Abstract
The mortality of central nervous system (CNS) aspergillosis approaches 100%, requiring improved therapies. Voriconazole gives superior efficacy and survival in invasive aspergillosis, compared with amphotericin B. Also, in contrast to other antifungal drugs, voriconazole penetrates well into the CNS. We evaluated, retrospectively, the outcome and survival of 81 patients who were treated with voriconazole for definite (n = 48) or probable (n = 33) CNS aspergillosis. Complete and partial responses were recorded in 35% of patients and varied by the underlying disease group: hematologic malignancies (54%), other underlying conditions (50%), chronic immunosuppression (45%), solid organ transplantation (36%), and hematopoietic stem cell transplantation (16%). Thirty-one percent of patients survived CNS aspergillosis for a median observation time of 390 days. There were 31 patients who underwent neurosurgical procedures, including craniotomy/abscess resection (n = 14), abscess drainage (n = 12), ventricular shunt (n = 4), and Ommaya-reservoir (n = 1). Multifactorial analysis revealed that neurosurgery was associated with improved survival (P = .02). Patients who underwent hematopoietic stem cell transplantation had a poorer survival (P = .02), but 7 (22%) of 32 survived for a median of 203 days. We conclude from this large cohort of patients that voriconazole treatment together with neurosurgical management, whenever feasible, is currently the best approach to treat patients with CNS aspergillosis.
Introduction
Intensive chemotherapy and hematopoietic stem cell transplantation procedures and the increasing use of immunosuppressive therapy in patients with solid organ transplants or autoimmune diseases parallel a rising mortality caused by invasive aspergillosis. By 1997, invasive fungal infections were the seventh most frequent cause of infectious disease–related deaths in the United States, and invasive aspergillosis has shown a dramatic increase as a cause of death in recent years.1 Invasive aspergillosis is an air-borne disease and the majority of patients develop pneumonia or sinusitis.2 However, central nervous system (CNS) aspergillosis occurs at a frequency of 14% to 42% among patients with invasive aspergillosis and acute leukemia or allogeneic stem cell transplantation.3,4 Moreover, Aspergillus has been identified as the most frequent agent causing brain abscesses in bone marrow transplant patients.5 Likewise, autopsy studies in cancer patients reveal that the CNS is the second most frequent organ affected in invasive aspergillosis.6 The prognosis of invasive aspergillosis is related to the pattern of organ involvement. Localized pulmonary disease has the lowest reported mortality, whereas disseminated or CNS aspergillosis has a mortality approaching 100%.7,8 There are no specific treatment recommendations, as detailed data on patient survival with CNS aspergillosis are only available from case reports.9
Poor penetration of antifungal drugs into the CNS contributes to the adverse prognosis of CNS aspergillosis. Amphotericin B, itraconazole, and caspofungin show negligible levels in cerebrospinal fluid or brain tissue.10-13 Voriconazole, a novel triazole, penetrates into the CNS in humans with a cerebrospinal fluid-to-plasma ratio of 0.22 to 1. In addition, high voriconazole brain tissue levels (11.8 μg/g and 58.5 μg/g) were found at autopsy in 2 patients with pulmonary aspergillosis, indicating that voriconazole is enriched in brain tissue.14 In a large randomized trial, initial therapy with voriconazole has shown superior efficacy compared to that with amphotericin B in all forms of invasive aspergillosis.15 Moreover, voriconazole has been used successfully in a few patients with CNS aspergillosis.16-19 Thus, we conducted a retrospective analysis of 81 patients who were treated with voriconazole for CNS aspergillosis.
