T cells directed against hematopoietic-restricted minor histocompatibility antigens (mHags) may mediate graft-versus-leukemia (GVL) reactivity without graft-versus-host disease (GVHD). Recently, the HLA-A24–restricted mHag ACC-1 and the HLA-B44–restricted mHag ACC-2 encoded by separate polymorphisms within the BCL2A1 gene were characterized. Hematopoietic-restricted expression was suggested for these mHags. We demonstrate BCL2-related protein A1 (BCL2A1) mRNA expression in mesenchymal stromal cells (MSCs) that was up-regulated by the inflammatory cytokines tumor necrosis factor α (TNF-α) and/or interferon γ (IFN-γ). Analysis of cytotoxicity and IFN-γ production illustrated that ACC-2–specific T cells did not recognize untreated MSCs or IFN-γ–treated MSCs but showed specific recognition and killing of MSCs treated with TNF-α plus IFN-γ. We hypothesize that under steady-state circumstances BCL2A1-specific T cells may exhibit relative specificity for hematopoietic tissue, but reactivity against nonhematopoietic cells may occur when inflammatory infiltrates are present. Thus, the role of BCL2A1-specific T cells in differential induction of GVL reactivity and GVHD may depend on the presence of inflammatory responses that may occur during GVHD.
Introduction
Allogeneic stem cell transplantation (SCT) and donor lymphocyte infusion (DLI) are effective treatments for patients with hematologic malignancies,1-4 but can be complicated by graft-versus-host-disease (GVHD).5,6 Following HLA-identical SCT both the graft-versus-leukemia (GVL) effect and GVHD can be mediated by donor-derived T cells recognizing polymorphic minor histocompatibility antigens (mHags) on recipient cells. Immunotherapy directed against mHags with hematopoietic tissue-restricted expression may eradicate the leukemic cells without inducing GVHD.7
Recently, the HLA-A24–restricted mHag ACC-1 and the HLA-B44–restricted mHag ACC-2, encoded by separate polymorphisms within the BCL2A1 gene, were identified, and hematopoietic-restricted expression was suggested for these mHags.8 However, in a retrospective analysis, GVHD relapse and disease-free survival were not statistically different between patients receiving ACC-1–compatible or –incompatible transplants.9 Previous studies have indicated expression of the human BCL2A1 gene not only in hematopoietic cells, but also in nonhematopoietic tissues, including lung, small intestine, and testis, and expression could be induced in endothelial cells by the inflammatory cytokines tumor necrosis factor α (TNF-α) and interleukin 1β (IL-1β).10-12
Induction of BCL2A1 expression in nonhematopoietic tissues by inflammatory cytokines may have important consequences for the role of T cells recognizing ACC-1 and ACC-2 in differential induction of GVL and GVHD. Local inflammatory cytokine production can be triggered by T cells recognizing host antigen-presenting cells (APCs) in nonhematopoietic tissues.13-15
In this study, we characterized the recognition pattern of mHag ACC-2–specific T cells isolated from a patient during a combined GVHD and GVL response after DLI. We show that ACC-2–specific T cells recognized not only leukemic cells but also nonhematopoietic cells under inflammatory conditions and, therefore, could play a role in the etiology of GVHD.
Study design
Isolation of T-cell clones and cell culture
Cytotoxic T-lymphocyte (CTL) clones were isolated from an HLA-A2 A3 B7 B44+ male patient with relapsed chronic myeloid leukemia (CML) during a clinical response to DLI from his HLA-identical female donor.16
Epstein-Barr virus (EBV)–transformed B-cell lines (EBV-LCLs) and phytohemagglutinin (PHA)–activated T cells (PHA blasts) were generated as described.17 Mesenchymal stromal cells (MSCs) were derived from bone marrow of healthy donors or CML patients as previously described.18 To induce gene expression in response to inflammatory cytokines, MSCs were cultured for 48 hours with 10 ng/mL TNF-α and/or 100 IU/mL IFN-γ (Boehringer Ingelheim, Ingelheim, Germany). Prior to use as targets or stimulators, cells were thoroughly washed to remove all added cytokines. Colon tumor cell line CCL-228 was obtained from the American Type Culture Collection (Rockville, MD). The primary airway smooth muscle (ASM) cells treated with or without 20 ng/mL TNF-α, IFN-γ, and IL-1β (each cytokine 20 ng/mL) were generated by Sylvia Lazeroms (Department of Pulmonary Disease, Leiden University Medical Center [LUMC], Leiden, The Netherlands). Approval was obtained from the LUMC Institutional Review Board for these studies. Informed consent was provided according to the Declaration of Helsinki.
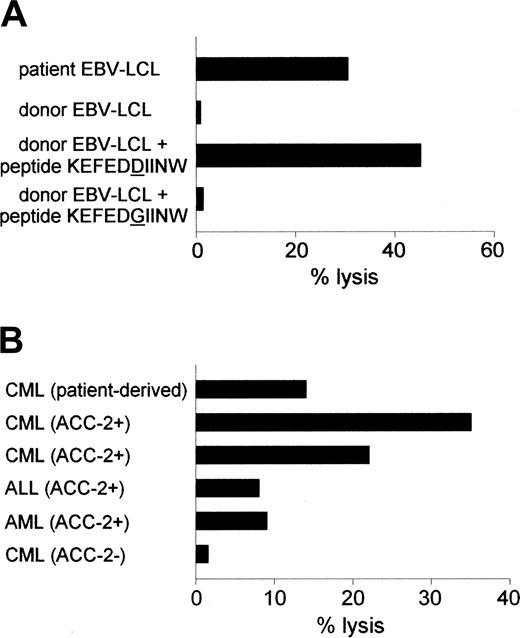
Reactivity of the ACC-2–specific CTL clone against hematopoietic cells. (A) Cytolytic activity of CTL clone 65 against patient-derived EBV-LCLs and donor-derived EBV-LCLs loaded with ACC-2 peptide KEFEDDIINW but not against donor-derived EBV-LCLs loaded with ACC-2 control peptide KEFEDGIINW. (B) Lysis of patient-derived CML cells and leukemic cells from unrelated HLA-B44+, mHag ACC-2+ individuals but not of leukemic cells from an unrelated HLA-B44+, mHag ACC-2- individual by CTL clone 65. Cytotoxicity was measured at an E/T ratio of 10:1.
Reactivity of the ACC-2–specific CTL clone against hematopoietic cells. (A) Cytolytic activity of CTL clone 65 against patient-derived EBV-LCLs and donor-derived EBV-LCLs loaded with ACC-2 peptide KEFEDDIINW but not against donor-derived EBV-LCLs loaded with ACC-2 control peptide KEFEDGIINW. (B) Lysis of patient-derived CML cells and leukemic cells from unrelated HLA-B44+, mHag ACC-2+ individuals but not of leukemic cells from an unrelated HLA-B44+, mHag ACC-2- individual by CTL clone 65. Cytotoxicity was measured at an E/T ratio of 10:1.
Genotyping of BCL2A1 polymorphisms and quantitative real-time RT-PCR
RNA isolation and cDNA synthesis were performed as described.19 A 311-bp amplication product containing all known BCL2A1 polymorphisms was generated using forward primer 5′-ttggatatatttacaggctggctca-3′ and reverse primer 5′-gggcaatttgctgtcgtagaag-3′. Polymerase chain reaction (PCR) products were sequenced as described.20
Quantitative real-time reverse transcriptase (RT)–PCR for BCL2A1 was performed as described21 using primers 5′-gtcccgtagacactgccagaacact-3′ and 5′-ccattttcccagcctccgt-3′ and probe 5′-TET-ttctacgacagcaaattgccccgg-TAMRA-3′.
Analysis of cytotoxicity and IFN-γ production
To determine cytotoxicity, 51Cr-release assays were performed.22 As effector cells, CTL clone 65 and the HLA-A2–specific control CTL clone MBM13 were used. To analyze cytotoxicity against ACC-2 peptide KEFEDDIINW or ACC-2 control peptide KEFEDGIINW, donor-derived EBV-LCLs were loaded with 1 μg/mL peptide during 51Cr-labeling. IFN-γ production was measured as described.16
Results and discussion
Recently, we isolated mHag-specific CTL clones from a male patient with relapsed CML during the clinical response to DLI from his HLA-identical female donor.16,23 The DLI induced a complete remission but was complicated by GVHD resulting in organ damage. Not only CTL clones specific for the mHags HA-1 and HY-B7 were isolated. CTL clone 65 appeared to be specific for ACC-2 because it lysed donor-derived EBV-LCLs labeled with ACC-2 peptide KEFEDDIINW (Figure 1A).
The ACC-2–specific T cells lysed patient-derived EBV-LCLs and CML cells and leukemic cells from unrelated HLA-B44+ mHag ACC-2+ individuals but not HLA-B44+ mHag ACC-2- leukemic cells (Figure 1). These data are in line with the reactivity reported by Akatsuka et al.8
To investigate potential cytolytic activity against nonhematopoietic cells, first B-cell lymphoma 2 (BCL2)–related protein A1 (BCL2A1) mRNA expression levels in nonhematopoietic and hematopoietic cells were measured by quantitative real-time RT-PCR. A screen of commercially available tissue samples indicated BCL2A1 expression in normal and leukemic hematopoietic cells, lung, and heart (data not shown). We analyzed in more detail hematopoietic cells, ASM cells, and MSCs. High BCL2A1 mRNA expression was found in patient-derived EBV-LCLs and PHA blasts. In primary ASM cells expression was 15 to 40 times lower but treatment with TNF-α plus IFN-γ plus IL-1β resulted in expression levels even exceeding those of EBV-LCLs. MSCs showed a 300- to 1000-fold lower expression as compared to EBV-LCLs, but expression was 5-fold up-regulated in MSCs treated with IFN-γ or TNF-α plus IFN-γ.
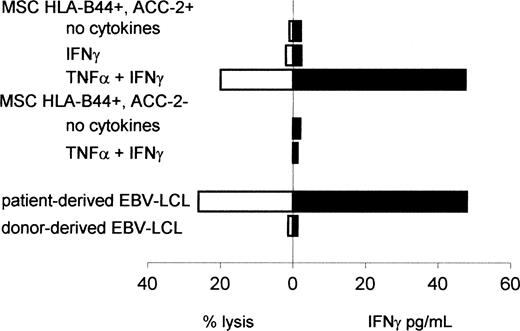
To determine whether this up-regulation of BCL2A1 mRNA expression in MSCs was sufficient for recognition of these nonhematopoietic cells, we measured lysis of untreated or inflammatory cytokine-treated MSCs and IFN-γ production by the ACC-2–specific CTL clone (Figure 2). Similar to the results from Akatsuka et al,8 the ACC-2–specific CTL clone 65 did not recognize untreated or IFN-γ–treated HLA-B44+, mHag ACC-2+ MSCs. However, HLA-B44+, mHag ACC-2+ MSCs treated with TNF-α plus IFN-γ were lysed by the ACC-2–specific CTL clone, which also produced IFN-γ at levels comparable to those after stimulation with patient-derived EBV-LCLs. Nonspecific reactivity was excluded because ACC-2–specific CTLs showed no IFN-γ production in response to untreated or cytokine-treated HLA-B44+ ACC-2- MSCs. Untreated or cytokine-treated MSCs were not lysed and did not induce IFN-γ production by nonreactive control clones (data not shown). To determine whether the ACC-2–specific T cells may have contributed to the GVHD observed in the patient after DLI, we also analyzed the reactivity against patient-derived MSCs. We found no recognition of untreated or IFN-γ–treated MSCs but low lysis of MSCs treated with TNF-α plus IFN-γ (14% at an effector-target [E/T] ratio of 10:1).
In this report, we demonstrate that in nonhematopoietic tissue under steady-state circumstances expression of BCL2A1 is relatively low but can be up-regulated during inflammation. Although we could not determine whether the ACC-2–specific T cells were involved in the GVHD in the patient studied, which may also have been due to the presence of several other mHag-specific T cells including male-specific T cells, we hypothesize that the concomitant disease state of a patient poses a risk factor for GVHD and may therefore determine whether BCL2A1–specific T cells exclusively exhibit a GVL effect or also contribute to GVHD. Especially the presence of inflammatory cells such as monocytes or dendritic cells in nonhematopoietic tissues may pose a risk factor for inducing or enhancing GVHD. Recognition of the inflammatory cells by donor-derived T cells may provide the necessary cytokines leading to up-regulated expression of the BCL2A1-derived mHags in damaged tissue.13-15 The subsequent recognition by ACC-1– or ACC-2–specific T cells may then enhance GVHD. Accordingly, the patient from whom we isolated the ACC-2–specific CTLs suffered from skin and pulmonary GVHD. A recent report24 on patients treated with mHag-specific T-cell clones may also be indicative of inflammatory cytokine-induced expression in nonhematopoietic tissue of mHags that under steady-state circumstances are preferentially expressed in hematopoietic tissue. In one of the patients treated for relapsed leukemia with donor T cells recognizing a mHag preferentially expressed in hematopoietic cells, pulmonary toxicity developed, which was attributed to recognition of the mHag in the lung microenvironment.24
Reactivity of the ACC-2–specific CTL clone against nonhematopoietic cells. Cytolytic activity or IFN-γ production by CTL clone 65 against MSCs treated with or without TNF-α and/or IFN-γ for 48 hours prior to labeling with 51Cr. MSCs were derived from an unrelated HLA-A2-HLA-B44+ACC-2+ individual or an HLA-A2-HLA-B44+ACC-2- individual. Cytotoxicity was measured at an E/T ratio of 10:1. IFN-γ production was measured by determining the concentration of IFN-γ in the supernatant after 24 hours of coculture.
Reactivity of the ACC-2–specific CTL clone against nonhematopoietic cells. Cytolytic activity or IFN-γ production by CTL clone 65 against MSCs treated with or without TNF-α and/or IFN-γ for 48 hours prior to labeling with 51Cr. MSCs were derived from an unrelated HLA-A2-HLA-B44+ACC-2+ individual or an HLA-A2-HLA-B44+ACC-2- individual. Cytotoxicity was measured at an E/T ratio of 10:1. IFN-γ production was measured by determining the concentration of IFN-γ in the supernatant after 24 hours of coculture.
Our study does not necessarily exclude the use of BCL2A1-specific T cells in adoptive immunotherapy to eliminate leukemic cells. In the absence of inflammatory cells in nonhematopoietic tissues, ACC-1 or ACC-2 CTLs may act as hematopoietic-specific T cells eradicating leukemia. However, when patients suffer from signs of GVHD the production of inflammatory cytokines may be triggered, resulting in the up-regulated expression of BCL2A1 in target tissues of GVHD.
Prepublished online as Blood First Edition Paper, August 11, 2005; DOI 10.1182/blood-2004-09-3749.
F.M.K. designed the research, performed research, analyzed data, and wrote the paper; S.A.P.v.L.-H., R.A.v.S., and H.M.E.v.E. performed research and analyzed data; R.W. designed research and wrote the paper; and J.H.F.F. designed research, analyzed data, and wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal