Abstract
Among 2333 consecutive patients with myelofibrosis with myeloid metaplasia (MMM) seen at our institution, 91 fulfilled the World Health Organization (WHO) criteria for leukemic transformation (LT). All episodes of LT were myeloid in origin (acute myeloid leukemia [AML]) with all French-American-British (FAB) subtypes represented except M3; the most frequent subtypes were M7 (25.4%), M0 (22.4%), and M2 (17.9%). Cytogenetic studies during LT were available in 56 patients and revealed a clonal abnormality in 51 (91%): 30 patients had complex karyotype, 2 had core-binding factor gene lesions, and 18 had abnormalities of chromosome 5 or 7. Karyotypic evolution was documented in the majority of the patients in whom serial analysis was possible. In general, LT was fatal in 98% of the cases after a median of 2.6 months (range, 0-24.2 months). Twenty-four patients received AML-like induction chemotherapy that resulted in no complete remission: 41% reverted into chronic-phase disease and the incidence of treatment-related mortality was 33%. The remaining 67 patients received either supportive care alone (48 patients) or low-intensity chemotherapy (19 patients). Overall, survival was similarly poor in all 3 treatment categories. The outcome of LT in MMM with current therapies is dismal and either supportive care alone or appropriate clinical trials should be considered.
Introduction
Myelofibrosis with myeloid metaplasia (MMM) is currently classified as a myeloproliferative disorder and can present de novo (agnogenic myeloid metaplasia) or develop in the setting of either polycythemia vera (postpolycythemic myeloid metaplasia) or essential thrombocythemia (postthrombocythemic myeloid metaplasia) at a rate of 10% to 20% after 15 to 20 years of follow-up.1,2 The disease represents stem cell–derived clonal myeloproliferation that is accompanied by intense bone marrow stromal reaction including collagen fibrosis, osteosclerosis, and angiogenesis.3 Clinical manifestations include progressive anemia, massive splenomegaly, hepatosplenic as well as nonhepatosplenic extramedullary hematopoiesis, and a leukoerythroblastic blood smear.4 Average life expectancy is estimated at 5 to 7 years but may approach 15 years in young patients with good prognostic factors.5 Neither drug therapy nor allogeneic hematopoietic stem cell transplantation (AHSCT) has been shown, in a controlled setting, to positively influence survival in MMM.6 Cause of death in MMM includes leukemic transformation (LT) that occurs in 8% to 23% of patients in the first 10 years of diagnosis.7,8 Little data exist regarding the details of the pathologic and cytogenetic presentations as well as treatment outcome of LT in MMM.
Patients, materials, and methods
After obtaining approval from the Mayo Clinic Institutional Review Board, the study patients were retrospectively identified from our institutional database of medical diagnoses. Specifically, patients were required to have both a histologically confirmed case of MMM initially and subsequently have been evaluated at our institution between 1980 and 2003 after LT. The diagnosis and classification of LT were according to the World Health Organization (WHO) criteria (ie, 20% blasts in bone marrow or peripheral blood).9 Cases of acute myelofibrosis, overt myelodysplastic syndrome, chronic myelomonocytic leukemia, and bcr/abl-positive leukemia were excluded. Details regarding clinical and laboratory presentation during both initial diagnosis of MMM and at the time of LT were abstracted from medical records. All therapeutic interventions were recorded and assessed after the time of transformation and treatment categories included supportive care only, low-intensity chemotherapy (ie, goal of therapy was palliation rather than obtaining remission), and acute myeloid leukemia (AML)–like induction chemotherapy. Survival of LT patients was defined as the interval from the date of diagnosis of LT to either death or last contact. An event was defined as a death from any cause, unless otherwise indicated. Kaplan-Meier methodology was used to estimate survival distributions. Bone marrow histology and cytogenetic details at time of initial MMM diagnosis and LT and after therapy were rereviewed in each instance for diagnostic confirmation, French-American-British (FAB) classification of LT, myeloblast percentage, degree of reticulin fibrosis and osteosclerosis, and evidence of karyotypic evolution.