Patients, materials, and methods
Patients and case definitions
Cases of CNS aspergillosis were identified from a database containing 747 patients with invasive aspergillosis who were treated with voriconazole in clinical trials or compassionate-use programs.15,17-19 Approval for these studies was obtained from the institutional review boards of all participating centers affiliated with the authors and listed in the appendix. All patients were classified (by S.S. and M.R.) as having definite or probable CNS aspergillosis using predefined diagnostic criteria. In definite CNS aspergillosis there was cultural or histologic (fungal elements with typical morphologic characteristics) evidence for Aspergillus in cerebrospinal fluid or brain biopsy specimens. Patients classified as definite CNS aspergillosis without positive fungal cultures from the CNS required a positive histologic or cytologic specimen from brain/CNS and a positive culture with growth of Aspergillus from other sterile body sites, or bronchoalveolar lavage. Patients with probable CNS aspergillosis had radiologic signs of CNS infection in computed tomographic or magnetic resonance imaging scans plus a definite Aspergillus infection at body sites outside the CNS. Diagnostic criteria proposed by the European Organization for Research and Treatment of Cancer Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group were used to categorize definite aspergillosis outside the CNS.20 In addition, immunocompromised patients (those having undergone allogeneic stem cell transplantation or with neutropenic hematological disease) with imaging suggesting invasive aspergillosis (lung-halo or air-crescent sign) plus signs and symptoms of a CNS infection were also classified as probable CNS aspergillosis if they had a positive Aspergillus culture from a nonsterile site or an adequate antigen or polymerase chain reaction result from cerebrospinal fluid or brain abscess specimens. Patients not fulfilling these criteria were classified as possible CNS aspergillosis and excluded from further analysis. All patients provided informed consent, according to the Declaration of Helsinki.
Voriconazole treatment
Voriconazole was administered intravenously as monotherapy at the standard or protocol-specified dose of 6 mg/kg body weight every 12 hours on day 1 followed by 3 mg/kg to 4 mg/kg body weight every 12 hours from day 2 onwards. Alternatively, an initial oral loading dose of 400 mg voriconazole twice a day in patients weighing at least 40 kg, or 200 mg voriconazole twice a day in patients weighing less than 40 kg, was given on day 1. The recommended oral maintenance doses were 200 mg voriconazole twice a day in patients weighing at least 40 kg and 100 mg voriconazole twice a day for patients weighing less than 40 kg. Provided that there were no treatment-related adverse events and in case of a lack of response after 3 days of voriconazole, maintenance doses could be increased to 5 mg/kg intravenously every 12 hours, 300 mg orally twice a day (body weight at least 40 kg), or 150 mg orally twice a day (body weight < 40 kg).
Assessment of treatment response and survival
The treatment response was assessed by the local investigators at the end of antifungal therapy. A complete response was defined as complete resolution of all clinical signs and radiologic lesions. A partial response was defined as a major improvement in all clinical signs and radiologic lesions. All other patients not complying with these definitions were classified as failures. The time from start of voriconazole therapy to the last date of follow-up reported by the local investigators was used for survival analysis.
The treatment response and survival were assessed in 5 different disease groups: (1) hematopoietic stem cell transplantation, (2) hematologic malignancy, (3) solid organ transplantation, (4) chronic immunosuppression due to underlying disease or drug therapy (eg, corticosteroids), and (5) miscellaneous diseases or conditions.
Statistical analyses
Proportional hazards analysis was used to investigate survival and to calculate the risk ratios in the multifactor situation. Factors used were underlying disease, certainty of diagnosis, neurosurgery, baseline steroid therapy at any dosage, and baseline neutropenia (neutrophil count < 0.5/nL). A log-rank test was used to see which of these factors had a statistically significant influence on survival. Kaplan-Meier plots of survival were also produced. P values less than .05 were considered to indicate statistically significant differences. Calculations were performed using SAS 6.09 (SAS Institute, Cary, NC).
Results
Baseline characteristics
A total of 81 patients with definite (n = 48) or probable (n = 33) CNS aspergillosis were identified from clinical trials (n = 36) or compassionate-use programs (n = 45) and evaluated in detail (Table 1).
Baseline characteristics of 81 patients with CNS aspergillosis treated with voriconazole
. | Data . |
|---|---|
| Total no. of patients | 81 |
| Definite diagnosis, no. (%) | 48 (59) |
| Probable diagnosis, no. (%) | 33 (41) |
| Age | |
| Median | 43 y |
| Range | 9 mo-81 y |
| Sex, no. | |
| Male | 47 |
| Female | 34 |
| Antifungal pretreatment, no. (%) | 78 (96) |
| Duration | |
| Median | 31 d |
| Range | 2 d-88 mo |
| Agent, no. | |
| Amphotericin B (or lipid derivatives) | 76 |
| Itraconazole | 37 |
| 5-fluorocytosine | 22 |
| Caspofungin | 5 |
| Underlying conditions, no. (%) | |
| Hematopoietic stem cell transplantation | 32 (39) |
| Hematologic malignancy | 13 (16) |
| Solid organ transplantation | 11 (14) |
| Chronic immunosuppression | 11 (14) |
| Other* | 14 (17) |
. | Data . |
|---|---|
| Total no. of patients | 81 |
| Definite diagnosis, no. (%) | 48 (59) |
| Probable diagnosis, no. (%) | 33 (41) |
| Age | |
| Median | 43 y |
| Range | 9 mo-81 y |
| Sex, no. | |
| Male | 47 |
| Female | 34 |
| Antifungal pretreatment, no. (%) | 78 (96) |
| Duration | |
| Median | 31 d |
| Range | 2 d-88 mo |
| Agent, no. | |
| Amphotericin B (or lipid derivatives) | 76 |
| Itraconazole | 37 |
| 5-fluorocytosine | 22 |
| Caspofungin | 5 |
| Underlying conditions, no. (%) | |
| Hematopoietic stem cell transplantation | 32 (39) |
| Hematologic malignancy | 13 (16) |
| Solid organ transplantation | 11 (14) |
| Chronic immunosuppression | 11 (14) |
| Other* | 14 (17) |
Other underlying diseases/conditions: having just had surgery (n = 2), trauma (n = 2), renal insufficiency (n = 2), polyradiculopathy (n = 1), liver cirrhosis (n = 1), tuberculosis (n = 1), obstructive pulmonary disease (n = 1), chronic sinusitis (n = 1), and unknown (n = 3).
Most patients (96%; 78 of 81) had received antifungal pretreatment with drugs other than voriconazole for a median of 31 days. Antifungal pretreatment consisted of conventional amphotericin B (n = 57) or its lipid derivatives liposomal amphotericin B (n = 38) and amphotericin B lipid complex (n = 8); itraconazole (n = 37); 5-fluorocytosine (n = 22); or caspofungin (n = 5). Most patients (n = 54; 67%) received more than one of these antifungal agents during pretreatment. Reasons for a changeover to voriconazole treatment were efficacy failure (n = 62), intolerance (n = 1), initiation of scheduled study treatment (n = 10), and unknown (n = 8).
The majority of patients had underlying diseases or conditions resulting in severe immunosuppression (Table 1). Of the 32 patients who had undergone hematopoietic stem cell transplantation, 30 had received allogeneic and 2 autologous transplants. In 13 patients with hematologic malignancies, 11 had acute leukemia, 1 had myelodysplastic syndrome, and 1 had malignant lymphoma. Eleven patients had undergone solid organ transplantation of the liver (n = 6), kidney (n = 3), or heart (n = 2). In the patient group with chronic immunosuppression (n = 11), the following underlying diseases/conditions were reported: chronic granulomatous disease (n = 4), corticosteroid therapy (n = 5), HIV-infection (n = 1), and congenital agranulocytosis (n = 1). Finally, 14 patients had a variety of underlying diseases or conditions, including having just had surgery (n = 2), trauma (n = 2), renal insufficiency (n = 2), polyradiculopathy (n = 1), liver cirrhosis (n = 1), tuberculosis (n = 1), obstructive pulmonary disease (n = 1), chronic sinusitis (n = 1), and unknown (n = 3).
Treatment responses and survival
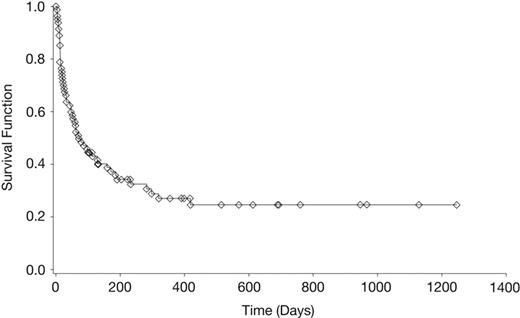
The duration of voriconazole therapy ranged from 1 to 1128 days (median, 51 days). A complete or partial response occurred in 28 (35%) of 81 patients (Table 2; Figure 1). At last follow-up, 25 (31%) of 81 patients were alive and the reported survival ranged from 3 days to 1245 days (median, 69 days; Table 2; Figure 2). For the 25 patients not known to have died, the reported median survival time was 390 days. Of these, 15 had a reported median survival after termination of voriconazole therapy of 237 days (range, 1-473 days). Those with hematologic malignancies or who had undergone solid organ or hematopoietic stem cell transplantation had lower survival rates of 15%, 27%, and 22%, respectively, than those with other underlying conditions and chronic immunosuppression (Table 2). Seven of the 32 patients with CNS aspergillosis as a complication of hematopoietic stem cell transplantation survived for a median of 203 days (range, 17-689 days). Of the 13 patients with hematologic malignancies, 7 (54%) of 13 responded to voriconazole therapy, which contrasts with a survival rate of only 15% in this subgroup (Table 2). Four (31%) of these 13 patients died due to CNS aspergillosis, whereas 7 (54%) patients died of other causes (eg, relapsed acute leukemia, bacterial infections). In the remaining subgroups, death due to causes other than CNS aspergillosis occurred at a lower frequency (9%-22%).
Treatment responses, survival, and causes of death in 81 patients treated with voriconazole for definite or probable central nervous system aspergillosis
Underlying condition . | No. patients . | Median duration of VRC, d (range) . | No. CR/no. PR . | No. stable/no. failure . | Median survival, d (range) . | No. patients died due to aspergillosis, (%) . | No. patients with reported survival (%) . |
|---|---|---|---|---|---|---|---|
| Hematologic malignancy | 13 | 96 (5-522) | 2/5 | 2/4 | 113 (7-759) | 4 (31) | 2 (15) |
| Other | 14 | 82 (1-946) | 2/5 | 3/4 | 130 (11-969) | 5 (36) | 6 (43) |
| Chronic immune suppression | 11 | 122 (9-1128) | 1/4 | 3/3 | 222 (10-1128) | 3 (27) | 7 (64) |
| Solid organ transplantation | 11 | 39 (7-825) | 0/4 | 2/5 | 78 (8-1245) | 7 (64) | 3 (27) |
| Stem cell transplantation | 32 | 20 (3-390) | 2/3 | 3/24 | 28 (3-689) | 18 (56) | 7 (22) |
| Total | 81 | 51 (1-1128) | 7/21 | 13/40 | 69 (3-1245) | 37 (46) | 25 (31) |
Underlying condition . | No. patients . | Median duration of VRC, d (range) . | No. CR/no. PR . | No. stable/no. failure . | Median survival, d (range) . | No. patients died due to aspergillosis, (%) . | No. patients with reported survival (%) . |
|---|---|---|---|---|---|---|---|
| Hematologic malignancy | 13 | 96 (5-522) | 2/5 | 2/4 | 113 (7-759) | 4 (31) | 2 (15) |
| Other | 14 | 82 (1-946) | 2/5 | 3/4 | 130 (11-969) | 5 (36) | 6 (43) |
| Chronic immune suppression | 11 | 122 (9-1128) | 1/4 | 3/3 | 222 (10-1128) | 3 (27) | 7 (64) |
| Solid organ transplantation | 11 | 39 (7-825) | 0/4 | 2/5 | 78 (8-1245) | 7 (64) | 3 (27) |
| Stem cell transplantation | 32 | 20 (3-390) | 2/3 | 3/24 | 28 (3-689) | 18 (56) | 7 (22) |
| Total | 81 | 51 (1-1128) | 7/21 | 13/40 | 69 (3-1245) | 37 (46) | 25 (31) |
VRC indicates voriconazole; CR, complete response; PR, partial response.
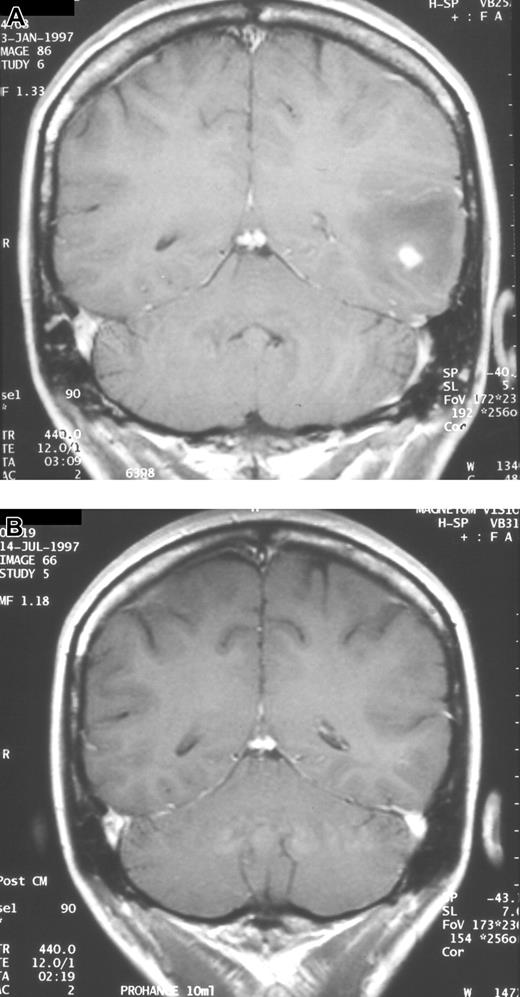
Serial neuroradiologic studies in a patient with CNS aspergillosis. Coronal MRI scans showing complete resolution of a single brain abscess after 133 days of voriconazole therapy. (A) Prior to start of voriconazole. (B) After therapy.
Serial neuroradiologic studies in a patient with CNS aspergillosis. Coronal MRI scans showing complete resolution of a single brain abscess after 133 days of voriconazole therapy. (A) Prior to start of voriconazole. (B) After therapy.
Multifactor risk analysis
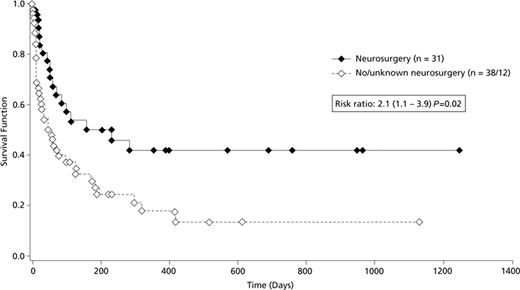
A variety of risk factors possibly related to outcome was evaluated in a multifactorial analysis. Diagnostic certainty (definite/probable) and baseline steroid therapy had no statistically significant impact on survival (hazard ratios, 1.1 and 1.3; confidence intervals, 0.6-1.9 and 0.7-2.4; P values, 0.82 and 0.35, respectively). There was a trend toward increased mortality with baseline neutropenia (hazard ratio, 1.9; confidence interval, 0.9-4.3; P = 0.12). Survival in patients who had undergone hematopoietic stem cell transplantation was significantly less than that of all other patients with a hazard ratio of 2 (confidence interval, 1.1-3.6=; P = .02) Interestingly, neurosurgical intervention (at any time after initial diagnosis of CNS aspergillosis) was associated with improved survival (hazard ratio, 2.1; confidence interval, 1.1-3.9; P = .02) compared with patients without or with unknown neurosurgery (Figure 3). A total of 31 patients underwent neurosurgical interventions, including craniotomy/abscess resection (n = 14), abscess drainage (n = 12), ventricular shunt (n = 4), and placement of an Ommaya-reservoir (n = 1). Slightly more of these 31 patients with neurosurgery had single brain lesions (single lesion, 14; multiple lesions, 11; unknown, 6) in contrast to the 50 patients without or with unknown neurosurgery (single lesion, 12; multiple lesions, 27; unknown, 11).
Kaplan-Meier survival curve of patients with CNS aspergillosis. Survival curve of 81 patients with primary/salvage voriconazole therapy.
Kaplan-Meier survival curve of patients with CNS aspergillosis. Survival curve of 81 patients with primary/salvage voriconazole therapy.
Microbiology
The infecting fungus was identified to the species level in 50 patients (A fumigatus = 44, A nidulans = 5, A terreus = 3, A flavus = 2; including 1 triple and 2 dual infections). In vitro susceptibility testing to voriconazole was available for 13 (A fumigatus = 10, A nidulans = 2, A terreus = 1) of these isolates and was measured using the National Committee for Clinical Laboratory Standards (NCCLS) M38A testing method.21 The voriconazole minimal inhibitory concentration range (0.03-0.5 μg/mL; IC90 0.5 μg/mL) for these isolates is consistent with published data22 and no isolate showed resistance to itraconazole (range, 0.03-1.0 μg/mL; IC90 0.25 μg/mL) or amphotericin B (range, 0.5-2.0 μg/mL; IC90 1.0 μg/mL) despite sometimes extensive prior exposure to these agents.
Adverse events during voriconazole therapy
Overall, 485 all causality adverse events were recorded from 73 (90%) of 81 patients with CNS aspergillosis during voriconazole therapy. The investigators ascribed most adverse events to the underlying condition or CNS aspergillosis, but 59 (12%) adverse events in 32 patients were considered possibly voriconazole related. The most common voriconazole-related adverse events were raised liver function tests (n = 11), visual events (n = 8), and rash (n = 7). In 21 patients who discontinued voriconazole due to adverse events, the events were considered voriconazole-related by the investigator in 8 (elevated liver function tests: 4; cutaneous eruption: 1; acute pancreatitis: 1; bone marrow aplasia: 1; tachyarrhythmia: 1).
Discussion
This study evaluated the clinical characteristics, response to therapy, and survival of the largest cohort of patients with CNS aspergillosis reported to date. Recognized diagnostic criteria were used and adapted to patients with CNS aspergillosis to strengthen the certainty of the diagnoses.20 Thus, 59% of patients in this series had definite CNS aspergillosis with positive results from diagnostic CNS specimens. Moreover, most patients (83%) suffered from severe disease- or drug-induced immunosuppression.
Treatment responses in patients with CNS aspergillosis have rarely been observed in the past. In a recent review, complete and partial responses, mainly to amphotericin B- or itraconazole-based therapy, occurred in only 3 (9%) of 34 patients with CNS aspergillosis.23 Moreover, prolonged survival in such patients is also uncommon, with mortality in a large retrospective analysis reaching 99%.7 Given these unpromising historical data and the high percentage of patients in the present study who failed prior therapy, the results in the current series of voriconazole-treated patients are encouraging, with complete and partial responses occurring in 35% of patients and 31% surviving for a median observation time of 390 days. Amongst these were some long-term survivors who were potentially cured from CNS aspergillosis, although they were mostly patients with low or chronic underlying immune suppression. A variety of factors determine the prognosis of patients with invasive aspergillosis and might also have impacted the results of the present study. However, the significantly improved survival and long-term survival after termination of therapy in a subset of patients suggests that voriconazole treatment had a major impact on the outcome.
Patients with established risk factors, such as having undergone hematopoietic stem cell transplantation, still had high mortality rates from CNS aspergillosis. Complete and partial responses have been previously reported in only 15% of patients with bone marrow transplantation and invasive aspergillosis at any site.23 The response rate of 16% in patients with hematopoietic stem cell transplantation seen in our series is thus at least as high as for patients with bone marrow transplantation and any type of invasive aspergillosis. Moreover, 7 of the 32 voriconazole-treated patients who had undergone hematopoietic stem cell transplantation survived CNS aspergillosis for a median of 203 days, demonstrating that long-term survival may occasionally be achieved in these high-risk patients.
Resection of infected tissue or abscesses eliminates areas with low drug penetration that might contain viable fungi. Surgery has been advocated particularly in patients with invasive aspergillosis of the lung, sinuses, and bone.2 However, the significance of neurosurgical procedures in CNS aspergillosis has not previously been evaluated and detailed data are only available from case reports. Coleman et al reported on a patient who survived CNS aspergillosis, and additionally reviewed 25 previously published case reports on surviving patients.9 Interestingly, 20 of these 26 patients underwent neurosurgical interventions, including 15 with craniotomy and 6 with stereotactic drainages or intracavitary catheters. It is noteworthy that, in the present series, patients with neurosurgical procedures at some time during the course of their CNS infection had a significantly improved survival, although a variety of interventions were performed.
Kaplan-Meier survival curves of patients with CNS aspergillosis according to application of neurosurgical interventions. Survival curves comparing voriconazole-treated patients who had neurosurgery (n = 31) versus those with no or unknown neurosurgery (n = 50).
Kaplan-Meier survival curves of patients with CNS aspergillosis according to application of neurosurgical interventions. Survival curves comparing voriconazole-treated patients who had neurosurgery (n = 31) versus those with no or unknown neurosurgery (n = 50).
This retrospective review is, of course, subject to selection bias regarding which patients underwent neurosurgical procedures. However, the ability of voriconazole therapy to slow down the progression of CNS aspergillosis may allow more effective management of the complications of CNS infections such as elevated intracranial pressure or prevention of fatal hemorrhage. More detailed studies of the use of surgical interventions in combination with voriconazole therapy of CNS aspergillosis are needed.
In conclusion, voriconazole is a promising treatment option in patients with CNS aspergillosis. However, the optimal treatment duration in CNS aspergillosis, like in other forms of invasive aspergillosis, remains unknown. Prolongation of therapy beyond the resolution of all lesions and until reversal of underlying predispositions appears to be a meaningful approach and should be carefully considered in individual patients.2 The results from the present study show that a more intensified treatment approach, which should include neurosurgical interventions whenever feasible, rather than palliative treatment, might improve the prognosis. Further studies to enhance treatment efficiency by using a combination of voriconazole with additional antifungals, higher doses of medication, and better adjunctive therapy appear warranted.24-27
Appendix
The following centers participated in the study: Australia: T. Gottlieb (Concord); Belgium: H. Spapen (Brussels); Canada: I. Salit (Toronto, ON), M. Salvatori (Toronto, ON); France: S. Blanche (Paris), C. Cordonnier (Créteil), B. Dupont (Paris), R. Herbrecht (Strasbourg), S. Lariven (Paris), N. Milpied (Nantes), J. Piette (Paris), D. Vachon (Paris), F. Witz (Vandoeuvre); Germany: V. Aumann (Magdeburg), A. Bender (Munich), H. Bertz (Freiburg), H. Breithaupt (Giessen), G. Fätkenheuer (Cologne), G. Fleischhack (Bonn), A. Grote-Metke (Hamm), A. Haas (Potsdam), H. Hebart (Tübingen), W. Huber (Munich), W. Kern (Ulm), H. Kolb (Munich), D. Milatovic (Munich), R. Naumann (Dresden), M. Nowrousian (Essen), N. Peter (Cottbus), G. Ruckdeschel (Munich), G. Silling (Münster), A. Simon (Bonn), A. Zander (Hamburg); Israel: A. Nagler (Jerusalem); Italy: D. Scevola (Pavia); Netherlands: A. Hoepelman (Utrecht), P. Verweij (Nijmegen); Portugal: M. Abecasis (Lisbon); Switzerland: U. Flückiger (Basel), M. Glauser (Lausanne); United Kingdom: A. Goldstone (London), P. Gower (London), F. Nye (Liverpool), A. Parker (Glasgow); USA: M. Boeckh (Seattle. WA), J. Brown (Stanford, CA), J. Dossett (Hershey, PA), P. Chandrasekar (Detroit, MI), P. Flomenberg (Philadelphia, PA), K. Marr (Seattle, WA), J. Moreb (Gainesville, FL), and S. Murphey (Livingston, NJ).
Prepublished online as Blood First Edition Paper, July 5, 2005; DOI 10.1182/blood-2005-02-0733.
P.T. is employed by Pfizer, whose product, voriconazole, was studied in the present work. O.A.C. received a research grant from Pfizer.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors are grateful to A. Espinel-Ingroff and E. Johnson for Aspergillus identification and susceptibility testing, K. Greenhalgh for statistical analyses, B. Dodell for clinical data collection, and to the centers that participated in the study; a complete list of the participating centers appears in “Appendix.”

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal