Abstract
The mechanism of action of intravenous immunoglobulin (IVIg) and polyclonal anti-D–mediated reversal of immune thrombocytopenia (ITP) is still unclear. However, in a murine model of ITP, the therapeutic effect of IVIg appears to be wholly dependent upon the expression of the inhibitory Fc receptor, FcγRIIB. We previously demonstrated that, similar to anti-D in humans, 2 erythrocyte-reactive monoclonal antibodies (TER119 and M1/69) ameliorated murine ITP and inhibited reticuloendothelial system (RES) function at doses that protected against thrombocytopenia. The current study evaluated the involvement of the inhibitory and activating Fc receptors, FcγRIIB and FcγRIIIA, respectively, in the TER119 and M1/69-mediated inhibition of thrombocytopenia. In contrast to IVIg, in FcγRIIB-deficient mice, both monoclonal antibodies ameliorated ITP and both significantly down-regulated the level of expression of the activating FcγRIIIA in splenic macrophages. These results indicate that anti-erythrocyte antibodies that ameliorate ITP act independently of FcγRIIB expression but are dependent upon the activating FcγRIIIA.
Introduction
Intravenous immunoglobulin (IVIg) is used widely in the treatment of various autoimmune conditions, including immune thrombocytopenic purpura (ITP). Many mechanisms have been suggested to explain the therapeutic effectiveness of IVIg in increasing platelet counts in ITP. The predominant hypotheses are Fcγ receptor (FcγR) blockade, anti-idiotypic regulation, and immunomodulation.1-5 It also has been suggested that the major histocompatibility complex (MHC) class I–like Fc receptor (FcRn) may play a role in the action of IVIg.6 Recently, the ratio of activating FcγRIII and the inhibitory FcγRIIB expression on splenic macrophages has been proposed to be a key to the therapeutic effectiveness of IVIg, as IVIg up-regulates FcγRIIB expression in wild-type mice (FcγRIIB+/+) and the absence of FcγRIIB (FcγRIIB–/–) completely abrogates the therapeutic efficacy of IVIg in thrombocytopenic mice.7
IVIg and anti-D may use different pathways to produce their therapeutic effects.7-15 We have previously demonstrated that 2 monoclonal anti-erythrocyte antibodies, TER119 and M1/69, mimic the effect of anti-D in reversing thrombocytopenia in a murine model of ITP; amelioration of thrombocytopenia correlated with an impaired ability to clear sensitized erythrocytes.16 The current study shows that, unlike IVIg, these therapeutic anti-erythrocyte monoclonal antibodies reverse thrombocytopenia in a FcγRIIB-independent fashion, possibly by blocking or decreasing the level of functional FcγRIIIA expression. This suggests that, in contrast to IVIg, anti-D–like monoclonal antibodies ameliorate ITP via inhibition of activating Fcγ receptors.
Study design
Mice
FcγRIIB–/– mice (B6;129S4-Fcgr2btm1Rav/J) and C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and housed at the St Michael's Hospital Research Vivarium.
Induction and reversal of ITP
Thrombocytopenia was induced by daily intraperitoneal injection of 2 μg rat anti–mouse integrin αIIb antibody (PharMingen, Mississauga, ON, Canada) as previously described.16-18 On day 2, mice were injected intravenously with 50 μg monoclonal antibody TER119 or M1/69 (PharMingen) or intraperitoneally with 2 g/kg IVIg (Gamimune, 10%; Bayer, Elkhart, IN). Whole blood was collected daily via the saphenous vein into capillary tubes preloaded with 5 μL 1% EDTA (ethylenediaminetetraacetic acid) in phosphate-buffered saline (PBS); pH 7.2; 50 μL blood was diluted in 1200 μL 1% EDTA in PBS (1:25), and centrifuged at room temperature at 170g for 2 minutes to isolate platelet-rich plasma (PRP). Fifty microliters of PRP was diluted into 9.95-mL Isoton II diluent (Coulter Corporation, Miami, FL) and platelet count determined using a Beckman Z2 Coulter Counter (Coulter Corporation).
Induction and prevention of erythrocyte clearance
To block erythrocyte clearance, mice were injected intravenously with 50 μg 2.4G2 (an FcγRIIB/FcγRIIIA blocking antibody)19,20 or rat IgG as negative control, or intraperitoneally with 2 g/kg IVIg. After 2 hours, erythrocyte clearance was induced with the intravenous administration of 50 μg TER119. After a further 24 hours, the blood was collected and erythrocytes enumerated as previously reported.16
Preparation of splenocytes and flow cytometric analysis
Twenty-four hours after treatment with TER119, M1/69, or 30-F1 (PharMingen) or IVIg as indicated, the spleen was removed, mechanically disrupted in 5 mL PBS containing 0.5% bovine serum albumin (BSA), and filtered through 70-μm nylon mesh strainer.21 Erythrocytes were lysed using 0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM Na2 EDTA (ACK) lysis buffer22 and washed in PBS/BSA. One million cells in 50 μL were incubated with a murine macrophage marker (PE-CY5–anti-F4/80; Cedarlane Laboratories, Hornby, ON, Canada) and fluorescein isothiocyanate (FITC)–2.4G2 antibody (PharMingen) for 30 minutes at 4°C with constant shaking. The cells were washed and acquired on a FACScan flow cytometer (Becton Dickinson, Mountain View, CA).
Results and discussion
Salama et al23 initially postulated that the success of IVIg in treating ITP was due to reduced macrophage binding of sensitized platelets by competitive blockade from immunoglobulin-coated erythrocytes. This was supported by observations that infusion of IVIg in patients with ITP prolonged the clearance of radiolabeled anti–Rh (D)–sensitized erythrocytes in vivo.1 Recently, a clear requirement for FcγRIIB has been demonstrated7 and confirmed18 for the therapeutic effect of IVIg in murine ITP.
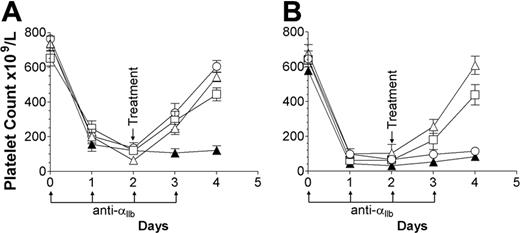
We have shown that the monoclonal erythrocyte-reactive antibodies TER119 and M1/69 successfully ameliorate murine ITP.16 In the current study we tested these antibodies, as well as IVIg, for efficacy in treating thrombocytopenic FcγRIIB+/+ and FcγRIIB–/– mice. In agreement with previous publications, 7,18 IVIg successfully reversed the disease in wild-type mice (Figure 1A, ○) but not in FcγRIIB–/– mice (Figure 1B, ○). In contrast, TER119 and M1/69 demonstrated a successful therapeutic effect in both thrombocytopenic FcγRIIB+/+ (Figure 1A, ▵, □) and FcγRIIB–/– mice (Figure 1B, ▵, □), indicating that TER119 and M1/69 can function fully independent of FcγRIIB expression. Since the antiplatelet antibody used in this study is completely dependent on FcγRIIIA expression for its pathologic effect (A.R.C., unpublished observations, December 2003), we hypothesized that, since TER119 and M1/69 do not rely on FcγRIIB expression, they might function by blocking or down-modulating FcγRIIIA expression, leading to increased platelet survival.
Anti-erythrocyte antibodies (TER119 and M1/69) do not require the inhibitory Fc receptor FcγRIIB in the amelioration of murine ITP. (A) FcγRIIB+/+ (C57BL/6) and (B) FcγRIIB–/– mice were injected with 2 μg antiplatelet antibody on days 0 to 3 (↑). All mice received an injection of 200 μL PBS (▴) or 2 g/kg IVIg (○) or 50 μg TER119 (▵) or M1/69 (□) on day 2 (↓). The x-axis denotes the days, the y-axis denotes the platelet count; n = 7 for each group. Data are expressed as mean ± SEM.
Anti-erythrocyte antibodies (TER119 and M1/69) do not require the inhibitory Fc receptor FcγRIIB in the amelioration of murine ITP. (A) FcγRIIB+/+ (C57BL/6) and (B) FcγRIIB–/– mice were injected with 2 μg antiplatelet antibody on days 0 to 3 (↑). All mice received an injection of 200 μL PBS (▴) or 2 g/kg IVIg (○) or 50 μg TER119 (▵) or M1/69 (□) on day 2 (↓). The x-axis denotes the days, the y-axis denotes the platelet count; n = 7 for each group. Data are expressed as mean ± SEM.
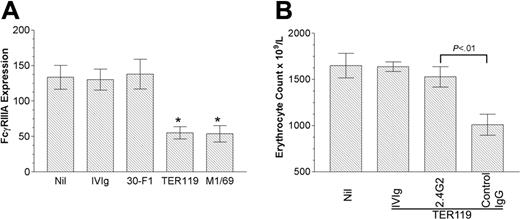
FcγR-bearing splenic macrophages are the main cells mediating platelet destruction in ITP.24 We have reported a positive correlation between the increase in platelet count and the clearance of erythrocytes by therapeutic anti-erythrocyte antibody in thrombocytopenic mice.16 Hence we presumed that, unlike IVIg, antierythrocyte antibodies may functionally block and/or down-regulate the expression of FcγRIIIA, resulting in the decreased platelet phagocytosis. To define the possible modulating effect of these anti-erythrocyte antibodies on FcγRIIIA expression, we analyzed the expression of FcγRIIIA on splenic macrophages in FcγRIIB–/– mice after injection of the ITP-ameliorating antibodies (TER119, M1/69), as well as after injection of an erythrocyte-reactive antibody (30-F1) that does not ameliorate ITP.16 The administration of TER119 and M1/69, but not IVIg or 30-F1, significantly decreased the level of expression of FcγRIIIA (Figure 2A). Antibody 2.4G2 reacts specifically with a common nonpolymorphic epitope on the extracellular domains of the mouse FcγRIII and FcγRII19,25 and blocks binding of immune complexes to the FcγRIII and FcγRII.20 In contrast, the antibody-coated erythrocytes engage the FcγRIIIA on macrophage through the Fc region of IgG. Thus, selective blockade of FcγRIIIA, using 2.4G2 in FcγRIIB–/– mice, would prevent the phagocytosis of antibody-coated erythrocytes by macrophages. As shown in Figure 2B, pretreatment of FcγRIIB–/– mice with 2.4G2 significantly inhibited the erythrocyte destruction by subsequent administration of TER119; in contrast, control IgG had no effect on erythrocyte destruction induced by TER119. These data support the contention that anti-erythrocyte antibodies that ameliorate ITP may do so by engaging FcγRIIIA and blocking and/or down-regulating the receptor. To establish a stronger link between the observed effects on erythrocyte destruction and the correction of platelet counts, we have investigated these phenomena in the same animals and observed the expected relationship (Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article).
Anti-erythrocyte antibodies inhibit the functional expression of the activating FcγRIIIA on splenic macrophages. (A) FcγRIIB–/– mice were injected with 2 g/kg IVIg intraperitoneally or 50 μg TER119, M1/69, or 30-F1 intravenously. After 24 hours, splenic cells were isolated and incubated with FITC-2.4G2 and PE-CY5-F4/80. The x-axis denotes the treatments given to each group of mice, the y-axis denotes FcγRIIIA expression (mean channel fluorescence) on F4/80-positive cells. Nil indicates no treatment. n = 5 for each column. *P < .005 versus Nil. (B) FcγRIIB–/– mice were injected with 2 g/kg IVIg intraperitoneally or 50 μg 2.4G2 or control IgG (rat IgG) intravenously. After 2 hours, 50 μg TER119 was injected, and erythrocyte count was evaluated on flow cytometry. The x-axis denotes the treatments given to each group of mice, the y-axis denotes the erythrocyte count. Nil indicates no treatment. n = 7 for each column. In both panels A and B, data are expressed as mean ± SEM.
Anti-erythrocyte antibodies inhibit the functional expression of the activating FcγRIIIA on splenic macrophages. (A) FcγRIIB–/– mice were injected with 2 g/kg IVIg intraperitoneally or 50 μg TER119, M1/69, or 30-F1 intravenously. After 24 hours, splenic cells were isolated and incubated with FITC-2.4G2 and PE-CY5-F4/80. The x-axis denotes the treatments given to each group of mice, the y-axis denotes FcγRIIIA expression (mean channel fluorescence) on F4/80-positive cells. Nil indicates no treatment. n = 5 for each column. *P < .005 versus Nil. (B) FcγRIIB–/– mice were injected with 2 g/kg IVIg intraperitoneally or 50 μg 2.4G2 or control IgG (rat IgG) intravenously. After 2 hours, 50 μg TER119 was injected, and erythrocyte count was evaluated on flow cytometry. The x-axis denotes the treatments given to each group of mice, the y-axis denotes the erythrocyte count. Nil indicates no treatment. n = 7 for each column. In both panels A and B, data are expressed as mean ± SEM.
It should be noted that the model of ITP used here is a simple model of immune thrombocytopenia without all of the complex attributes of a full-blown autoimmune disease such as human ITP. Thus, while this model provides a selective and powerful approach to understanding specific pathophysiologic mechanisms of IVIg action in ITP, the model may also be limited by virtue of this simplicity.
In this report, we have used several monoclonal anti-erythrocyte–specific antibodies to mimic the therapeutic effects of anti-D in ITP. The TER119 antigen is a molecule associated with cell-surface glycophorin A, and the monoclonal antibody TER119 specifically recognizes murine erythrocytes.26 Our previous study has demonstrated that TER119 mimics the action of anti-D to effectively ameliorate murine thrombocytopenia.16 A small prospective study to test a single human monoclonal anti-D in 7 D-positive patients with chronic ITP was unsuccessful,27 and this is in general agreement with our previous work, which has demonstrated that not all monoclonal anti-erythrocyte antibodies can ameliorate murine ITP.16 The monoclonal antibodies that effectively ameliorated the thrombocytopenia did react with the murine red cells and bound more strongly than did IVIg (Figure S2). In contrast, the antibody less reactive (equivalent to the batch of IVIg (lot #26N1LL1) used throughout these experiments with murine red cells did not ameliorate ITP. Therefore, antibodies that bind poorly to red cells (eg, 30-F1 and IVIg) may, in contrast to those that bind strongly to red cells (eg, TER119 and M1/69), not mediate “anti-D–like” effects. However, the mechanisms of action of IVIg therapy may be more complex in humans than in mice, and it is difficult to rule out the possibility that IVIg does not have anti-D–like effects in humans.
In summary, the anti-D–like antibodies TER119 and M1/69 require activating FcγRIIIA but not the inhibitory FcγRIIB in suppressing murine ITP. Since there appears to be different mechanisms of action involved with IVIg and anti-erythrocyte antibodies in ITP, we speculate that patients who do not benefit from one treatment may benefit from the other, or there may be an additive effect, although this remains to be demonstrated.
Prepublished online as Blood First Edition Paper, October 12, 2004; DOI 10.1182/blood-2004-05-1886.
Supported by an operating grant from the Canadian Blood Services—Canadian Institutes of Health Research Request for Proposal (RFP) program.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Ms Alison F. Starkey for assistance with the mouse work; Mr Hoang Le-Tien, Mr Davor Brinc, and Dr Zoë Cohen for assistance and helpful discussion; Dr Paul Doherty for critical review of the manuscript; and the St Michael's Hospital Research Vivarium staff.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal