PB of rat no. 14. Rats were injected intrafemorally with SV(Nef-FLAG), or SV(BUGT) as a control, as in Figure 4 legend. PB and BM cells were obtained 22 weeks later and immunostained for FLAG or FLAG+ granulocytes as in Figure 5 legend. Shown are the comparative percentages of total cells positive for FLAG in the unfractionated population and in the granulocyte population, with background values (from SV(BUGT) recipients) subtracted.
FACS analyses for FLAG expression in BM and PB of rat no. 14. Rats were injected intrafemorally with SV(Nef-FLAG), or SV(BUGT) as a control, as in Figure 4 legend. PB and BM cells were obtained 22 weeks later and immunostained for FLAG or FLAG+ granulocytes as in Figure 5 legend. Shown are the comparative percentages of total cells positive for FLAG in the unfractionated population and in the granulocyte population, with background values (from SV(BUGT) recipients) subtracted.
Retrovirus-mediated gene transfer to HSC by direct in vivo administration was first reported in 1997, when Nelson and coworkers succeeded in achieving long-term transduction, as measured by reverse transcriptase–polymerase chain reaction (RTPCR), for up to 20 months in rabbits.40 A recent report described in vivo retroviral gene transfer to murine HSCs using BM-targeted gene delivery. This study used intrafemoral BM injection after stimulation of BM cell division by pretreatment with 5-fluorouracil. Effective in vivo gene transfer into short-term reconstituting HSCs with multiple lineage potential was demonstrated.41 In that study, gene delivery to correct a defect in Jak3 was used as an effective approach to provide a selective advantage to transduced cells. In vivo retroviral gene transfer to HSCs of neonatal sheep using BM injection also has been reported.42 Newborn lambs were used in that work because they are particularly susceptible to induction of tolerance. The investigators sought to avoid immunologically mediated inactivation of the oncoretroviral vector. This study demonstrated long-term in vivo transgene expression for 16 months. On the average, 0.9% of peripheral blood cells were transgene-expressing lymphocytes, and 1.8% were transgene-expressing granulocytes/monocytes.
We have pursued a similar strategy, except that we used rSV40 viruses to deliver a marker gene. These vectors were employed in the current study because they are capable of transducing cells in G0 with high efficiency and integrating into their genomes to provide permanent transgene expression to both parent and daughter cells. We observed efficient, sustained gene transfer to rat BM progenitor cells: direct injection of a BM compartment with 10% to 15% of the total BM pool resulted in an average of 6% of nucleated blood cells expressing the transgene. This may be attributed to the efficiency of rSV40 viral vectors in transducing the targeted BM progenitor cells.
However, the efficiency of marking of cells from some series was underscored by calculating FLAG+ cells as a percentage of lineage+ cells (as opposed to as a percentage of total PB cells). Thus, an average of 20.4% of PB granulocytes were FLAG+ and 1% of PB CD3+ cells were FLAG+ over the 13 months of PB analyses. Analysis of BM from other sites suggests more widespread transduction of marrow progenitor cell pools than would be expected from femoral injection alone. The mechanism by which intrafemoral administration yielded such extensive PB cell, especially granulocyte, marking, is not clear. Cells of other organs (eg, liver, lungs) were not appreciably transduced (J.-P. Louboutin, B.L., B. A. S. Reyes, E. V. Bockstaele, and D.S.S., manuscript in preparation).
The marker transgene used, the FLAG epitope, was appended to a carrier protein, in this case, HIV-1 Nef. This approach, identifying marking using an antibody-recognized intracellular antigen, differs from techniques used in other studies. “Traditional” marker genes, for example, green or red fluorescent proteins, β-galactosidase, are not expressed well when delivered by rSV40s. The reasons for this are not entirely clear, but COS-7 cells, which package these vectors, appear to recognize and inactivate—in part by methylation and in part by other means—invertebrate or prokaryote DNAs43 (M.S. Strayer, G. Ren, M. K. White, and D.S.S., manuscript in preparation). As a result, such easily visualized markers cannot be used in this system. Other mammalian DNAs and genes from mammalian viruses are generally expressed effectively by these vectors. Nonetheless, levels of protein expression, though durable and not prone to posttransduction silencing,35 tend to be lower than are seen with, for example, adenoviral vectors, perhaps for the same reasons.
Our study demonstrated that in vivo gene transfer to pluripotential BM progenitor cells can be achieved in rats using direct BM injection of rSV40 viral vectors, with no selection or additional treatment. The percentages of FLAG-expressing PB cells in treated rats were high and persisted continuously throughout the follow-up period. Substantial FLAG expression was observed in BM cells at the time of killing.
FLAG was expressed in both granulocyte and lymphocyte PB populations throughout. These data indicate that these vectors, injected directly into the femoral BM cavity in vivo, transduced cells capable of differentiating into these diverse lineages. Whether these cells were true “stem” cells is not clear and cannot be considered to have been unequivocally established by these studies: HSCs are a very low percentage of the BM cell pool (<< 1%), and rodent HSCs are generally considered to be lineage-negative (ie, they lack an agreed-upon cell membrane marker), so that coexpression of FLAG + an HSC marker cannot easily be demonstrated. If they were not HSCs, however, they were long-lived progenitors and capable of both dividing and differentiating into mature cells of lymphoid and myeloid series.
Although rSV40 vectors are very effective in transducing mature lymphocytes,31,44,45 the percentages of total cells in the blood and BM that expressed both FLAG and lymphocyte markers were low, compared with granulocytes. This difference may reflect several factors. Lymphocytes are relatively long-lived, compared with granulocytes, so that the transduced lymphoid progenitors in the BM might not necessarily proliferate and differentiate in the absence of a systemic stress that would generate an increased demand for more lymphocytes. Bone marrow source(s) of lymphoid progenitors may fluctuate or change with time so that the femoral marrow may be responsible for a decreasing percentage of blood lymphocytes. It is also possible that expression of HIV-1 Nef carrier protein may have some lineage-specific toxicity. Such toxicity has been found for endothelial cells46 but not, to our knowledge, for T cells. This issue could be further addressed by doing serial transplantation with transduced BM cells of injected animals (which was not possible in these outbred rats) or by inducing transduced hematopoietic stem cells or by pretreatment with cytotoxic drugs or antibodies against specific lineages to deplete circulating pools of mature cells, either before or after transduction.
Percentages of transgene-expressing cells in the BM of injected rats were much higher than in the PB. A second study reported the same results.47 Although femoral marrow, the target in this study, is only 10% to 15% of the total marrow pool, it is of interest to note that at 16 months after transduction, another BM site (the tibia) contained similar percentages of FLAG+ cells. These findings, together with the very high levels of, for example, granulocyte marking, suggest that intrafemoral marrow injection may reach more progenitor cells than simply those present there at the time of injection. As well, resident marrow progenitors or their immature derivatives may circulate among the marrow-containing bones. Migration of HSCs has been studied in transplant situations,48-50 but this experimental system may allow for its study in resident HSCs.
The levels of FLAG expression (ie, the intensity of immunopositivity) were notably higher in the BM FLAG-expressing cells than in the FLAG+ cells in the blood. The reasons for this intriguing observation are unknown but suggest that maturation of these cells, which coincides with their release to the circulation, may be attended by alterations in levels of gene expression and that transduced genes may be affected by this process. If others can confirm this observation, implications for BM–directed gene delivery are substantial. Modifications, for example, of promoters or other facets of the vector or expression cassette may be necessary.
Percentages of transgene-marked cells may be further increased by multiple injections, preferably into different BM sites. We attempted to inject other bones in the rat, but more distal accessible long bones (eg, tibia) contain much less marrow than the femur, and other bones we tried (eg, ilium, sternum) proved too fragile for our injection technique. Studies are in progress to test this issue in larger animals, in which the flat bones should be stronger.
Repeated vector dosing also may be tried, since the majority of BM cells, even in the femur, were not transduced with a single injection. Increased percentages of rSV40-transduced cells have been seen using other rSV40 vectors in vivo targeting other organs.51,52 In this respect, rSV40 vectors have a unique advantage: repeated studies have shown that they do not elicit detectable neutralizing immune responses and so can be given multiple times without loss of gene delivery efficiency.53,54 The mechanistic basis for this observation is speculative but may lie in the unique method of cell entry employed by these vectors.55-60 This lack of antigenicity and the ability to transduce nondividing cells make SV40 vectors attractive vehicles for HSC gene transfer.
In conclusion, in our present study, we demonstrated efficient and sustained in vivo gene transfer using BM injection of rSV40 vectors. Direct in vivo BM injection may be an alternative to current gene delivery approaches to the BM, and SV40-derived vectors may be useful tools for this purpose.
Prepublished online as Blood First Edition Paper, June 30, 2005; DOI 10.1182/blood-2005-01-0028.
Supported by grants AI48244 and AI41399 from the National Institute of Allergy and Infectious Diseases.
B.L., a postdoctoral fellow, did most of the work: she conducted the research in question, assisted with—and then performed—the bone marrow injection, collected and analyzed the data, and wrote the preliminary version of the manuscript. J.D., Director of the Office of Animal Resources, worked with B.L. to develop and make operational the marrow injection technique under anesthesia. C.N.N., a postdoctoral researcher, was instrumental in data compilation, acquisition, and organization. D.S.S., the corresponding author and principal investigator (PI) in whose lab these studies were performed, directed the work, planned experiments with B.L. and J.D., and is responsible for the completed version of this manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank the Division of AIDS at the National Institute of Allergy and Infectious Diseases (NIAID), in particular Drs Sandra Bridges, Scott Cairns, and the late Nava Sarver, for their help and encouragement with these studies. Discussions with other investigators and the contributions of other laboratory members have contributed greatly to these studies: Drs Pierre Cordelier, John Rossi, Alice Tarantal, and John Zaia. The technical assistance of Ms Dawn Geverd is gratefully acknowledged.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal