Abstract
Anti–β2–glycoprotein I antibodies are known to have a heterogeneous reactivity against β2–glycoprotein I. We performed this study to characterize the epitope on β2–glycoprotein I to which pathologic anti–β2–glycoprotein I antibodies are directed. Plasma samples from 198 patients with various systemic autoimmune diseases were tested for the presence of lupus anticoagulant and anti–β2–glycoprotein I immunoglobulin G (IgG) antibodies. The reactivity of the anti–β2–glycoprotein I–positive samples was further tested by coating recombinant full-length β2–glycoprotein I and 8 deletion mutants of β2–glycoprotein I onto hydrophilic and hydrophobic enzyme-linked immunosorbent assay (ELISA) plates. Full-length β2–glycoprotein I with point mutations in domain I at positions 8, 40, and 43 were used in inhibition experiments. Fifty-two patients with anti–β2–glycoprotein I IgG antibodies could be divided into 2 patterns. Type A antibodies only recognize domain I when coated onto hydrophobic plates; they do not recognize domain I coated onto hydrophilic plates. Type B antibodies have heterogeneous reactivity for all domains. Type A antibodies recognize the epitope around amino acids Gly40-Arg43 and cause lupus anticoagulant activity. In contrast to type B antibodies, those of type A strongly correlated with thrombosis. In conclusion, antibodies directed at domain I (epitope comprising Gly40 and Arg43) have lupus anticoagulant activity and strongly associate with thrombosis.
Introduction
The antiphospholipid syndrome (APS) is an autoimmune disease characterized by the presence of circulating antiphospholipid antibodies (aPLs), notably anticardiolipin antibodies (aCLs) and antibodies that prolong phospholipid coagulation tests (lupus anticoagulant [LAC]) in patients with thrombosis and/or well-defined pregnancy morbidity.1-3 Initially it was thought that aPLs bind directly to negatively charged phospholipids. However, it is now generally accepted that the clinically relevant aPLs bind to proteins with affinity for phospholipids.4-7 Probably the most important epitope of APS-related aPL resides on β2–glycoprotein I (β2GPI).4 Most authorities do agree that anti-β2GPI antibodies of the immunoglobulin G (IgG) class are more related to clinical symptoms like thrombosis than IgM-class antibodies.
β2GPI is a 50-kDa glycoprotein that is present in plasma at a concentration of approximately 200 μg/mL. The protein is a member of the complement control proteins (CCPs).8 β2GPI consists of 5 CCP repeats in which the first 4 domains are regular repeats consisting of 60 amino acids. The fifth domain is different from the other 4 domains as it consists of 82 amino acids, 4 conserved hydrophobic amino acids (313-316), 3 disulfide bonds instead of 2, and a cluster of positively charged amino acids (282-287), which is responsible for binding of β2GPI to phospholipids.9-12
To understand the pathophysiology of APS, it is necessary to know the domains involved in anti-β2GPI antibody binding and to know whether pathologic antibodies are all directed toward the same epitope. There is substantial controversy on the specificity of anti-β2GPI antibodies. Some groups claim that the epitope for binding of anti-β2GPI antibodies is located on domain IV.13,14 Other studies showed that binding of anti-β2GPI antibodies to β2GPI was abrogated when there was a clip at 316-317 in domain V, suggesting that domain V is involved.15 Arvieux et al16 found that anti-β2GPI antibodies inhibited the binding of β2GPI to cardiolipin, suggesting again that domain V harbors the epitope for binding of anti-β2GPI antibodies. Blank et al17 found that anti-β2GPI antibodies react with peptides that cover sequences present in domains I, II, III, and IV. Most of these groups used murine monoclonal antibodies or a restricted number of affinity-purified patient anti-β2GPI antibodies for their studies. Interestingly, studies using only anti-β2GPI antibodies purified from patients concluded that domain I is the major domain of β2GPI involved in the binding of anti-β2GPI antibodies.18-20
Recently it was suggested that the positively charged epitope R39-R43 on domain I is important in binding of anti-β2GPI antibodies.21 Here we further investigate that suggestion by testing the specificity of anti-β2GPI antibodies in patients with systemic lupus erythematosus, lupuslike disease, and primary antiphospholipid syndrome by using deletion mutants of β2GPI, recombinant full-length β2GPI, point-mutated recombinant full-length β2GPI, and both hydrophilic and hydrophobic enzyme-linked immunosorbent assay (ELISA) plates. Findings were related to the presence or absence of a history of thrombosis and anti-β2GPI–dependent LAC activity.
