Abstract
Early recognition of cobalamin (Cbl)–responsive disorders in the ambulatory care setting is essential to prevent irreversible neurologic deficits. However, diagnostic algorithms using Cbl, methylmalonic acid (MMA), and homocysteine (HCys) measurements reflect studies in academic centers, and their negative predictive values have not been established. Thus, records of 456 ambulatory patients evaluated for Cbl deficiency at a staff model HMO were reviewed. Pretherapy Cbl, MMA, and HCys values in individual patients varied by 23%, 23%, and 17%, respectively, over 2 to 6 weeks. Hematologic or neurologic responses to pharmacologic doses of Cbl occurred in 37 of the 95 evaluable patients. In these patients, pretherapy Cbl, MMA, and HCys values were normal in 54%, 23%, and 50%, respectively. If therapy had been restricted to symptomatic patients with both low or intermediate Cbl levels and increased metabolite values, 63% of responders would not have been treated. Twenty-five patients did not respond to treatment, including 5 of 11 patients (45%) with low Cbl, 22 of 49 patients (45%) with high MMA, and 13 of 30 patients (43%) with high HCys values. It is concluded that Cbl, MMA, and HCys levels fluctuate with time and neither predict nor preclude the presence of Cbl-responsive hematologic or neurologic disorders.
Introduction
While cobalamin (Cbl) deficiency classically causes macrocytic anemia, anemia can occur without macrocytosis, and both neurologic and cognitive dysfunction often develop in the absence of hematologic changes.1-5 Symptoms consistent with Cbl deficiency, including paresthesias, unsteady gait, fatigue, and impaired memory, are commonly encountered in the ambulatory care setting, and early recognition of Cbl deficiency is important to prevent progressive, irreversible neurologic and cognitive impairment.6,7
Although treatment with Cbl is often initiated after the finding of a low serum Cbl level, many patients with low Cbl levels are not Cbl deficient.8,9 As a result, measurements of the Cbl-dependent metabolites, methylmalonic acid (MMA) and homocysteine (HCys), have recently been used to confirm the presence of Cbl deficiency. Retrospective studies of patients at academic medical centers with low serum Cbl levels and clinically overt Cbl deficiency found MMA and/or HCys levels more than 3 standard deviations (SD) above the normal mean in almost all patients.8,10-12 Thus, algorithms for the diagnosis of Cbl deficiency were developed that suggest initial measurement of serum Cbl followed by determination of MMA and HCys when Cbl levels are intermediate or when suspicion of clinically significant Cbl deficiency is high.8,13-20 However, because little attention has been given to the study of patients with clinical findings consistent with Cbl deficiency but normal vitamin and metabolite levels, the negative predictive value of these tests is uncertain. Indeed, Cbl levels are normal in some patients with overt deficiency,11 and currently available Cbl assay kits may not be reliable.21,22 Moreover, because studies from academic centers usually employed special diagnostic or research laboratory facilities, their findings may not be representative of those obtainable in other ambulatory care settings.
Thus, a retrospective review of patients evaluated for Cbl deficiency during a 10-year period at a staff model HMO utilizing a national commercial laboratory was performed. Initially, only patients with low serum Cbl levels or elevated metabolite levels received therapeutic trials of Cbl. However, in the seventh year of the study period, one patient with total absence of vibratory sensation in the iliac crest, knees, and ankles had complete recovery following 2 months of Cbl therapy despite normal Cbl, MMA, and HCys levels. Thereafter, therapy was offered to all patients with hematologic or neurologic abnormalities consistent with Cbl deficiency regardless of the results of screening studies.
Patients, materials, and methods
Laboratory methods
Serum Cbl was measured by the ADVIA Centaur chemiluminescence assay (Bayer Diagnostics, Tarrytown, NY). Normal Cbl values were 200 to 1100 pg/mL, but higher reference ranges have been suggested,11,13,16,17,20,23,24 and the statement “patients with values less than 300 pg/mL may experience unexplained neuropsychiatric or hematologic abnormalities due to occult B12 deficiency” was appended to test reports during the last 4 years of the study. Thus, Cbl levels were defined as low (below 201 pg/mL), intermediate (201 to 300 pg/mL), or normal (above 300 pg/mL).
Serum MMA was measured by gas chromatography/mass spectroscopy with a reference range of 90 to 250 nmol/L during the first 4 years of the study and 90 to 279 nmol/L during the last 6 years. For a brief time, normal MMA values were reported as below 400 nmol/L. Thus, MMA levels below 251 nmol/L and those reported as below 400 nmol/L were considered to be normal, while values of 251 to 376 nmol/L were considered to be moderately increased (ie, 2 to 3 SD above the normal mean) and values above 376 nmol/L were considered to be elevated (ie, more than 3 SD above the normal mean).
Plasma HCys was measured by the Abbott AxSYM fluorescent polarization immunoassay (HPIA; Abbott Park, IL). Plasma was removed by centrifugation and frozen immediately after the sample was obtained. The reference range varied during the study period but was 5.4 to 12.1 μmol/L at the time most evaluations were performed. Thus, HCys levels below 12.2 μmol/L were considered to be normal, values of 12.2 to 13.6 μmol/L were considered to be moderately increased (ie, 2 to 3 SD above the normal mean), and values above 13.6 μmol/mL were considered to be elevated (ie, more than 3 SD above the normal mean).
Patients
A retrospective review of the medical records of 456 patients evaluated on 510 occasions by the author for Cbl deficiency between August 1, 1993, and September 30, 2003, was conducted. A total of 152 asymptomatic hematologically healthy patients were screened for Cbl deficiency because of the presence of conditions associated with Cbl depletion. Of the remaining 304 patients with clinical or laboratory abnormalities, 49 patients were excluded from this review because they were evaluated for cognitive dysfunction or nonspecific systemic complaints that were not objectively measured.
The remaining 255 patients had hematologic and/or neurologic findings consistent with Cbl deficiency. Cbl therapy was not offered to 127 of these patients because of the presence of one or more of the following: normal serum Cbl or MMA levels in 125 patients (98%); loss to follow-up in 26 patients (20%); resolution of signs, symptoms, or laboratory abnormalities prior to Cbl therapy in 12 patients (9%); and/or the presence of other causes for the clinical findings in 60 patients (47%). Of the 128 patients who received therapeutic trials of Cbl, 33 were considered unevaluable because of inadequate follow-up or noncompliance in 16 patients (50%); response associated with the use of another therapeutic modality in addition to Cbl in 8 patients (25%); and/or the presence of another disorder that could explain the clinical picture in 10 patients (30%). The remaining 95 patients received at least 3 months of Cbl therapy and were evaluable for hematologic and/or neurologic responses to treatment.
Cbl treatment
Patients with suspected deficiency were treated with cyanocobalamin 2 mg per day orally or 1 mg intramuscularly 3 times a week for 2 weeks, weekly for 8 weeks, and monthly thereafter. All patients were reevaluated within 3 months of beginning Cbl therapy.
Criteria for Cbl-responsive clinical disorders
Criteria for clinically significant responses to Cbl therapy were the same as those used in previous reports except that peripheral smears were not systematically reviewed for the presence of macroovalocytes or hypersegmented neutrophils.1,2,8,11,12 Thirty-seven patients were considered to have a definite response to therapy (responders). Hematologic improvement in these patients during Cbl therapy was considered to be significant if mean corpuscular volume (MCV) values above 98 fL decreased by at least 5 fL or if hematocrit values increased by at least 0.05 hematocrit points from values below 0.36 in women and below 0.39 in men. Neurologic improvement in these patients during Cbl therapy was considered to be significant if paresthesias completely resolved, observed ataxic gait became normal, and/or vibratory sensation that was completely absent at least in the ankles prior to therapy became easily detectable within 6 months of treatment. In contrast, 25 patients with macrocytosis, anemia, or neurologic deficits who had no other apparent causes for these abnormalities and who had no improvement after at least 3 months of Cbl therapy were considered nonresponders.
An additional 33 patients were considered possible responders because of less striking improvements in clinical findings, including a decrease in MCV of 3 to 4 fL; an increase in hematocrit of 0.03 to 0.04 hematocrit points; and partial improvement in paresthesias, ataxic gait, or vibratory sensation. Laboratory findings in these patients are shown in “Appendix 3.”
Statistical methods
Average differences between repeat measurements of Cbl, MMA, and HCys were expressed as the mean ± 1 standard deviation (SD). Statistical significance was assessed by the 2-tailed Student t test.
Results
Intraindividual variation in serum Cbl and metabolites
Repeat measurements of Cbl, MMA, and HCys prior to therapy were obtained within 2 to 6 weeks to confirm abnormal values or when the suspicion of Cbl deficiency was high despite initial normal test results. Average intraindividual variations in all 3 parameters were considerable, and a wide range of values was noted even when outliers with marked differences in the 2 test results obtained were omitted from analysis (Table 1). The variations in MMA and HCys were significantly greater in patients with at least one elevated value than in those in whom both values were within the normal reference range (P < .001 and P = .011, respectively). Overall, differences in Cbl values were above 100 pg/mL in 21% of patients, while differences in MMA levels above 100 nmol/L occurred in 24% of patients and differences in HCys levels above 2.5 μmol/L were present in 22% of patients (data not shown).
Moreover, test variability significantly affected whether or not a patient might be considered to be Cbl deficient. Thus, normal MMA and HCys values were associated with higher metabolite levels in 37% and 26% of events, respectively, while 49% of patients with Cbl levels above 300 pg/mL also had values in the low or intermediate range (Table 2).
Patients with clinical responses to Cbl therapy
Clinical responses to Cbl therapy occurred in 37 patients. Hematologic responses occurred in 8 patients, including 6 patients with a fall in MCV, 1 patient with a fall in MCV associated with an increase in hematocrit, and 1 patient with an increase in hematocrit alone. In all 7 patients with macrocytosis, MCV values decreased to values below 98 fL. Neurologic responses occurred in 30 patients, including 21 patients with resolution of paresthesias, 13 patients with improvement in vibratory sensation, and 7 patients with correction of ataxic gait. Ten patients had 2 or more neurologic abnormalities that resolved with Cbl therapy. One patient had improvement of both hematologic and neurologic abnormalities. Cbl was administered intramuscularly to 30 patients (81%), including 24 of the 30 patients (80%) with neurologic responses. Other medical problems that may be associated with hematologic or neurologic abnormalities were present in 15 of these patients (“Appendix 1”). However, in every case, the response to Cbl therapy occurred without concurrent changes in the severity of these disorders and in the absence of other therapeutic interventions.
Serum Cbl was measured in all 37 patients, and a second pretherapy determination was obtained in 24 of them (65%). MMA and HCys were measured in 35 patients and 34 patients, with second pretherapy determinations obtained in 22 patients (63%) and 17 patients (50%), respectively. To maximize the sensitivity of these tests, the lowest pretherapy Cbl levels and the highest metabolite values obtained were used for analysis.
As shown in Table 3, serum Cbl values were above 200 pg/mL in 84% of responders, including 62% of patients with hematologic responses and 90% of patients with neurologic responses. Only 1 patient had a serum Cbl level below 150 pg/mL. In fact, 20 patients had serum Cbl levels above 300 pg/mL (54%), including 15 patients with Cbl levels above 400 pg/mL (41%).
Pretherapy MMA values were considered to be normal using reference laboratory criteria at the time of evaluation in 8 of the 35 responders studied (23%), including 2 of 7 patients with hematologic responses (29%) and 6 of 29 patients with neurologic responses (21%) (Table 3). Moreover, MMA values were above 376 nmol/L in only 13 responders (37%). Only 1 of the 22 patients with normal or moderately increased MMA values was receiving antibiotics at the time of evaluation while the remaining 21 patients had not received antibiotics for at least 6 weeks prior to testing and 19 had not received antibiotics for at least 3 months. Similarly, HCys values were normal in 17 of the 34 responders studied (50%) (Table 3).
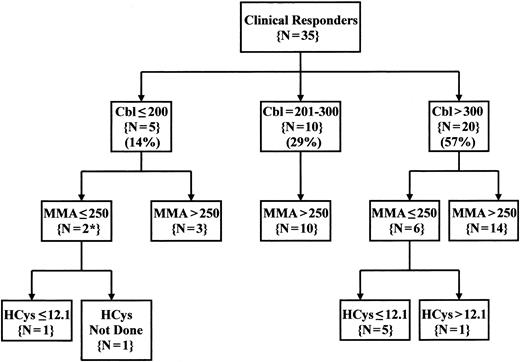
Overall, both metabolites were normal in 7 of the 33 evaluable patients (21%). If algorithms were used to limit Cbl therapy to patients with both Cbl levels below 301 pg/mL and MMA levels above 250 nmol/L, 22 of the 35 evaluable Cbl-responsive patients would not have received a therapeutic trial (63%) (Figure 1). Even extending criteria for treatment to include patients with Cbl levels below 401 pg/mL and MMA levels above 250 nmol/L would have missed 17 patients with clinical responses to Cbl therapy (49%) (data not shown). An increased HCys value identified only one additional responder with normal Cbl and MMA levels.
Pattern of Cbl, MMA, and HCys values in patients with clinical responses to Cbl therapy. Serum, Cbl, and MMA were measured on at least one occasion in 35 of the 37 patients with clinical responses to Cbl therapy, and serum HCys was also measured in 34 of these patients. The values shown represent the lowest pretherapy serum Cbl value and the highest pretherapy serum metabolite values obtained for each patient. Serum Cbl levels were defined as low (<201 pg/mL), intermediate (201 to 300 pg/mL), or normal (above 300 pg/mL). Normal metabolite levels were <251 nmol/L for MMA and <12.2 μmol/L for HCys. *MMA values in both of these cases were reported as below 400 nmol/L. Cbl indicates cobalamin; MMA, methylmalonic acid; HCys, homocysteine.
Pattern of Cbl, MMA, and HCys values in patients with clinical responses to Cbl therapy. Serum, Cbl, and MMA were measured on at least one occasion in 35 of the 37 patients with clinical responses to Cbl therapy, and serum HCys was also measured in 34 of these patients. The values shown represent the lowest pretherapy serum Cbl value and the highest pretherapy serum metabolite values obtained for each patient. Serum Cbl levels were defined as low (<201 pg/mL), intermediate (201 to 300 pg/mL), or normal (above 300 pg/mL). Normal metabolite levels were <251 nmol/L for MMA and <12.2 μmol/L for HCys. *MMA values in both of these cases were reported as below 400 nmol/L. Cbl indicates cobalamin; MMA, methylmalonic acid; HCys, homocysteine.
Predictors of clinical response to Cbl therapy
An additional 25 patients received Cbl therapy without significant improvement, including 12 patients with MCV values above 98 fL (4 of these patients also had anemia with hematocrit values of 0.34 to 0.38); 12 patients with otherwise unexplained normocytic anemia; and 5 patients with neurologic abnormalities, including paresthesias in 3 patients, paresthesias associated with impaired vibration sensation in 1 patient, and ataxic gait alone in 1 patient. Four patients had both hematologic and neurologic abnormalities. All patients received at least 3 months of Cbl therapy, and 20 patients were treated for at least 6 months, including 4 of the 5 patients with neuropathy. Cbl was administered intramuscularly in 16 of these patients (65%), including all 5 patients with neuropathy.
As shown in Table 4, 14 of the 31 patients with serum Cbl levels below 301 pg/mL (45%) and 5 of 11 patients with serum Cbl levels below 201 pg/mL (45%) did not improve with Cbl therapy. Similarly, 22 of 49 patients (45%) with MMA values above 250 nmol/L and 13 of 30 patients (43%) with HCys values above 12.1 μmol/L were nonresponders (Table 4). The same pattern was seen in patients with more marked increases in metabolite levels. Thus, 54% of patients with MMA values above 376 nmol/L were nonresponders despite the fact that Cbl therapy led to a fall in MMA of at least 200 nmol/L within 1 month in 12 of the 13 nonresponders (data not shown) and in 11 of the 12 responders studied (“Appendix 2”). Similarly, 37% of patients with HCys values above 13.6 μmol/L were nonresponders.
Moreover, within each group, the proportion of patients with abnormal test results was similar. Thus, low or intermediate Cbl levels were present in 46% of responders and 56% of nonresponders, increased MMA values were present in 73% of responders and 88% of nonresponders, and increased HCys values were present in 50% of responders and 62% of nonresponders.
Discussion
Recent approaches to Cbl deficiency reflect the recognition that neither macrocytosis nor anemia is consistently present in patients with neurologic or cognitive dysfunction.1-5 In addition, assays for serum MMA and HCys have become available and algorithms have been developed for the use of these tests in patients with an appropriate clinical picture.9-11,13-20,25 In general, initial measurement of serum Cbl has been suggested followed by determination of MMA and HCys when Cbl levels are intermediate or when suspicion of clinically significant Cbl deficiency is high.
Cost-effective clinical decision making often leads to reliance on a single test result particularly when more expensive tests such as metabolite assays are involved. Thus, it is important to appreciate how greatly test results may fluctuate over time in individual subjects. As shown in Table 1, serum Cbl levels varied significantly in individual patients. In fact, the large variations observed using the current chemiluminescence assay were similar to those previously reported more than 40 years ago using early microbiologic methods.26,27 Large intraindividual variations in MMA and HCys were also observed in the present study (Table 1). While the changes observed in patients with normal metabolite levels were similar to those previously reported in healthy subjects,28-34 fluctuations were even greater in patients with at least one elevated metabolite value (Table 1). Most significantly, many patients with normal Cbl or metabolite values also had abnormal test results when a second pretherapy measurement was obtained (Table 2). Thus, short-term fluctuations of Cbl, MMA, and HCys may obscure the presence of Cbl deficiency. The variation in MMA and HCys levels may also lead to erroneous conclusions regarding responses of these metabolites to Cbl or folate therapy.
Previous studies of serum metabolites in patients with clinical responses to Cbl therapy found MMA and/or HCys levels more than 3 SD above the normal mean in almost all patients.1,8,10-12 However, patients in these studies were preselected for the presence of low serum Cbl levels, and survey studies of ambulatory populations have found elevated MMA levels in many subjects with serum Cbl levels well within the normal range.16,23,24,35-38 Only one study contrasted the serum metabolite levels in 86 patients with clinical responses to Cbl therapy with those in 59 patients who did not improve with Cbl therapy.9 While 94% of responders had increases of one or both metabolites at least 3 SD above the normal mean, it was also notable that 12 of 61 patients (21%) with normal or less striking increases in MMA were responders, as were 13 of 51 patients (25%) with normal or less striking increases in HCys. All of the subjects in this study had serum Cbl levels below 200 pg/mL. Thus, reports of Cbl therapy in patients with either intermediate or high Cbl levels and/or normal metabolite levels are limited, and the negative predictive value of normal test results remains to be established.
Strikingly, one patient in the present series had total absence of vibratory sensation in the iliac crest, knees, and ankles, which resolved completely after 2 months of Cbl therapy despite normal Cbl and metabolite levels. Thus, in the present study, patients with a clinical picture consistent with Cbl deficiency were evaluated with metabolite measurements and often given Cbl therapy regardless of their Cbl, MMA, and HCys values. Indeed, only 16% of patients with clinical responses to Cbl therapy had “low” serum Cbl levels, and values were above 300 pg/mL in 54% of cases (Table 3). Moreover, MMA and HCys values were normal in 23% and 49% of cases, respectively, and both metabolite levels were normal in 21% of evaluable cases even though more modest metabolite cutoff levels (ie, more than 2 SD) were used (Table 3). In fact, commonly employed algorithms would have excluded 57% of these patients from metabolite testing and 63% from Cbl therapy (Figure 1). Furthermore, the low incidence of increased MMA levels in responders cannot be explained by decreased production of proprionic acid by gut flora because concurrent antibiotic use occurred in only one patient in this group (“Results” and Lindenbaum et al11 ). Finally, high MMA and HCys values were as frequent in the 25 patients who did not respond to Cbl therapy as they were in the 37 responders (Table 4). Moreover, all but 1 of the 15 nonresponders tested with MMA values above 376 nmol/L had a marked decrease in the level of this metabolite after Cbl therapy, suggesting a dissociation of metabolic and clinical responses to treatment. Thus, low Cbl and increased metabolite levels are neither sensitive markers of Cbl-responsive disorders nor are they predictive of a subsequent clinical response to Cbl therapy.
While these inconsistencies in serum Cbl and metabolite levels as indicators of clinical Cbl deficiency may reflect either biologic variability or inaccuracies associated with large-scale commercial testing, alternate mechanisms of the response to Cbl therapy may also explain the findings in this report, particularly when it is recognized that high pharmacologic doses of Cbl were used in these therapeutic trials (Table 5). First, classic vitamin deficiency should result in both low serum Cbl levels and elevated metabolite values. Second, vitamin-dependent disorders associated with inherited or acquired abnormalities of Cbl metabolism should result in elevated metabolite levels but normal serum Cbl values.39-42 Finally, it is speculated that in vitamin-responsive disorders, normal Cbl and metabolite levels would be expected if the high therapeutic doses of Cbl currently used in clinical practice have pharmacologic effects on Cbl-related processes or on cellular functions completely unrelated to the known physiologic effects of Cbl. In this regard, “functional” deficiency of Cbl has recently been suggested in the pathogenesis of both Alzheimer disease and AIDS myelopathy.43,44 Moreover, pharmacologic doses of methylcobalamin can improve measures of both cardiac autonomic dysfunction and peripheral neuropathy in diabetic subjects,45-47 and high doses of methylcobalamin also accelerate nerve regeneration in rats with acrylamide neuropathy.48 The observation that methylcobalamin can serve as a methyl donor in vitro in the DNA methylase reaction provides one possible mechanism for a pharmacologic effect of Cbl therapy.49 Analogously, pharmacologic doses of B6 vitamers have been shown to alter enzymes of vitamin B6 metabolism, B6-dependent apoenzymes, and unrelated enzyme proteins both in vitro and in vivo.50-55
It is concluded that (1) short-term intraindividual variations in Cbl, MMA, and HCys values can affect the recognition of Cbl deficiency; (2) Cbl levels well within the reference range are common in patients with clinical disorders responsive to Cbl therapy; (3) metabolites are frequently normal in patients with hematologic or neurologic abnormalities responsive to pharmacologic doses of Cbl; and (4) the presence of elevated metabolite levels are not predictive of a clinical response to Cbl therapy. Thus, therapeutic trials of Cbl are warranted when clinical findings consistent with Cbl deficiency are present, and further studies of the pharmacologic effects of Cbl coenzymes in the treatment of these disorders are suggested.
Prepublished online as Blood First Edition Paper, October 5, 2004; DOI 10.1182/blood-2004-04-1641.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The assistance with data analysis and the helpful suggestions of Peter Solomon are gratefully acknowledged.