Although the Abelson (Abl) tyrosine kinase inhibitor imatinib mesylate has improved the treatment of breakpoint cluster region–Abl (Bcr-Abl)–positive leukemia, resistance is often reported in patients with advanced-stage disease. Although several Src inhibitors are more effective than imatinib and simultaneously inhibit Lyn, whose overexpression is associated with imatinib resistance, these inhibitors are less specific than imatinib. We have identified a specific dual Abl-Lyn inhibitor, NS-187 (elsewhere described as CNS-9), which is 25 to 55 times more potent than imatinib in vitro. NS-187 is also at least 10 times as effective as imatinib in suppressing the growth of Bcr-Abl–bearing tumors and markedly extends the survival of mice bearing such tumors. The inhibitory effect of NS-187 extends to 12 of 13 Bcr-Abl proteins with mutations in their kinase domain but not to T315I. NS-187 also inhibits Lyn without affecting the phosphorylation of Src, Blk, or Yes. These results suggest that NS-187 may be a potentially valuable novel agent to combat imatinib-resistant Philadelphia-positive (Ph+) leukemia.
Introduction
The Philadelphia (Ph) chromosome results from a reciprocal translocation between chromosomes 9 and 22 that generates the breakpoint cluster region–Abelson (Bcr-Abl) chimeric protein, which in turn causes chronic myeloid leukemia (CML) and Ph+ acute lymphoblastic leukemia (ALL).1,2 Imatinib mesylate (STI571, Gleevec) specifically inhibits the autophosphorylation of Abl tyrosine kinase and is not only highly efficacious in treating these diseases but also generally produces only mild side effects.3 Within a few years of its introduction to the clinic, imatinib dramatically altered the first-line therapy for CML.4 Most newly diagnosed CML patients in the chronic phase achieve durable responses when treated with imatinib.4 However, a small percentage of these patients as well as most advanced-phase patients relapse on imatinib therapy.5,6 Bcr-Abl–dependent mechanisms of resistance to imatinib include overexpression of Bcr-Abl and amplification of the bcr-abl gene and, most intriguingly, point mutations within the Abl kinase domain that interfere with imatinib binding.7-10
To overcome imatinib resistance, higher doses of imatinib and combination therapy with other agents have been used with some efficacy. However, these strategies are limited in their application and effectiveness, especially for patients with Abl point mutations.11-13 Therefore it is necessary to develop more effective Abl tyrosine kinase inhibitors. Several Src inhibitors from various chemical classes, including PD166326,14 SKI-606,15 AP23464,16 and BMS-354825,17 have been found to be 100 to 300 times more effective than imatinib in blocking Bcr-Abl tyrosine kinase autophosphorylation, and these effects extend to point mutants of Bcr-Abl. However, while imatinib binds to only the inactive form of Abl, these dual Src-Abl inhibitors also bind to the active form, which shares considerable conformational similarity with the active forms of diverse tyrosine kinases, including the Src-family proteins.16,18 This characteristic of dual Src-Abl inhibitors has some advantage with respect to Lyn kinase, a Src-family protein, because overexpression of Lyn is associated with imatinib resistance.19-21 It has been shown that dual Src-Abl inhibitors can simultaneously inhibit Lyn18 and this may enhance their ability to induce apoptosis in CML cells. However, the effect of lower specificity against Src-family kinases is not yet fully understood because these kinases play many important roles in vivo.22-26
We sought to identify a novel orally bioavailable compound for treating Ph+ leukemias that (1) is more potent than imatinib in blocking Bcr-Abl kinase activity, including variants with point mutations in the kinase domain; (2) has fewer adverse effects; and (3) inhibits Lyn while otherwise remaining highly specific for Bcr-Abl. The crystal structure of the Abl kinase domain in complex with imatinib shows that some alterations to the structure of the drug are likely to be tolerated.27,28 Chemical modifications made with the guidance of molecular modeling yielded several promising compounds. Among them, a potent and specific dual Abl-Lyn inhibitor, NS-187 (Figure 1A), was selected on the basis of its overall characteristics, including its pharmacokinetics and toxicity as determined in animal studies. NS-187 was found to be much more effective than imatinib and to inhibit Lyn at clinically relevant concentrations without affecting the phosphorylation of Src, Blk, or Yes. Its inhibitory effect extended to almost all Bcr-Abl point mutants tested.
Materials and methods
Reagents and cell lines
NS-187 (Figure 1A) and imatinib (Figure 1B) were synthesized and purified at Nippon Shinyaku (Kyoto, Japan). The compounds were dissolved as 10-mM aliquots in dimethyl sulfoxide (Sigma-Aldrich, St Louis, MO) and stored at -80°C until required for use. K562 (chronic myeloid leukemia), U937 (histiocytic lymphoma), NCI-H526 (small cell lung cancer), and A431 (epidermoid carcinoma) cells were obtained from the American Type Culture Collection (Manassas, VA). KU812 (chronic myeloid leukemia) and NHDF (normal fibroblast) cells were obtained from the Japanese Collection of Research Biosources (Osaka, Japan) and Kurabo (Osaka, Japan), respectively. BaF3/wt, BaF3/E255K, and BaF3/T315I cells were kindly provided by Dr Martin Ruthardt of Frankfurt University. K562, KU812, U937, BaF3/wt, BaF3/E255K, and BaF3/T315I cells were cultured in RPMI 1640 (Nissui, Tokyo, Japan) with 2 mM L-glutamine (Nacalai Tesque, Kyoto, Japan) and 10% fetal bovine serum (FBS; Vitromex, Vilshofen, Germany). NCI-H526 cells were cultured in RPMI 1640 with 2 mM L-glutamine, 4.5 g/L glucose (Nacalai Tesque), 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; Sigma-Aldrich), 1.0 mM sodium pyruvate (ICN Pharmaceuticals, Costa Mesa, CA), and 10% FBS. A431 and NHDF cells were cultured in Dulbecco modified Eagle medium (DMEM; Nissui) with 4 mM L-glutamine and 10% FBS. All cell lines were maintained at 37°C in a fully humidified atmosphere of 5% CO2. Cells undergoing exponential growth were used in the experiments.
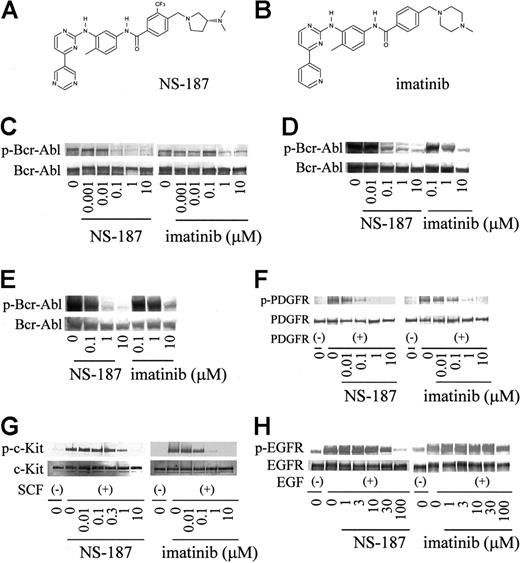
Structure and activity of NS-187. Chemical structures of NS-187 (A) and imatinib (B). K562 cells (C), BaF3/wt cells (D), and BaF3/E255K cells (E) were incubated for 1.5 hours with the indicated concentrations of each compound. Serum-starved NHDF cells (F), NCI-H526 cells (G), and A431 cells (H) were incubated for 1.5 hours with each compound prior to stimulation for 10 minutes with PDGF, SCF, or EGF, respectively. Cells were then lysed and subjected to Western blot analysis with antiphosphotyrosine antibody (C-H), anti–c-Abl antibody (C-E), anti-PDGFR antibody (F), anti–c-Kit antibody (G), or anti-EGFR antibody (H). p indicates phosphorylated.
Structure and activity of NS-187. Chemical structures of NS-187 (A) and imatinib (B). K562 cells (C), BaF3/wt cells (D), and BaF3/E255K cells (E) were incubated for 1.5 hours with the indicated concentrations of each compound. Serum-starved NHDF cells (F), NCI-H526 cells (G), and A431 cells (H) were incubated for 1.5 hours with each compound prior to stimulation for 10 minutes with PDGF, SCF, or EGF, respectively. Cells were then lysed and subjected to Western blot analysis with antiphosphotyrosine antibody (C-H), anti–c-Abl antibody (C-E), anti-PDGFR antibody (F), anti–c-Kit antibody (G), or anti-EGFR antibody (H). p indicates phosphorylated.
Generation and purification of Abl kinase domains
The Abl kinase domain, which consists of c-Abl amino acids 229-515, was amplified by polymerase chain reaction with K562 cDNA as a template and subcloned into the pENTR/D-TOPO vector (Invitrogen, Carlsbad, CA). Each mutation (E244V, G250E, Q252H, Y253F, E255K, E255V, T315I, F317L, M351T, E355G, F359V, H396P, and F486S) was introduced by using the QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). The presence of the mutations was confirmed by sequencing. The wild-type (wt) and mutated kinase domains in the pENTR/D-TOPO vector were transferred to the pDEST10 vector (Invitrogen) by recombination with Gateway LR clonase (Invitrogen). Sf9 cells were infected with baculovirus generated from each kinase domain pDEST10 by using the Bac-to-Bac Baculovirus Expression System (Invitrogen). After inoculation, Sf9 cells were lysed and the kinase domain was purified on a Q-Sepharose Fast Flow anion-exchange column (Amersham Biosciences, Piscataway, NJ) and a HisTrap HP column (Amersham Biosciences). The protein concentration of each kinase domain was determined by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) followed by staining with Coomassie Brilliant Blue.
Kinase assay
Bcr-Abl kinase assays were performed in 25 μL of reaction mixture containing 250 μM peptide substrate, 740 Bq/μL [γ-33P]ATP, and 20 μM cold adenosine triphosphate (ATP) by using the SignaTECT protein tyrosine kinase assay system (Promega, Madison, WI). Each Bcr-Abl kinase was used at a concentration of 10 nM. Kinase assays for Abl, Src, and Lyn were carried out with an enzyme-linked immunosorbent assay (ELISA) kit from Carna Biosciences (Kobe, Japan). The inhibitory effects of NS-187 against 79 tyrosine kinases were tested with KinaseProfiler (Upstate, Dundee, United Kingdom).
Inhibition of intracellular tyrosine kinases
K562 cells, BaF3/wt cells, BaF3/E255K cells, and BaF3/T315I cells were treated with serial dilutions of the compounds for 1.5 hours. Serum-starved NHDF cells, NCI-H526 cells, and A431 cells were treated with serial dilutions of the compounds for 1.5 hours followed by stimulation with 50 ng/mL platelet-derived growth factor (PDGF; Upstate), 100 ng/mL stem-cell factor (SCF; R&D Systems, Minneapolis, MN), or 100 ng/mL epidermal growth factor (EGF; R&D Systems) for 10 minutes. Cells were harvested and lysed with radioimmunoprecipitation assay (RIPA) buffer, and equal amounts of cell lysate protein were analyzed by Western blotting with antiphosphotyrosine antibody PY20 conjugated with horseradish peroxidase (BD Transduction Laboratories, San Jose, CA) and anti–c-Abl antibody, anti–c-Kit antibody, anti–CT10 regulator of kinaselike (anti-CrkL) antibody, anti–EGF receptor (anti-EGFR) antibody, anti–extracellular signal-related kinase 1 (anti-ERK1) antibody (Santa Cruz Biotechnology, Santa Cruz, CA), or anti-PDGFR type A/B (Upstate).
Cell proliferation assays
K562, BaF3/wt, BaF3/E255K, and BaF3/T315I cells were plated in triplicate at 1 × 103 cells/well in 96-well plates, whereas KU812 and U937 cells were plated in triplicate at 5 × 103 cells/well in 96-well plates. Cells were incubated with serial dilutions of the compounds for 3 days. Cell proliferation was measured by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Nacalai Tesque) assay, and the 50% inhibitory concentration (IC50) values were calculated by fitting the data to a logistic curve.
Subcutaneous xenograft model and BaF3 leukemia models
Approvals for the following in vivo studies were obtained from the institutional review board at Kyoto University Hospital.
Balb/c-nu/nu female mice 8 weeks of age were purchased from Japan Clea (Osaka, Japan). KU812 xenograft was established by subcutaneous injection of 2.5 × 107 cells into the right flank of the mice. Seven days after inoculation, the mice were randomized into groups of 5, and NS-187, imatinib, or vehicle (0.5% methylcellulose) was administered orally twice a day by gavage for 10 consecutive days. Tumor sizes were assessed at least twice a week by caliper measurement and their volumes calculated from the formula, tumor size (cm3) = (d2 × D)/2/1000, where d and D are the shortest and longest diameter of the tumor, respectively. Significant differences between samples were determined with the Dunntt test.
BaF3/wt leukemia was established by intravenous injection of 1 × 106 cells into the tail vein of Balb/c-nu/nu mice. The next day the mice were randomized into groups of 7, and NS-187, imatinib, or vehicle (0.5% methylcellulose) was administered orally twice a day for 11 consecutive days. BaF3/E255K leukemia was established by intravenous injection of 5 × 104 cells into the tail vein of Balb/c mice. The next day the mice were randomized into groups of 3, and NS-187, imatinib, or vehicle was administered orally twice a day for 26 consecutive days. Survival analysis was performed by the Kaplan-Meier method and statistical significance was assessed by the log-rank test.
Molecular modeling
All computations were performed with Molecular Operating Environment (MOE) version 2003.01 (Chemical Computing Group, Montreal, QC, Canada). Sequence alignment and homology modeling of Src-family proteins were performed with MOE-Align and MOE-Homology, respectively. The X-ray coordinates of the imatinib/Abl cocrystal structure28 were used as the template. NS-187 was manually docked into the binding site. Because of the structural similarity between NS-187 and imatinib, the mode of binding of NS-187 was assumed to be very similar to that of imatinib, and typical hydrogen-bonding interactions were retained: the nitrogen of the terminal pyrimidine ring with the backbone-NH of Met318, the nitrogen of the anilino group with the OH of Thr315, the amide-NH with the side-chain carboxylate of Glu286, and the amide-CO with the backbone-NH of Asp381. The energy of the models thus obtained was minimized by using the Merck Molecular Force Field (MMFF94s) method.29 The amino acids within 0.7 nm (7 Å) of NS-187 were fully minimized, and changes in the structures of NS-187 and around the amino acids were small. Figures were prepared with PyMOL version 0.97 (DeLano Scientific, South San Francisco, CA).
Results
Chemical structure of NS-187
NS-187 (Figure 1A) is N-[3-(4,5′-bipyrimidin-2-ylamino)-4-methylphenyl]-4-{[3S]-3-(dimethylamino)pyrrolidin-1-yl}methyl)-3-(trifluoromethyl)benzamide (C30H31F3N8O; molecular weight [MW], 576.62). Structure-activity studies leading to the identification of NS-187 as a promising lead compound and a description of its synthesis will be published elsewhere.
NS-187 blocks wt Bcr-Abl autophosphorylation and its downstream kinase activity
We compared the ability of NS-187 and imatinib to inhibit the tyrosine kinase activity of wt Bcr-Abl by first testing by Western blot analysis whether the protein is autophosphorylated in Bcr-Abl–positive K562 cells and BaF3 cells transfected with wt Bcr-Abl (BaF3/wt) when the cells are treated with either drug (Figure 1C-D). Its autophosphorylation status in 293T cells transfected with wt Bcr-Abl was also examined. The IC50 values of NS-187 against wt Bcr-Abl in K562 and 293T cells were 11 and 22 nM, respectively (Table 1). The corresponding values for imatinib were 280 and 1200 nM. Thus, NS-187 was 25 and 55 times more potent than imatinib, respectively, in blocking Bcr-Abl autophosphorylation (Table 1).
The effect of NS-187 and imatinib on the tyrosine kinase activity of platelet-derived growth factor receptor (PDGFR), c-Kit, and epidermal growth factor receptor (EGFR) were also examined. NS-187 suppressed PDGFR and c-Kit phosphorylation with IC50 values very similar to those of imatinib (Figure 1F-G). The ranking of IC50 with respect to imatinib is PDGFR > c-Kit > Bcr-Abl, whereas the ranking with respect to NS-187 is Bcr-Abl > PDGFR > c-Kit (Table 1). Neither NS-187 nor imatinib inhibited EGFR at clinically relevant concentrations (Figure 1H).
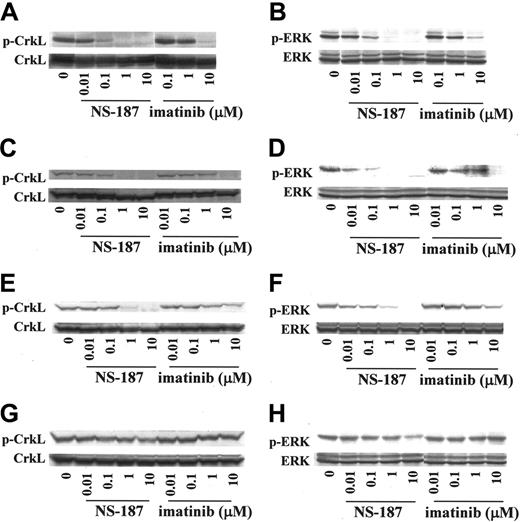
The downstream mediators of Bcr-Abl, CrkL, and ERK are dephosphorylated on treatment with imatinib.30 Examination of the intracellular phosphorylation status of CrkL and ERK revealed that NS-187 inhibited the phosphorylation of CrkL and ERK in K562 cells at much lower concentrations than did imatinib (Figure 2A-B). The inhibition of phosphorylation was also observed in BaF3/wt cells (Figure 2C-D). Thus, NS-187 was much more potent and specific against Bcr-Abl than was imatinib.
NS-187 inhibits the tyrosine phosphorylation of 12 of 13 known kinase domain point mutants
More than 30 point mutations within the Abl kinase domain have been reported.31 The ability of NS-187 to inhibit 13 of these mutant Bcr-Abl kinases was tested by constructing and purifying the mutant kinase domains and then subjecting them to in vitro kinase assays in the presence of NS-187 or imatinib. NS-187 at physiologically obtainable concentrations inhibited the phosphorylation of Bcr-Abl harboring the M244V, G250E, Q252H, Y253F, E255K, E255V, F317L, M351T, E355G, F359V, H396P, or F486S mutations but it did not inhibit the phosphorylation of the T315I mutant (Table 2). For all mutants except T315I, the IC50 for imatinib was at least 5 times as high as the corresponding value for NS-187.
The effect of NS-187 on 2 of the mutants was also examined in whole cells by assessing the phosphorylation of Bcr-Abl with the E255K or the T315I mutation in BaF3 cells (BaF3/E255K and BaF3/T315I). Unlike imatinib, NS-187 inhibited the phosphorylation of the E255K mutant at clinically relevant concentrations (Figure 1E). However, neither imatinib nor NS-187 had any effect on the phosphorylation of the T315I mutant (data not shown). NS-187 also inhibited the phosphorylation of CrkL and ERK in BaF3/E255K cells at much lower concentrations than did imatinib (Figure 2E-F). In contrast, the phosphorylation of CrkL and ERK in BaF3/T315I cells was only slightly inhibited by 10 μM NS-187 and not at all by up to 10 μM imatinib (Figure 2G-H). Thus, NS-187 was effective against all Bcr-Abl mutants tested except for T315I.
Effect of NS-187 on intracellular phosphorylation of CrkL and ERK. NS-187 inhibits the phosphorylation of CrkL and ERK in cells expressing wt Bcr-Abl or the E255K Bcr-Abl mutant but not in cells expressing the T315I Bcr-Abl mutant. K562 (A-B), BaF3/wt (C-D), BaF3/E255K (E-F), or BaF3/T315I (G-H) cells were incubated for 1.5 hours with the indicated concentrations of each compound. Cells were then lysed and subjected to Western blot analysis with antiphosphotyrosine antibody (A-H), anti-CrkL antibody (A,C,E,G), or anti-ERK1 antibody (B,D,F,H). The phosphorylated forms of CrkL (p) migrate more slowly than the unphosphorylated forms.
Effect of NS-187 on intracellular phosphorylation of CrkL and ERK. NS-187 inhibits the phosphorylation of CrkL and ERK in cells expressing wt Bcr-Abl or the E255K Bcr-Abl mutant but not in cells expressing the T315I Bcr-Abl mutant. K562 (A-B), BaF3/wt (C-D), BaF3/E255K (E-F), or BaF3/T315I (G-H) cells were incubated for 1.5 hours with the indicated concentrations of each compound. Cells were then lysed and subjected to Western blot analysis with antiphosphotyrosine antibody (A-H), anti-CrkL antibody (A,C,E,G), or anti-ERK1 antibody (B,D,F,H). The phosphorylated forms of CrkL (p) migrate more slowly than the unphosphorylated forms.
NS-187 inhibits Abl more specifically than does imatinib
To test the ability of NS-187 to inhibit other tyrosine kinases, 79 tyrosine kinases were assayed with KinaseProfiler (Upstate). These included the 5 Src-family kinases Blk, Src, Fyn, Lyn, and Yes. The effects of 0.1 μM NS-187 and 10 μM imatinib were tested because these concentrations gave equal inhibition of Abl. At a concentration of 0.1 μM, NS-187 inhibited 4 of the 79 tyrosine kinases, that is, Abl, Abl-related gene (Arg), Fyn, and Lyn. Notably, at 0.1 μM, NS-187 did not inhibit PDGFRα, PDGFRβ, Blk, Src, or Yes (Figure 3A-B). In contrast, 10 μM imatinib inhibited 9 tyrosine kinases, that is, Abl, Arg, Blk, FMS-like TK-3 (Flt3), Fyn, Lyn, PDGFRα, PDGFRβ, and p70S6K (data not shown), indicating that NS-187 inhibited Abl more specifically than did imatinib. To confirm the specificity of NS-187 against Lyn, the inhibitory effects of NS-187 against Abl, Src, and Lyn were tested. The IC50 values of NS-187 for these kinases were 5.8 nM, 1700 nM, and 19 nM, respectively, and those of imatinib were 106 nM, greater than 10 000 nM, and 352 nM, respectively. These findings suggest that NS-187 acts as an Abl-Lyn inhibitor while otherwise remaining highly specific for Bcr-Abl.
NS-187 suppresses the in vitro growth of leukemic cell lines expressing wt Bcr-Abl or the E255K mutant
NS-187 suppressed the growth of the Bcr-Abl–positive cell lines K562 (Figure 4A), KU812 (Figure 4B), and BaF3/wt (Figure 4D) much more potently than did imatinib, but neither drug affected the proliferation of the Bcr-Abl–negative U937 cell line (Figure 4C). As to Bcr-Abl point mutants, NS-187 exhibited a concentration-dependent antiproliferative effect against BaF3/E255K cells (Figure 4E) but had no effect on BaF3/T315I cells within the concentration range tested (Figure 4F). Imatinib was less effective against all of the point mutants tested (Figure 4E-F).
In vivo effects of NS-187
In our preliminary experiments, when Balb/c mice were given NS-187 orally at a dose of 30 mg/kg, the pharmacokinetic parameters were as follows: Tmax, 2 hours; maximum concentration (Cmax), 661 ng/mL; area under the curve (AUC)0-∞, 2294 ng · h/mL; T1/2, 1.0 hour; and bioavailability value (BA), 32%. The maximal tolerated dose (MTD) of NS-187 in Balb/c or Balb/c-nu/nu mice was 200 mg/kg/d (100 mg/kg twice a day). Cmax for NS-187 was estimated at 2226 ng/mL (4.0 μM) when mice were treated twice a day orally with 100 mg/kg of NS-187.
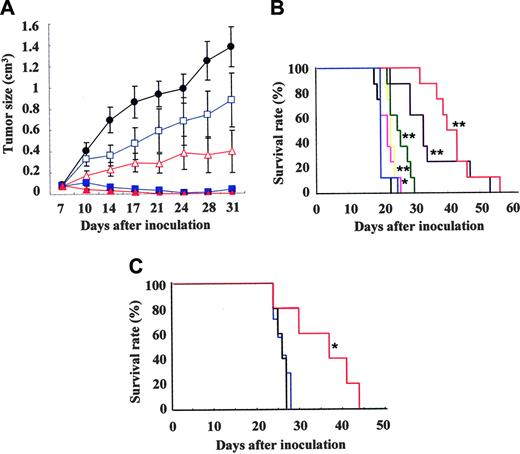
To test the effect of NS-187 on in vivo tumor growth, Balb/c-nu/nu mice were injected subcutaneously with Bcr-Abl–positive KU812 cells on day 0 and given NS-187 or imatinib orally twice a day from day 7 to day 17. At 20 mg/kg/d, imatinib inhibited tumor growth slightly, whereas at 200 mg/kg/d, it inhibited tumor growth almost completely. In contrast, at only 0.2 mg/kg/d NS-187 significantly inhibited tumor growth, whereas at 20 mg/kg/d it completely inhibited tumor growth without any adverse effects (Figure 5A). When mice were treated with 0.2 or 20 mg/kg/d of NS-187, the estimated Cmax was 4 nM or 400 nM, respectively, suggesting that the in vivo effects of NS-187 were comparable to its in vitro effects (Figure 4B). The body weights of the treated tumor-bearing mice were not significantly different from those of untreated mice, even at a dosage of 200 mg/kg/d NS-187 (data not shown). Thus, NS-187 was at least 10-fold more potent than imatinib in vivo with complete inhibition of tumor growth as the end point and at least 100-fold more potent with partial inhibition as the end point. NS-187 was well tolerated by the mice.
We also tested the ability of NS-187 to suppress tumor growth in another murine tumor model, namely, Balb/c-nu/nu mice that received BaF3/wt cells intravenously. The mice were treated orally with NS-187 or imatinib for 11 days starting on day 1. All 7 untreated mice had died by day 23 due to leukemic cell expansion, whereas all mice treated with 400 mg/kg/d imatinib had died by day 25. Thus, imatinib had little effect on tumor growth in this model, whereas NS-187 significantly prolonged the survival of the mice in a dose-dependent manner compared with untreated mice (Figure 5B). We also examined the effect of longer exposure to NS-187 (25 days) in mice inoculated with 5 × 104 instead of 1 × 106 BaF3/wt cells. NS-187 also prolonged the survival of these mice in a dose-dependent manner (data not shown).
Kinase inhibition profile and the docking model. (A-B) Kinase inhibition profile of NS-187 and Abl (panel B is the continuation of panel A). Seventy-nine tyrosine kinases, including 5 Src-family kinases (Blk, Src, Fyn, Lyn, and Yes; indicated by Δ on the x-axis) were assayed by KinaseProfiler. At 0.1 μM, NS-187 inhibited 4 of the 79 tyrosine kinases, namely, Abl, Arg, Fyn, and Lyn (indicated by red on the x-axis). (C) Close view of the docking model of NS-187 and Abl. Hydrogen-bonding interactions are shown as white broken lines. Movement of the P-loop shown in pink and the hydrogen-bonding interactions of the amino acids shown in sky blue contribute to stabilize the complex.
Kinase inhibition profile and the docking model. (A-B) Kinase inhibition profile of NS-187 and Abl (panel B is the continuation of panel A). Seventy-nine tyrosine kinases, including 5 Src-family kinases (Blk, Src, Fyn, Lyn, and Yes; indicated by Δ on the x-axis) were assayed by KinaseProfiler. At 0.1 μM, NS-187 inhibited 4 of the 79 tyrosine kinases, namely, Abl, Arg, Fyn, and Lyn (indicated by red on the x-axis). (C) Close view of the docking model of NS-187 and Abl. Hydrogen-bonding interactions are shown as white broken lines. Movement of the P-loop shown in pink and the hydrogen-bonding interactions of the amino acids shown in sky blue contribute to stabilize the complex.
We next examined the ability of NS-187 to block the in vivo growth of BaF3/E255K cells in Balb/c-nu/nu mice. BaF3/E255K-bearing mice were treated with NS-187 or imatinib for 26 days starting on day 1. The untreated mice and mice treated with 200 mg/kg/d imatinib had all died by day 28 (Figure 5C). In contrast, NS-187 at 120 mg/kg/d significantly prolonged survival compared with untreated mice (Figure 5C). NS-187 was also well tolerated by the mice. Thus, NS-187 was more potent than imatinib and could override the point mutation–based imatinib-resistance mechanism in vivo.
Discussion
In view of the frequent development of imatinib resistance during the treatment of Ph+ leukemia, we sought to develop a novel agent to treat this disease that fulfills the following requirements for a “post-imatinib” drug: (1) it inhibits Bcr-Abl, including variants with point mutations in the kinase domain, more effectively than does imatinib; (2) it has at most mild toxicity; and (3) it simultaneously inhibits another kinase whose activity is associated with imatinib resistance while remaining at least as specific for Bcr-Abl as is imatinib. With the aid of Bcr-Abl–docking modeling we have identified a candidate drug, NS-187, that inhibited the autophosphorylation of wt Bcr-Abl in K562 and 293T/wt cells more potently than did imatinib (Table 1). It also inhibited the phosphorylation of CrkL and ERK in K562 and BaF3/wt cells more strongly than did imatinib (Figure 2A-D; Table 1). Moreover, it suppressed the in vitro growth of Bcr-Abl–positive KU812 and K562 cells at much lower concentrations than did imatinib (Figure 4A-B). Significantly, NS-187 was at least 10-fold more potent than imatinib in mice bearing Bcr-Abl–positive tumors (Figure 5A-B) and had no adverse effects. Furthermore, not only was NS-187 effective against wt Bcr-Abl but it also blocked the activity of Bcr-Abl with point mutations in the kinase domain (Figure 1E, 2E-F; Tables 1, 2). In addition, it inhibited the in vitro growth of cells expressing mutant Bcr-Abl proteins (Figure 4E) and prolonged the survival of mice engrafted with BaF3/E255K cells (Figure 5C). The only point mutant that NS-187 failed to significantly inhibit was the T315I Bcr-Abl mutant. Imatinib failed to inhibit any of the point mutants tested. These findings show that NS-187 fulfills the first 2 requirements of a post-imatinib drug that were listed above.
Effect of NS-187 on in vitro growth of cell lines harboring Bcr-Abl. (A) Bcr-Abl–positive K562 cells, (B) Bcr-Abl–positive KU812 cells, (C) Bcr-Abl–negative U937 cells, (D) BaF3/wt cells, (E) BaF3/E255K cells, and (F) BaF3/T315I cells were treated with serial dilutions of NS-187 (•) or imatinib (○) for 3 days. Cell proliferation was assessed by means of the MTT assay.
Effect of NS-187 on in vitro growth of cell lines harboring Bcr-Abl. (A) Bcr-Abl–positive K562 cells, (B) Bcr-Abl–positive KU812 cells, (C) Bcr-Abl–negative U937 cells, (D) BaF3/wt cells, (E) BaF3/E255K cells, and (F) BaF3/T315I cells were treated with serial dilutions of NS-187 (•) or imatinib (○) for 3 days. Cell proliferation was assessed by means of the MTT assay.
Effect of NS-187 on in vivo tumor growth. (A) Balb/c-nu/nu mice received subcutaneous transplants of KU812 cells (day 0) and were given vehicle (•), NS-187 (▵, 0.2 mg/kg/d; ▴, 20 mg/kg/d), or imatinib (□, 20 mg/kg/d; ▪, 200 mg/kg/d) daily from day 7 to day 17. Data points represent the mean ± standard deviation calculated for 7 mice. Effect of NS-187 in BaF3-leukemia model. (B) Survival of Balb/c-nu/nu mice that received transplants of BaF3/wt cells and given vehicle (black), NS-187 (pink, 6 mg/kg/d; yellow, 20 mg/kg/d; green, 60 mg/kg/d; purple, 120 mg/kg/d; red, 200 mg/kg/d), or imatinib (blue; 400 mg/kg/d) daily from day 1 to day 11. (C) Survival of Balb/c-nu/nu mice that received transplants of BaF3/E255K cells and given vehicle (black), NS-187 (red; 120 mg/kg/d), or imatinib (blue; 200 mg/kg/d) daily from day 1 to day 26. *P < .05; **P < .01.
Effect of NS-187 on in vivo tumor growth. (A) Balb/c-nu/nu mice received subcutaneous transplants of KU812 cells (day 0) and were given vehicle (•), NS-187 (▵, 0.2 mg/kg/d; ▴, 20 mg/kg/d), or imatinib (□, 20 mg/kg/d; ▪, 200 mg/kg/d) daily from day 7 to day 17. Data points represent the mean ± standard deviation calculated for 7 mice. Effect of NS-187 in BaF3-leukemia model. (B) Survival of Balb/c-nu/nu mice that received transplants of BaF3/wt cells and given vehicle (black), NS-187 (pink, 6 mg/kg/d; yellow, 20 mg/kg/d; green, 60 mg/kg/d; purple, 120 mg/kg/d; red, 200 mg/kg/d), or imatinib (blue; 400 mg/kg/d) daily from day 1 to day 11. (C) Survival of Balb/c-nu/nu mice that received transplants of BaF3/E255K cells and given vehicle (black), NS-187 (red; 120 mg/kg/d), or imatinib (blue; 200 mg/kg/d) daily from day 1 to day 26. *P < .05; **P < .01.
Other post-imatinib agents such as PD166326,14 SKI-606,15 AP23464,16 and BMS-35482517,18 were developed from Src inhibitors on the basis of the similarity between Abl and the Src-family proteins.32,33 It has been shown that the simultaneous inhibition of any 2 of the Ras, signal transducers and activators of transcription 5 (STAT5), and phosphatidylinositol 3–kinase (PI3K) pathways leads to a synergistic enhancement of apoptosis in CML cells.34-36 At present, research aimed at the development of effective therapies for Ph+ leukemias is focused not only on these signaling pathways but also on Lyn kinase, since acquired imatinib resistance may be mediated in part through the overexpression of Lyn.19,20 Moreover, short interfering RNA (siRNA) targeting Lyn induces apoptosis in imatinib-resistant Ph+ leukemia cells.21 Imatinib minimally inhibits Lyn (IC50, 352 nM). However, the dual Src-Abl inhibitor BMS-354825 significantly suppresses Lyn phosphorylation, and this is consistent with its greater antitumor activity in CML samples.18 These observations suggest that the ability of an agent to act simultaneously against Lyn and Abl will be advantageous in Ph+ leukemia therapy. However, it may be important for the activity of such a therapeutic agent to be limited to Abl and Lyn to avoid the possibility of adverse effects. It is well known that imatinib inhibits not only Abl but also PDGFR and c-Kit, and this may be responsible for adverse effects such as bone marrow suppression, periorbital edema, and hypopigmentation.37 In addition, Src proteins are known to play important roles in osteoclastogenesis,22 the regulation of Ca2+ signaling,23 the modulation of vascular adaptations,24 and signaling through a variety of cell surface receptors.25 For example, the vascular permeability activity of vascular endothelial growth factor (VEGF) specifically depends on the Src-family proteins Src or Yes.26 These observations suggest that inhibition of PDGFR, c-Kit, or Src-family proteins could cause unpredictable adverse effects. We set out to discover a compound that would inhibit Lyn and Abl while inhibiting as few other kinases as possible. Although another novel Abl-specific inhibitor, AMN107, which like NS-187 is a 2-phenylaminopyrimidine–class compound, does not inhibit Lyn,38,39 we found that NS-187 inhibited Lyn in addition to Fyn but did not inhibit PDGFR, Src, Blk, or Yes at 0.1 μM, a concentration that adequately inhibits Abl kinase activity (Figure 3A-B).
To investigate why NS-187 inhibited Lyn without simultaneously inhibiting Src, docking studies were performed using the cocrystal structure of imatinib bound to Abl.28 Our proposed docking model of the NS-187/Abl complex is shown in Figure 3C. The cocrystal structure reveals that the P-loop of Abl moves toward imatinib upon binding of imatinib to the protein.28 This induced fit contributes to increased van der Waals interactions and stabilization of the inactive conformation of the complex. Such an induced fit is also likely to occur with NS-187 due to its structural similarity to imatinib. Moreover, as with imatinib, the NS-187/Abl complex is likely to be stabilized by the hydrogen-bonding interaction between the OH of Tyr253 and the side-chain NH2 of Asn322 as well as the water-mediated hydrogen-bonding interaction between the side-chain CO of Gln252 and the side-chain carboxylate of Asp325 (Figure 3C). As to the variable binding of NS-187 to different Src-family proteins, it seems to be significant that while the amino acids at positions 253 and 325 are always Phe and Asp, respectively, the amino acid at position 252 is either Gln or Cys (Table 3). NS-187 inhibited the Gln252-bearing proteins Abl, Fyn, and Lyn but had lower activity against the Cys252-bearing Src and Yes. This is probably because Gln, unlike Cys, readily forms hydrogen bonds.40 As a result, the stabilization of the NS-187/Src complex by the induced movement of the P-loop is weaker when Cys252 is present than when Gln252 is present. Thus, induced fit of NS-187 to the kinase is likely to be important for its ability to block the activity of the kinase. Our docking models support the notion that NS-187 is more specific for Lyn than for Src.
NS-187 is therefore an excellent candidate for an antileukemia agent. However, NS-187 is limited in one respect; that is, it cannot inhibit the phosphorylation of T315I Bcr-Abl. To our knowledge, an ATP-competitive inhibitor that can inhibit the phosphorylation of T315I Bcr-Abl has not yet been developed. This is probably because the T315I mutation eliminates a crucial hydrogen bond and sterically blocks the imatinib-binding site. According to our docking model, there is little space around T315, suggesting that it would be difficult for an ATP-competitive inhibitor of Bcr-Abl to inhibit the T315I mutant (data not shown). Only ON012380, a non–ATP-competitive inhibitor of Bcr-Abl, is effective against Bcr-Abl/T315I.41 In addition, such non–ATP-competitive inhibitors may be less specific for Abl than are ATP-competitive inhibitors like NS-187. A compound that specifically inhibits Bcr-Abl/T315I is desirable even if it is difficult to develop.
In conclusion, NS-187 more potently inhibits Abl kinase activity than does imatinib, and its inhibitory activity is less affected by point mutations in the Abl kinase domain. Moreover, NS-187 also inhibits Lyn while otherwise maintaining a high specificity for Bcr-Abl. The efficacy and safety of NS-187 for Ph+ leukemias is expected to be verified by early-phase clinical trials.
Prepublished online as Blood First Edition Paper, August 16, 2005; DOI 10.1182/blood-2005-06-2209.
Supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan; the Public Trust; the Uehara Memorial Foundation; and the COE program of the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Several of the authors (H. Naito, Y.N., H. Naruoka, T.W., K.N., T.A., T.N., K.H.) are employed by a company whose potential product was studied in the present work.
S.K. designed research, performed research, and wrote the paper; H. Naito and T.Y. performed research and analyzed data; H.S., J.K., K.S., A.Y., Y.K., E.K., E.A., Y.N., and H. Naruoka performed research; T.W., K.N., and T.A. contributed a vital new reagent; T.N. designed research and computer science; and K.H. and T.M. designed research and wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Gerald E. Smyth for advice on the preparation of this manuscript.