Patients, materials, and methods
Patients
Included in this study were 198 patients with a diagnosis of systemic lupus erythematosus (SLE; n = 176), lupuslike disease (LLD; n = 16), and primary antiphospholipid syndrome (PAPS; n = 6) that were seen consecutively at the lupus clinic of the University Medical Center, Utrecht, the Netherlands. Patients with SLE meet at least 4 American College of Rheumatology (ACR) criteria for the classification of SLE, and patients with LLD meet at least 1 to 3 of these criteria. Patients with PAPS have aPLs and a history of thrombosis in absence of other signs for a systemic autoimmune disease. By chart review, the number of objectively verified thromboembolic events was recorded for each patient. Computed tomographic scanning or magnetic resonance imaging were used to diagnose a patient having thrombosis of intracerebral vessels. Typical electrocardiographic features and an elevated-fraction creatine kinase muscle and brain (MB) diagnosed myocardial infarction. Peripheral arterial thrombosis and thrombosis of the distal aorta was diagnosed by arteriography or thrombectomy at surgery. Retinal thrombosis was documented by fundoscopy and fluorescence angiography. Deep vein thrombosis was diagnosed by ultrasonography or venography, pulmonary embolism by radionuclide lung scanning, and portal vein thrombosis by angiography. The Institutional Review Board of the University Medical Center Utrecht approved this study, and informed consent was obtained from all patients.
Blood samples
Blood samples were taken at an arbitrary visit of the patients to the outpatient department and were collected by venipuncture using plastic tubes containing 3.8% trisodium citrate (0.129 M) as the anticoagulant (9:1; vol/vol). To obtain platelet-poor plasma, the samples were centrifuged twice at 2000g for 10 minutes and subsequently stored at –50°C until use.
Serologic assays to measure antiphospholipid antibodies
All patient samples were measured with 2 LAC assays: an activated partial thromboplastin time (APTT)–like assay (PTT-LA) and a dilute Russell's Viper Venom time (dRVVT). The APTT (PTT-LA; Diagnostica Stago, Gennevilliers, France) was performed as follows: 50 μL patient plasma was diluted 1:1 with normal pool plasma of 40 healthy volunteers and incubated with 50 μL APTT reagent (Diagnostica Stago). Coagulation was initiated by the addition of 50 μL CaCl2 (25 mM). As a control, samples were tested in an APTT with actin-FS (Dade-Behring, Marburg, Germany) as activator, a LAC-insensitive assay.22 Patients were considered positive when the ratioAPTT-LA/APTT-FS was greater than 1.20. The dRVVT was performed according to the instructions of the manufacturer (Gradipore, North Ryde, Australia) and considered positive when LAC screen/LAC confirm was greater than 1.20. A patient was considered LAC positive if 1 of the 2 LAC assays was positive.
Anticardiolipin antibodies were measured in an ELISA as described before.23 Nine IgG/IgM calibrators were used to report aCL levels as GPL or MPL units. Levels above 10 GPL or GPM units were considered positive.
IgG-class antibody binding to β2GPI and β2GPI mutants
Anti–β2GPI IgG antibody ELISA. IgG-class antibodies against β2GPI were measured in an ELISA.24 In short, plasma-purified β2GPI (10 μg/mL in Tris-based solution [TBS]: 50 mM Tris in 100 mM NaCl) was coated onto in a high-binding ELISA plate (9102; Costar, New York, NY) by incubation for 1 hour. Then the plates were washed with 0.1% Tween in TBS (washing solution) and incubated with 4% bovine serum albumin (BSA) in TBS for 1 hour (blocking buffer) to prevent aspecific binding. The plates were washed again, followed by incubation with patient plasma (1:50 diluted in blocking buffer) for 1 hour. Then the plates were washed and incubated with a goat antihuman alkaline phosphatase–labeled antibody (diluted 1:1000; Biosource, Camarillo, CA) followed by staining with para-nitrophenyl phosphatase (PnPP; 0.4 mg/mL). A sample was considered positive if the optical density (OD) was greater than 3SD above the mean OD obtained with plasma from 40 healthy volunteers.
Mutants of β2GPI. By a generous gift of Dr Iverson of La Jolla Pharmaceutical Company, we obtained recombinant full-length β2GPI (DI-V); 8 deletion mutants of β2GPI (comprising domain I [DI]; domains I and II [DI-II]; domains I, II, and III [DI-III]; domains I, II, III, and IV [DI-IV]; domains II, III, IV, and V [DII-V]; domains III, IV, and V [III-V]; domains IV and V [DIV-V]; and domain V [DV]); and 3 recombinant β2GPI molecules with different point mutations in domain I (aspartate-alanine mutation at position 8 [D8A], glycine-glutamate mutation at position 40 [G40E], and an arginine-glycine mutation at position 43 [R43G]).21
Anti–β2GPI IgG ELISA with β2GPI deletion mutants. To determine the IgG reactivity against β2GPI deletion mutants we used both 96-well hydrophilic high-binding ELISA plates (9102; Costar) and hydrophobic ELISA plates (2595; Costar). The plates were coated with deletion mutants in a concentration (10 μg/mL) that allows the use of the maximum binding capacity of the wells (1 hour at 37°C). The plates were washed 4 times with TBS/0.1% Tween and subsequently blocked with a 4% BSA/TBS solution. Patient plasma (diluted 1:50 in blocking solution) was added to the wells (50 μL/well) and incubated for 1 hour at 37°C. The plates were washed 4 times (0.1% Tween/TBS) and incubated with alkalic-phosphatase–labeled goat antihuman IgG antibodies (Abs; diluted 1:1000; Biosource) for 1 hour at 37°C. Staining was performed by using PnPP (Sigma, St Louis, MO) at a concentration of 0.6 mg/mL diluted in diethynolamine (DEA) buffer. Adding 2.4 M/L NaOH to the wells after 10 minutes at room temperature stopped the reaction. Absorption was measured at 405 nm. Extinctions obtained with normal pooled plasma were subtracted from those with patient plasma.
Inhibition experiments with point-mutated β2GPI coupled to Sepharose beads. Recombinant β2GPI and 3 different point-mutated forms of β2GPI were covalently coupled to chelating Sepharose beads by the following procedure. Chelating Sepharose beads (Pharmacia, Uppsala, Sweden) were centrifuged for 15 seconds at 10 000g followed by removal of ethanol and washing of the beads with 4 volumes 50 mM EDTA (ethylenediaminetetraacetic acid)/H2O. All washing procedures were done at room temperature by spinning down the beads at 57g. Subsequently beads were washed with 4 volumes H2O, 4 volumes 50 mM CoCl2/HO2, 4 volumes H2O, and 4 volumes PBS, followed by incubation for 15 minutes with point-mutated β2GPI or full-length recombinant β2GPI (2 volumes, 20 μM/mL) at room temperature during rotation. Then the beads were washed (with 6 volumes PBS) and incubated with 2 volumes 0.03% H2O2/PBS for 2 hours at room temperature during rotation, followed by washing with 4 volumes PBS, 1 volume 50 mM EDTA, 1 volume PBS, 1 volume 0.5 M imidazole/PBS, and 1 volume PBS. Blocking of aspecific binding places was done by incubation of beads with 4% BSA/PBS for 1 hour at room temperature during rotation.
Purified patient IgG was incubated for 1 hour at room temperature during rotation with Sepharose beads alone and Sepharose beads to which recombinant β2GPI or mutated β2GPI–Sepharose beads was coupled. Then the mixture was centrifuged for 1 minute at 57g. The supernatant was added to ELISA plates (Costar; high absorbing plates) coated with full-length recombinant β2GPI and incubated for 1 hour at 37°C. After washing with 0.1% Tween-20/TBS five times, the plates were incubated with 50 μL/well alkalic-phosphatase–labeled goat antihuman IgG diluted 1:1000 in 4% BSA/PBS for 1 hour at 37°C. Then plates were washed 5 times with 0.1% Tween/TBS, and color development was performed by adding 100 μL/well PNPP at a concentration of 6 mg/10 mL DEA buffer. After 20 minutes, addition of 50 μL/well 2.4 M/L NaOH stopped the coloring reaction. Absorption was measured at 405 nm. From extinctions with purified patient IgGs, extinctions obtained with normal pool IgG were subtracted.
Anti-β2GPI–specific LAC assay
To discriminate between a β2GPI-dependent LAC and a β2GPI-independent LAC we made use of an aPTT-based clotting test (PTT-LA; Diagnostica Stago), as described before.25 In short, 25 μL PTT-LA reagent, 50 μL patient plasma that was mixed 1:1 with normal pool plasma (of 40 healthy volunteers), and 25 μL of different concentrations (0, 25, 50, 100, 200 μM) of cardiolipin vesicles diluted in TBS were mixed and added to a KC-10 microcoagulometer (Amelung, Lemgo, Germany). The cardiolipin vesicles were made as described before.26 After 3 minutes of incubation at 37°C, coagulation was initiated by the addition of 50 μL CaCl2 and clotting time was measured. A LAC was considered β2GPI dependent when the ratio of coagulation times of patient plasma and normal pool plasma was decreased by at least 0.05 with addition of cardiolipin vesicles at a concentration of 25 μM cardiolipin vesicles.22 An example of a patient with a β2GPI-dependent LAC and a patient with a β2GPI-independent LAC is given in Table 1.
LAC assay with point-mutated β2GPI
First, β2GPI-deficient plasma was obtained by adding normal pool plasma (mixture of 40 healthy volunteers) to a Sepharose column coupled with 21B2 (a monoclonal murine anti-β2GPI antibody, generous gift of Prof J. Arnout). After this procedure, β2GPI could not be demonstrated anymore by chromatography. The β2GPI-deficient plasma was reconstituted with either recombinant β2GPI or mutated β2GPI (R43G). Of this mixture, 75 μL was added to the KC-10 (Amelung) and incubated at 37°C. After 3 minutes of incubation, a dRVVT solution (Gradipore, Frenchs Forrest, Australia) was added and the coagulation time was measured.
Statistical analysis
Chi-square statistics was used to compare the prevalence of thrombosis with serologic findings. Odds ratios and 95% confidence intervals were calculated by binary logistic regression. Student t test or Mann-Whitney test was used to calculate differences between 2 groups. For these calculations we used SPSS (Chicago, IL). P values less than .05 were considered significant.
Results
Patient population
The median age of the 198 patients (180 female) was 33 years. In 60 (30%) of 198 patients there was a history of objectively verified thrombosis. Thirty-two patients had a history of arterial thrombosis (stroke, n = 19; myocardial infarction, n = 5; peripheral artery, n = 7; distal aorta, n = 2; retinal artery, n = 3) and 38 patients had a history of venous thrombosis (deep venous thrombosis, n = 30; pulmonary embolism, n = 19; thrombophlebitis, n = 7; portal vein thrombosis, n = 1). Anticardiolipin antibodies were present in 112 (57%) of 198 patients, LAC in 63 (32%) of 198 patients, and anti–β2GPI IgG antibodies in 52 (26%) of 198 patients (Table 2). Table 3 shows the interassay variability as an indication for the quality of the assays used in this study.
Domain specificity of anti–β2GPI IgG antibodies
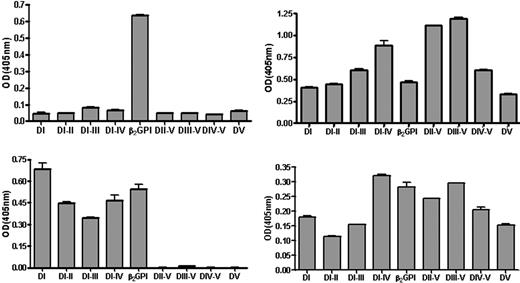
To investigate the domain specificity of the anti–β2GPI IgG antibodies that was found in 52 patients, separate wells of hydrophilic and hydrophobic ELISA plates were coated with 8 domain-deleted mutants of β2GP1 and full-length recombinant β2GP1. It appeared that the 52 samples with anti–β2GPI IgG antibodies could be divided into 2 types (designated A and B) based on the reactivity that these had (Figure 1). On a hydrophilic high-binding plate the samples of type A (n = 30) only recognized full-length recombinant β2GP1, whereas those of type B (n = 22) recognized full-length recombinant β2GP1, domains I and V, and also domains I and V extended with additional domains. When a hydrophobic plate was used, samples of type A recognized not only full-length recombinant β2GP1 (albeit the OD was lower) but also domain I. On a hydrophobic plate the reactivity of samples from type B was similar to that found with hydrophilic ELISA plates, albeit the ODs were lower. A typical example of the reactivity of samples of types A and B is presented in Figure 1.
Recognition of domain-deleted mutants by anti-β2GPI antibodies coated to different plates. Typical recognition of domain-deleted mutants of β2GPI and full-length β2GPI coated onto hydrophilic or hydrophobic ELISA plates by a sample from a patient with type A (left) and type B (right) reactivity. ODs are displayed after subtraction of the OD obtained by pooled normal plasma. Error bars represent mean ± SEM of triplicate points.
Recognition of domain-deleted mutants by anti-β2GPI antibodies coated to different plates. Typical recognition of domain-deleted mutants of β2GPI and full-length β2GPI coated onto hydrophilic or hydrophobic ELISA plates by a sample from a patient with type A (left) and type B (right) reactivity. ODs are displayed after subtraction of the OD obtained by pooled normal plasma. Error bars represent mean ± SEM of triplicate points.
Fine specificity of anti–β2GPI IgG antibodies
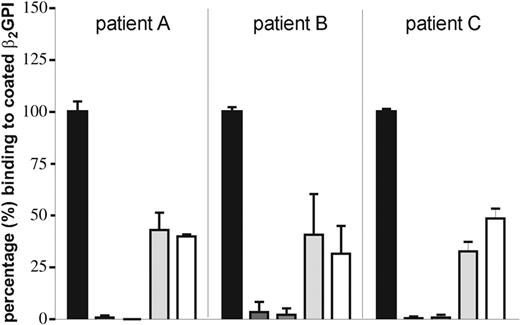
We hypothesized that patients of group A recognize a positive epitope on domain I that is shielded off from recognition when coated to hydrophilic plates. To demonstrate the involvement of this positively charged epitope on domain I in recognition by type A anti-β2GPI antibodies, we performed inhibition experiments. We coated Sepharose beads with native full-length recombinant β2GPI or 1 of 3 point mutations (D8A, G40E, or R43G) of full-length recombinant β2GPI, blocked the beads with BSA, and incubated them with IgG fractions isolated from plasma from 3 patients from type A. The supernatants were added to a hydrophilic plate that was coated with full-length recombinant β2GPI. We found that in all 3 samples with type A anti-β2GPI antibodies, the anti-β2GPI activity could be fully absorbed by full-length recombinant β2GPI and with D8A and only partially with G40E or R43G (Figure 2).
A mutation in domain I of β2GPI at positions 43 and 40 abrogates binding of anti-β2GPI antibodies. A hydrophilic ELISA plate was coated with 10 μg/mL recombinant full-length β2GPI. IgG (20 μg/mL) of 3 patients (A, B, C) was preincubated with Sepharose beads coated with full-length recombinant β2GPI or 1 of 3 β2GPI molecules with point mutations (D8A, G40E, or R43G) or only blocked with BSA. Supernatants were added to the plate and IgG binding was detected by an alkalic-phosphatase–labeled goat antihuman IgG antibody. The OD obtained with IgG incubated with BSA-blocked Sepharose beads was set at 100%. Binding of IgG anti-β2GPI antibodies to β2GPI coated onto a hydrophilic ELISA plate can be fully absorbed by full-length recombinant β2GPI and D8A but only partially by R43G and G40E. Error bars represent mean ± SEM of triplicate points.
A mutation in domain I of β2GPI at positions 43 and 40 abrogates binding of anti-β2GPI antibodies. A hydrophilic ELISA plate was coated with 10 μg/mL recombinant full-length β2GPI. IgG (20 μg/mL) of 3 patients (A, B, C) was preincubated with Sepharose beads coated with full-length recombinant β2GPI or 1 of 3 β2GPI molecules with point mutations (D8A, G40E, or R43G) or only blocked with BSA. Supernatants were added to the plate and IgG binding was detected by an alkalic-phosphatase–labeled goat antihuman IgG antibody. The OD obtained with IgG incubated with BSA-blocked Sepharose beads was set at 100%. Binding of IgG anti-β2GPI antibodies to β2GPI coated onto a hydrophilic ELISA plate can be fully absorbed by full-length recombinant β2GPI and D8A but only partially by R43G and G40E. Error bars represent mean ± SEM of triplicate points.
When we tested the affinity for β2GPI in an anti–β2GPI IgG-class ELISA in the presence of 1 M NaCl, 26 of 30 patients from group A recognized an epitope in the presence of 1 M NaCl (data not shown), indicating the recognition of a specific epitope rather than charge. Only 8 of 22 of the IgG of group B recognized β2GPI in the presence of 1 M NaCl, indicating that the binding of those antibodies was of lower affinity (data not shown).
Specific anti-β2GPI reactivity and LAC
Out of the 30 samples from type A, 28 (93%) were LAC positive. In 23 of 28 samples the LAC activity was dependent on β2GPI (Table 4). To give support to the association between anti-β2GPI reactivity and β2GPI-dependent LAC, we added anti–β2GPI IgG isolated from plasma of 3 patients with type A reactivity to β2GPI-depleted plasma that was reconstituted with either recombinant full-length β2GPI or point-mutated β2GPI (R43G). We observed an increase in coagulation time from 104.0 ± 3.4 (mean ± SEM, n = 4) seconds to 175.5 ± 32.4 (mean ± SEM, n = 4) seconds when plasma was reconstituted with 150 μg/mL recombinant β2GPI and 100 μg/mL type A anti–β2GPI IgG antibodies. However, little prolongation (104.0 ± 3.4 → 122.8 ± 17.3, mean ± SEM, n = 4) was seen when plasma was reconstituted with 150 μg/mL R43G and 100 μg/mL type A anti–β2GPI IgG antibodies. This experiment is representative of 4 experiments performed in the same way.
Correlation between thrombosis and presence of type A or type B anti-β2GPI antibodies
A history of thrombosis was present in 32 of 52 patients with IgG anti-β2GPI antibodies, in 25 (83%) of 30 patients IgG anti-β2GPI antibodies with type A reactivity, and in 7 (32%) of 22 patients with type B reactivity (Table 4). The odds ratios for thrombosis were 6.7 (95% confidence interval [95% CI]: 13.5-3.4) for IgG anti-β2GPI antibodies, 18.9 (95% CI: 53.2-6.8) for type A reactivity, and 1.1 (95% CI: 2.8-0.4) for type B reactivity.
Anti–domain I ELISA for clinical practice
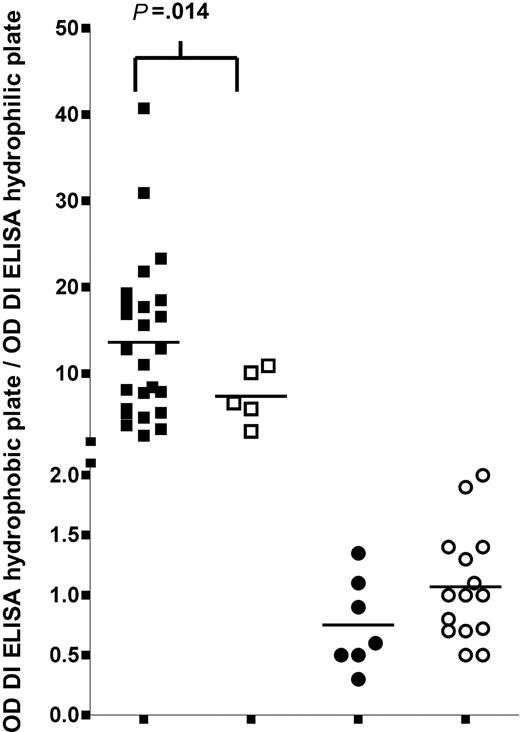
To enable differentiation between patients with IgG anti-β2GPI antibodies of type A and type B reactivity, one can coat domain I of β2GPI onto a hydrophilic and a hydrophobic ELISA plate. Plasma from patients with type A IgG anti-β2GPI antibodies do not recognize domain I on a hydrophilic plate but recognize domain I on a hydrophobic plate, whereas samples from patients with type B reactivity recognize domain I on both plates. A ratio of greater than 2 between the OD measured with the hydrophobic plate and the OD with the hydrophilic plate discriminates between type A and type B IgG anti-β2GPI antibodies. This ratio is an indication for the relative amount of IgG anti-β2GPI antibodies that recognize the positive charge on domain I. We determined a coefficient of variation of 3.7% (interassay variability) as an indication for the quality of this assay (Table 3). Figure 3 shows that within the group of patients with IgG anti-β2GPI antibodies with type A reactivity, the ratio in those with a thrombotic history is significantly higher than in patients without thrombosis (P = .014). Within the group of patients with IgG anti-β2GPI antibodies of type B reactivity, the ratios are low and there is no difference in ratio between patients with or without thrombosis.
Association between thrombosis and 2 different antibody populations. The association between the presence of a history of thrombosis and the ratio of ODs obtained with domain I coated onto hydrophobic or a hydrophilic ELISA plate, for type A and type B anti-β2GPI antibodies. Hydrophilic and hydrophobic ELISA plates were coated with 10 μg/mL domain I. Antibody binding was detected by an alkalic-phosphatase–labeled goat antihuman IgG antibody. The OD obtained with pooled plasma of 40 healthy volunteers was subtracted from the ODs obtained with patient plasma. As an estimation of the relative amount of antibodies reactive with the positive epitope on domain I, the OD obtained with the domain I ELISA on a hydrophobic plate was divided by the OD obtained with the domain I ELISA on a hydrophilic plate.
Association between thrombosis and 2 different antibody populations. The association between the presence of a history of thrombosis and the ratio of ODs obtained with domain I coated onto hydrophobic or a hydrophilic ELISA plate, for type A and type B anti-β2GPI antibodies. Hydrophilic and hydrophobic ELISA plates were coated with 10 μg/mL domain I. Antibody binding was detected by an alkalic-phosphatase–labeled goat antihuman IgG antibody. The OD obtained with pooled plasma of 40 healthy volunteers was subtracted from the ODs obtained with patient plasma. As an estimation of the relative amount of antibodies reactive with the positive epitope on domain I, the OD obtained with the domain I ELISA on a hydrophobic plate was divided by the OD obtained with the domain I ELISA on a hydrophilic plate.
Discussion
The identification of the domains of β2GPI that are involved in binding of anti-β2GPI antibodies has been the subject of many studies.13-21 The cumulative results show that each domain of β2GPI has been indicated as the location where anti-β2GPI antibodies may bind. A recent paper strongly suggests that for binding of anti-β2GPI antibodies purified from patient plasma, a positively charged epitope, spanning amino acids 40-43 on domain I of β2GPI, is very important. We tested this suggestion by studying the reactivity of 52 samples (52 patients) with anti–β2GPI IgG antibodies that were found within a group of 198 patients with SLE, LLD, or PAPS. The major conclusions from this study are as follows: (1) not all anti–β2GPI IgG antibodies have the same epitope specificity; (2) a substantial number of antibodies found in patients recognize an epitope around G40-R43 on domain I; (3) these latter antibodies seem to be the pathologic ones because their presence was highly correlated with a history of thrombosis; (4) the anti–β2GPI IgG antibodies against epitope G40-R43 in domain I are antibodies that cause LAC activity; and (5) anti–β2GPI IgG antibodies that do not recognize G40-R43 in domain I are not related to a history of thrombosis. For anti-β2GPI antibodies of the IgM class we did not find an increase in their association with thrombosis by using the anti–domain I ELISA. Based on these observations we have developed a simple assay to detect a subset of anti–β2GPI IgG antibodies that highly correlates with thrombosis. Although the proportion of patients with primary APS was very small in our population, previous studies found similar clinical and laboratory features for primary and secondary APS.27 It seems likely that this also accounts for this anti–domain I ELISA.
By coating 8 domain-deleted mutants of β2GPI and full-length recombinant β2GPI on both hydrophilic and hydrophobic ELISA plates we discriminated 2 different reactivity patterns (designated types A and B) for the anti-β2GPI antibodies. Samples with type A reactivity (present in 58% of anti-β2GPI antibody–positive samples) recognize full-length β2GPI but not deletion mutants when hydrophilic ELISA plates are used and a strong reactivity for domain I (and extensions of this domain) with use of hydrophobic ELISA plates (Figure 1). Samples designated type B do not seem to have a preferred binding site on β2GPI. With hydrophilic ELISA plates these antibodies react both with full-length β2GPI, domain I and V, and extensions of these. When hydrophobic ELISA plates are used, the pattern of reactivity against β2GPI and domain-deleted mutants is similar, albeit the ODs are much lower.
We hypothesized that our observation of the reactivity of type A antibodies is dependent on the type of ELISA plate used and could indicate that type A anti-β2GPI antibodies are directed against the G40-R43 epitope in domain I because it is conceivable that interactions between the positive charge of this epitope and the negative charge of a hydrophilic ELISA plate may hamper interaction of antibodies with that epitope.21 We showed that the reactivity of type A anti-β2GPI antibodies against β2GPI can be fully absorbed by full-length recombinant β2GPI and full-length recombinant β2GPI mutated at position 8 (D8A). When we absorbed type A anti-β2GPI antibodies with full-length recombinant β2GPI that had point mutations at 1 amino acid within the epitope G40-R43, namely R40E or R43G, 35% to 40% of the reactivity against β2GPI remained (Figure 2). This strongly suggests that these amino acids are part of the epitope to which type A anti-β2GPI antibodies are directed. We previously reported that β2GPI-dependent LAC activity is a strong risk factor for thrombosis.22 We now demonstrated that 23 (92%) of 25 samples with β2GPI-dependent LAC activity contained type A anti-β2GPI antibodies and that presence of type A antibodies is a strong risk factor for thrombosis (Table 4). The finding that reconstitution of β2GPI-depleted plasma with full-length β2GPI and type A anti-β2GPI antibodies induces more prolongation of the clotting time than reconstitution with R43G and type A anti-β2GPI antibodies suggested that type A anti-β2GPI antibodies cause LAC activity. As a β2GPI-dependent LAC activity correlates very strongly with thrombosis (odds ratio, 42.3), it also supports the concept that pathologic antibodies are directed against the epitope G40-R43.
Collectively, type B antibodies directed to β2GPI are not correlated with a history of thrombotic complications. Whether this population contains clinical relevant subpopulations of antibodies needs further studies.
In conclusion, our study shows that anti-β2GPI antibodies react with different epitopes on β2GPI. However, a substantial number of patient samples with anti-β2GPI antibodies (in our series 58%) recognize an epitope on domain I, including positions 40 and 43 (type A antibodies). The strong correlation between presence of such type A antibodies with histories of thrombosis and presence of β2GPI-dependent LAC activity strongly suggests that type A antibodies constitute a pathologic subset. Our observations form the basis for a simple assay (using domain I coated onto hydrophilic and hydrophobic ELISA plates) for the detection of type A anti-β2GPI antibodies.
Prepublished online as Blood First Edition Paper, October 26, 2004; DOI 10.1182/blood-2004-09-3387.
Supported by a grant of the Netherlands Organisation for Health Research and Development (ZonMw grant no. 902-26-290).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.