Results
Disease information prior to leukemic transformation
A total of 2333 patients with MMM were evaluated at our institution between 1980 and 2003. Among these, 91 fulfilled the WHO criteria for AML during the time of their visit at the Mayo Clinic. Among these individuals, 62 (68%) had been cared for at our institution during the chronic phase of their disease (MMM), whereas the remainder were referred at or near the time (< 2 months) of transformation. Patients who experienced LT elsewhere were not included in the current study. Patient characteristics are outlined in Table 1. The median interval between MMM diagnosis and LT was 31 months (range, 2-441 months) and the median age at transformation was 66 years (range, 41-82 years). As to be expected, most of the study patients had advanced disease at the time of LT, including the need for cytoreductive therapy in the majority, and pre-LT splenectomy was documented in 36% (Table 1). It is to be noted that 48% of the study patients had evidence of circulating blasts (a known adverse prognostic feature in MMM) at the time of MMM diagnosis.
Clinical and laboratory features of 91 patients with MMM who underwent LT
Patient characteristics . | Data . |
|---|---|
| Sex, no. (%) | |
| Male | 55 (60) |
| Female | 36 (40) |
| Median age, y (range) | |
| At diagnosis of MMM | 63 (21-81) |
| At time of LT | 66 (41-82) |
| Type of MMM, no. (%) | |
| Agnogenic myeloid metaplasia | 49 (54) |
| Postpolycythemic myeloid metaplasia | 22 (24) |
| Postthrombocythemic myeloid metaplasia | 20 (22) |
| Lille10prognostic score at diagnosis of MMM, available in 48 patients, no. (%) | |
| 0 | 26 (54) |
| 1 | 18 (38) |
| 2 | 4 (8) |
| Circulating blasts present at time of MMM diagnosis, available in 48 patients, no. (%) | 23 (48) |
| Median interval between diagnosis of MMM and LT, mos (range) | 31 (2-441) |
| Prior treatment for MMM, no. (%) | |
| Hydroxyurea | 47 (52) |
| Splenectomy | 33 (36) |
| Radioactive phosphorus, P32 | 12 (13) |
| Alkylators such as busulfan, cyclophosphamide, etc | 12 (13) |
Patient characteristics . | Data . |
|---|---|
| Sex, no. (%) | |
| Male | 55 (60) |
| Female | 36 (40) |
| Median age, y (range) | |
| At diagnosis of MMM | 63 (21-81) |
| At time of LT | 66 (41-82) |
| Type of MMM, no. (%) | |
| Agnogenic myeloid metaplasia | 49 (54) |
| Postpolycythemic myeloid metaplasia | 22 (24) |
| Postthrombocythemic myeloid metaplasia | 20 (22) |
| Lille10prognostic score at diagnosis of MMM, available in 48 patients, no. (%) | |
| 0 | 26 (54) |
| 1 | 18 (38) |
| 2 | 4 (8) |
| Circulating blasts present at time of MMM diagnosis, available in 48 patients, no. (%) | 23 (48) |
| Median interval between diagnosis of MMM and LT, mos (range) | 31 (2-441) |
| Prior treatment for MMM, no. (%) | |
| Hydroxyurea | 47 (52) |
| Splenectomy | 33 (36) |
| Radioactive phosphorus, P32 | 12 (13) |
| Alkylators such as busulfan, cyclophosphamide, etc | 12 (13) |
Clinical and laboratory features during leukemic transformation
All LT cases were pathologically confirmed to be myeloid in origin (AML) by direct examination of the bone marrow in 78 patients (86%) and the peripheral blood smear in the remaining 13 patients. Table 2 outlines the FAB classification details that reveal that the most frequent AML subtypes were M7, M0, and M2. However, all FAB subtypes except M3 were represented. Although an increase in circulating myeloblast percentage was prevalent (median, 25%; range, 0%-93%), LT in some patients was diagnosed by bone marrow examination in the absence of circulating blasts (Table 2). Clinically, LT was almost always accompanied by significant anemia and thrombocytopenia, contrasted by leukocytosis (Table 2). LT was also heralded by sharp progression of the disease-associated constitutional symptoms of fatigue, night sweats, involuntary weight loss, bone pain, and progression of organomegaly.
Laboratory and clinical details at the time of LT in 91 patients with MMM
Patient characteristics . | Data . |
|---|---|
| Method of LT diagnosis, no. (%) | |
| Bone marrow examination | 78 (86) |
| Peripheral blood examination | 13 (14) |
| Median peripheral blood counts | |
| Hemoglobin, g/L (range) | 92 (45-140) |
| Erythrocyte transfusion dependent, no. (%) | 59 (65) |
| Leukocyte count, × 109/L (range) | 4.9 (0.1-113.3) |
| Greater than 20 × 109/L, no. (%) | 43 (47) |
| Circulating myeloblasts (range) | 25 (0-92) |
| Platelet count, × 109/L (range) | 68 (4-797) |
| Less than 100 × 109/L, no. (%) | 60 (66) |
| Less than 50 × 109/L, no. (%) | 33 (36) |
| Median spleen and liver sizes, cm below the respective costal margins (range) | |
| Spleen | 6 (0-27) |
| Liver | 2 (0-20) |
| Bone marrow findings, % (range) | |
| Median marrow cellularity | 80 (5-95) |
| Median marrow myeloblast percentage | 43 (20-95) |
| Reticulin fibrosis of at least grade 2 | 37.5 |
| Osteosclerosis present | 30.3 |
| FAB classification of AML, no. patients (%) | |
| Total | 67 (100.0) |
| M0 | 15 (22.4) |
| M1 | 9 (13.4) |
| M2 | 12 (17.9) |
| M3 | 0 (0.0) |
| M4 | 8 (11.9) |
| M5 | 3 (4.5) |
| M6 | 3 (4.5) |
| M7 | 17 (25.4) |
| No. bone marrow karyotypic abnormalities, no. patients (%) | |
| Total | 53 (100.0) |
| Normal karyotype | 5 (9.4) |
| 1 | 16 (30.1) |
| 2 | 3 (5.6) |
| 3 or more | 29 (54.9) |
Patient characteristics . | Data . |
|---|---|
| Method of LT diagnosis, no. (%) | |
| Bone marrow examination | 78 (86) |
| Peripheral blood examination | 13 (14) |
| Median peripheral blood counts | |
| Hemoglobin, g/L (range) | 92 (45-140) |
| Erythrocyte transfusion dependent, no. (%) | 59 (65) |
| Leukocyte count, × 109/L (range) | 4.9 (0.1-113.3) |
| Greater than 20 × 109/L, no. (%) | 43 (47) |
| Circulating myeloblasts (range) | 25 (0-92) |
| Platelet count, × 109/L (range) | 68 (4-797) |
| Less than 100 × 109/L, no. (%) | 60 (66) |
| Less than 50 × 109/L, no. (%) | 33 (36) |
| Median spleen and liver sizes, cm below the respective costal margins (range) | |
| Spleen | 6 (0-27) |
| Liver | 2 (0-20) |
| Bone marrow findings, % (range) | |
| Median marrow cellularity | 80 (5-95) |
| Median marrow myeloblast percentage | 43 (20-95) |
| Reticulin fibrosis of at least grade 2 | 37.5 |
| Osteosclerosis present | 30.3 |
| FAB classification of AML, no. patients (%) | |
| Total | 67 (100.0) |
| M0 | 15 (22.4) |
| M1 | 9 (13.4) |
| M2 | 12 (17.9) |
| M3 | 0 (0.0) |
| M4 | 8 (11.9) |
| M5 | 3 (4.5) |
| M6 | 3 (4.5) |
| M7 | 17 (25.4) |
| No. bone marrow karyotypic abnormalities, no. patients (%) | |
| Total | 53 (100.0) |
| Normal karyotype | 5 (9.4) |
| 1 | 16 (30.1) |
| 2 | 3 (5.6) |
| 3 or more | 29 (54.9) |
FAB indicates French-American-British cooperative group; and AML, acute myeloid leukemia.
Karyotypic changes at time of initial MMM diagnosis and during leukemic transformation
Karyotypic analysis was available in 32 patients at the time of initial MMM diagnosis and 56 patients at the time of LT (Table 3). Karyotype analyses were unavailable at the time of LT in 35 patients due to either lack of availability of the assay (the series begins in 1980) or insufficient numbers of metaphases obtained from their diagnostic sample for evaluation. Cytogenetic findings at time of MMM diagnosis were similar to those previously described (Table 3).11 During LT, most (n = 51; 91%) but not all patients displayed a detectable cytogenetic abnormality (Table 3). The prevalence of “favorable in AML” cytogenetic abnormalities was very low and occurred in 2 patients, one each with t(8;21)(q22;q22) and inv(16)(p13;q22), neither of whom received AML-like induction chemotherapy due to poor performance status and both expired in less than one month of supportive care. The majority of patients studied at the time of LT displayed “unfavorable in AML” cytogenetic lesions (Table 3). Twenty-eight patients had karyotype analysis performed both at the diagnosis of MMM and at LT. At diagnosis of MMM, 20 patients had either normal chromosomes or a single chromosome anomaly, whereas only 7 displayed a complex karyotype with 3 or more chromosome anomalies. However, at LT all 28 patients had abnormal karyotypes, including 17 patients with markedly complex karyotypes and only 9 with simple clonal anomalies. The recurrent numeric and structural anomalies observed at transformation to AML most frequently involved chromosomes 5, 6, 7, 8, 17, 19, and 21. Additionally, among the 26 (93%) of 28 patients whose karyotype changed from MMM to LT, 11 patients (43%) displayed evolution of an existing clone and 15 (57%) developed one or more additional abnormal clones.
Cytogenetic findings at the time of initial diagnosis (Dx) of MMM and at the time of LT, where available, in a cohort of 91 patients
. | Findings at Dx of MMM, no. (%) . | Findings at Dx of LT, no. (%) . |
|---|---|---|
| No. | 32 (100.0) | 56 (100.0) |
| Normal (NN) | 12 (37.5) | 5 (8.9) |
| 1 abnormality | 12 (37.5) | 17 (30.3) |
| 2 abnormalities | 2 (6.2) | 4 (7.2) |
| 3 or more abnormalities | 6 (18.8) | 30 (53.6) |
| Specific cytogenetic lesions at time of MMM diagnosis | ||
| 20q- | 4 (12.5) | — |
| 13q- | 1 (3.1) | — |
| +8 | 1 (3.1) | — |
| +9 | 4 (12.5) | — |
| 12p- | 3 (9.4) | — |
| -5/-7 | 2 (6.3) | — |
| Specific cytogenetic lesions at time of LT | ||
| Favorable | — | 2 (3.6) |
| t(8;21)(q22;q22) | — | 1 (1.8) |
| inv(16)(p13;q22) | — | 1 (1.8) |
| Intermediate | — | 20 (35.7) |
| High risk | — | 34 (60.7) |
| 3 or more abnormalities | — | 30 (53.6) |
| -5/5q- | — | 11 (26.8) |
| -7/7q- | — | 7 (19.6) |
| +8 | — | 14 (23.2) |
. | Findings at Dx of MMM, no. (%) . | Findings at Dx of LT, no. (%) . |
|---|---|---|
| No. | 32 (100.0) | 56 (100.0) |
| Normal (NN) | 12 (37.5) | 5 (8.9) |
| 1 abnormality | 12 (37.5) | 17 (30.3) |
| 2 abnormalities | 2 (6.2) | 4 (7.2) |
| 3 or more abnormalities | 6 (18.8) | 30 (53.6) |
| Specific cytogenetic lesions at time of MMM diagnosis | ||
| 20q- | 4 (12.5) | — |
| 13q- | 1 (3.1) | — |
| +8 | 1 (3.1) | — |
| +9 | 4 (12.5) | — |
| 12p- | 3 (9.4) | — |
| -5/-7 | 2 (6.3) | — |
| Specific cytogenetic lesions at time of LT | ||
| Favorable | — | 2 (3.6) |
| t(8;21)(q22;q22) | — | 1 (1.8) |
| inv(16)(p13;q22) | — | 1 (1.8) |
| Intermediate | — | 20 (35.7) |
| High risk | — | 34 (60.7) |
| 3 or more abnormalities | — | 30 (53.6) |
| -5/5q- | — | 11 (26.8) |
| -7/7q- | — | 7 (19.6) |
| +8 | — | 14 (23.2) |
— indicates not applicable.
Bone marrow histologic changes during leukemic transformation
Serial bone marrow comparison was possible in 33 patients with the second bone marrow evaluation performed at a median of 24.5 months (range, 2.1-110.4 months) from the initial diagnosis of MMM. In general, bone marrow histology during LT displayed either a diffuse (Figure 1A-B) or focal (Figure 1D) increase in myeloblasts. In general, a substantial change in either overall bone marrow cellularity or degree of reticulin fibrosis/osteosclerosis was not evident. However, most patients displayed a change in megakaryocyte morphology as well as distribution pattern from large, round, and clumped at the time of MMM diagnosis to small, condensed, and nonclumped at the time of LT (Figure 1C,E).
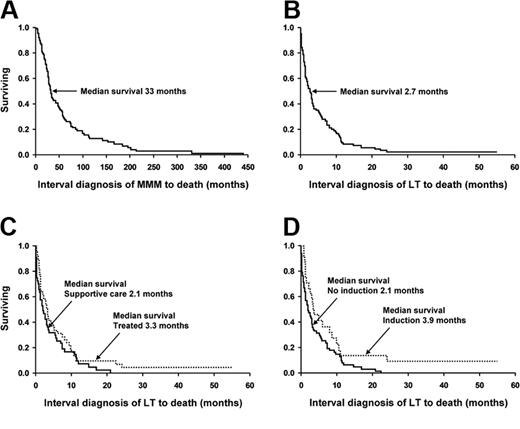
Kaplan-Meier survival curves of 91 patients with myelofibrosis with myeloid metaplasia. The survival curves show data from (A) the diagnosis of myelofibrosis with myeloid metaplasia (whole cohort); (B) the time of leukemic transformation (whole cohort); (C) the time of leukemic transformation (stratified by having received treatment versus supportive care alone); and (D) the time of leukemic transformation (stratified by having received induction chemotherapy).
Kaplan-Meier survival curves of 91 patients with myelofibrosis with myeloid metaplasia. The survival curves show data from (A) the diagnosis of myelofibrosis with myeloid metaplasia (whole cohort); (B) the time of leukemic transformation (whole cohort); (C) the time of leukemic transformation (stratified by having received treatment versus supportive care alone); and (D) the time of leukemic transformation (stratified by having received induction chemotherapy).
Outcome and treatment of leukemic transformation
Complete follow up was available in all 91 patients of which 98% (n = 89) have died from their disease- or treatment-related complications. Figure 2 outlines survival curves from time of initial MMM diagnosis (panel A), from the time of LT diagnosis (panel B), and comparison of survival curves between supportive care alone and actively treated (panel C) as well as between those who did and did not receive AML-like induction chemotherapy (panel D). Overall survival after LT was dismal with a median of 2.6 months (range, 0-24.2 months; Figure 2B). Specifically, mortality after LT (regardless of treatment intervention) was 26%, 52%, 70%, and 91% at 1, 3, 6, and 12 months, respectively, with only 2 patients (2.1%) alive with disease more than 2 years after LT and 41 and 57 months following treatment with either cytarabine-based induction chemotherapy or allogeneic transplantation.
Representative bone marrow histology in myelofibrosis with myeloid metaplasia (MMM) both at times of initial diagnosis and during leukemic transformation (LT). (A) Representative patient at time of diagnosis of MMM (B) Patient from panel A at time of LT. (C) Patient from panel A after induction chemotherapy reverting to chronic-phase MMM. (D) Focal transformation (top right-hand corner) in a background of typical MMM-related histologic changes (E) Megakaryocytes at time of diagnosis of MMM (large and clustered) compared with the smaller megakaryocytes seen in LT (B). (F) Focal residual leukemia (bottom right-hand corner) in background of regeneration in a patient treated with induction chemotherapy. Images were acquired with a Leitz Wetzler Orthoplan microscope (Leitz, Wetzler, Germany), with a 40×/0.75 NA objective, using an Orthocam camera, and processed with Adobe Photoshop 7.0 (San Jose, CA). Cells were stained with hematoxylin and eosin.
Representative bone marrow histology in myelofibrosis with myeloid metaplasia (MMM) both at times of initial diagnosis and during leukemic transformation (LT). (A) Representative patient at time of diagnosis of MMM (B) Patient from panel A at time of LT. (C) Patient from panel A after induction chemotherapy reverting to chronic-phase MMM. (D) Focal transformation (top right-hand corner) in a background of typical MMM-related histologic changes (E) Megakaryocytes at time of diagnosis of MMM (large and clustered) compared with the smaller megakaryocytes seen in LT (B). (F) Focal residual leukemia (bottom right-hand corner) in background of regeneration in a patient treated with induction chemotherapy. Images were acquired with a Leitz Wetzler Orthoplan microscope (Leitz, Wetzler, Germany), with a 40×/0.75 NA objective, using an Orthocam camera, and processed with Adobe Photoshop 7.0 (San Jose, CA). Cells were stained with hematoxylin and eosin.
For the purposes of discussion, we have categorized the patients into 3 treatment categories of supportive care (48 patients), low-intensity chemotherapy (19 patients), and AML-like induction chemotherapy (24 patients; Tables 4, 5). Therapy was chosen by the treating physician based upon clinical judgment and patient's performance status. Overall, survival was similarly poor in all 3 categories (Figure 2C-D). Supportive therapy included the use of erythrocyte or platelet transfusions, antibiotic therapy, and in most instances oral chemotherapy with hydroxyurea to prevent leukostasis. Median survival in this group was 2.0 months (range, 0-20.1 months). The goal of low-intensity therapy was palliation instead of remission. The treatment regimens in this regard (Table 4) were selected on the basis of performance status and patient choice. None of these low-intensity regimens produced a demonstrable and sustained response (median survival, 2.9 months; range, 0.4-22.5 months).
Treatment details of 91 patients with MMM who underwent LT
Treatment details . | No. (%) . |
|---|---|
| Supportive care only, transfusions ± hydroxyurea | 48 (53) |
| Noninduction chemotherapy | 19 (21) |
| Weekly vincristine, 2 mg/m2/weekly | 9 (47) |
| Oral alkylators such as melphalan or busulfan | 3 (16) |
| Low-dose cytarabine, subcutaneous | 2 (11) |
| Oral etoposide | 1 (5) |
| Other | 4 (21) |
| Induction chemotherapy | 24 (26) |
| Continuous infusion cytarabine, 5-7 d, plus anthracycline, 2-3 d | 18 (75) |
| High-dose cytarabine, greater than 1000 mg/m2/dose | 3 (13) |
| Mitoxantrone, VP-16, high-dose cytarabine | 3 (13) |
| Gemtuzumab | 3 (13) |
Treatment details . | No. (%) . |
|---|---|
| Supportive care only, transfusions ± hydroxyurea | 48 (53) |
| Noninduction chemotherapy | 19 (21) |
| Weekly vincristine, 2 mg/m2/weekly | 9 (47) |
| Oral alkylators such as melphalan or busulfan | 3 (16) |
| Low-dose cytarabine, subcutaneous | 2 (11) |
| Oral etoposide | 1 (5) |
| Other | 4 (21) |
| Induction chemotherapy | 24 (26) |
| Continuous infusion cytarabine, 5-7 d, plus anthracycline, 2-3 d | 18 (75) |
| High-dose cytarabine, greater than 1000 mg/m2/dose | 3 (13) |
| Mitoxantrone, VP-16, high-dose cytarabine | 3 (13) |
| Gemtuzumab | 3 (13) |
Survival among 91 patients with MMM with LT
. | Median mos (95% confidence interval) . |
|---|---|
| All patients with LT | |
| Survival from diagnosis of MMM | 33 (27.8-47.7) |
| Survival from LT | 2.7 (1.6-3.6) |
| Supportive care only, transfusions ± hydroxyurea | |
| Survival from diagnosis of MMM | 34.5 (28.9-57.6) |
| Survival from LT | 2.1 (1.1-3.4) |
| Noninduction chemotherapy | |
| Survival from diagnosis of MMM | 27.2 (14.5-48.7) |
| Survival from LT | 2.9 (0.8-5.3) |
| Induction chemotherapy | |
| Survival from diagnosis of MMM | 32.9 (21.7-57.4) |
| Survival from LT | 3.9 (1.6-8.9) |
. | Median mos (95% confidence interval) . |
|---|---|
| All patients with LT | |
| Survival from diagnosis of MMM | 33 (27.8-47.7) |
| Survival from LT | 2.7 (1.6-3.6) |
| Supportive care only, transfusions ± hydroxyurea | |
| Survival from diagnosis of MMM | 34.5 (28.9-57.6) |
| Survival from LT | 2.1 (1.1-3.4) |
| Noninduction chemotherapy | |
| Survival from diagnosis of MMM | 27.2 (14.5-48.7) |
| Survival from LT | 2.9 (0.8-5.3) |
| Induction chemotherapy | |
| Survival from diagnosis of MMM | 32.9 (21.7-57.4) |
| Survival from LT | 3.9 (1.6-8.9) |
For all patients with LT, n = 91; for supportive care only, n = 48; for noninduction chemotherapy, n = 19; and for induction chemotherapy, n = 24.
Twenty-four patients (26%) received AML-like induction chemotherapy (Table 4). Treatment-related mortality in this cohort was substantial (33%). Although several patients achieved posttreatment aplasia (ie, day-14 marrow after initiating therapy) without evidence of leukemia, none proceeded to complete remission. There were 10 patients (41%) who displayed posttreatment changes of bone marrow histology consistent with chronic-phase disease without an increase in blast percentage (Figure 1C,F). Among this latter group, survival after induction was a median of 6.2 months (95% confidence interval [CI] 1.4-10.9) as opposed to a median of 2.7 months (95% CI 1.1-3.6; P = not significant [NS]) for those patients who did not significantly reduce the marrow blast percentage. Additionally, among those who cleared the marrow of excess blasts (n = 10), 5 rapidly relapsed to acute leukemia whereas the rest died of end-stage MMM, profound cytopenias, or residual complications from induction. The use of either newer and presumably less toxic induction agents (gemtuzumab ozogamicin, R115777, on a clinical trial) or even autologous stem cell transplantation did not result in a better outcome. Median survival in patients receiving AML-like induction chemotherapy was 3.9 months (range, 1.6-57 months; Figure 2D).
Discussion
The current study was not designed to determine the rate of LT in MMM because only patients that were evaluated at the time of the particular event were considered. Instead, a detailed account of the clinical and laboratory features of LT, in the largest cohort of MMM patients ever studied, is presented. The representative nature of the study group is reflected by the expected distribution of baseline characteristics, at initial diagnosis of MMM, in terms of prognostic score categories, age, and cytogenetic findings.10-12 The overall findings were sobering in regards to both survival and treatment response. Almost all (98%) affected patients died after a median of fewer than 3 months and not a single patient achieved complete remission from standard induction chemotherapy. Such an outcome might be considered even worse than expected from other instances of poor-risk AML and possibly related to the high incidence of AML-M7 and AML-M0.13-15
It is difficult from the current uncontrolled study to suggest risk factors for LT in MMM. However, the relatively high proportion of patients (48%) who displayed circulating blasts during the initial diagnosis of MMM raises the possibility of significant association and is consistent with the previously recognized detrimental effect of the particular laboratory trait to survival in MMM.5,7 In general, there is limited information regarding risk factors for LT in MMM but both leukocytosis and abnormal karyotype have previously been implicated.10 However, the prognostic value of cytogenetic findings in MMM has been controversial and the discrepancies from various studies might relate to the presence of prognostically diverse specific lesions.11 Karyotypic information from the current study does not necessarily implicate any one abnormality as being associated with LT in MMM but instead confirms the process of clonal evolution and the appearance of new lesions that are infrequently detected at the time of initial diagnosis. Whether such lesions impart disease-promoting activity or reflect genetic instability during clonal progression is currently not delineated.
Another issue of relevance for LT in myeloproliferative disorders is its possible association with specific cytoreductive treatment agents. In the current study, 26% of patients had received potentially leukemogenic agents including radioactive phosphorus and oral alkylating agents, whereas 52% had received hydroxyurea prior to LT. However, short of a controlled study, it is difficult to separate drug leukemogenicity from disease-intrinsic clonal evolution that is associated with active disease.16 Another therapeutic modality in MMM that has been implicated in accelerating the occurrence of LT is splenectomy.17 However, the particular contention has not been supported by observations from other studies.10,18 In the current study, approximately one third of the patients with LT had been previously splenectomized without any evidence of temporal association with blastic transformation. The natural history of MMM, although quite variable depending upon presentation features, has been addressed by several large series and none have suggested the influence of treatment on either LT or survival.10,19,20
The major clinical findings from the current study demonstrate that LT in MMM usually occurs in the setting of advanced disease and is associated with dismal prognosis as well as poor response to current therapy. In particular, AML-like induction chemotherapy was associated with a high incidence of treatment-related mortality (33%) and did not appear to be superior to supportive care management. Allogeneic stem cell transplantation was used in only one patient. Age, comorbidities, organ dysfunction, lack of a suitable donor, concern of reduced efficacy of allogeneic transplantation in the setting of high disease burden, and the need for significant cytoreduction prior to transplantation limited this therapy being employed in the remaining patients. Given the poor outcomes we observed, it is indeed a small subset of patients who would (a) be a candidate for induction chemotherapy and (b) achieve a significant cytoreduction with induction and remain a candidate for transplantations after induction-related toxicities. This report is unable to address the utility of allogeneic transplantation as salvage therapy for transformed MMM. Nevertheless, our data would suggest, given the rapid mortality after LT, if a transplantation is to be considered in these patients, waiting until the time of LT is probably less than optimal. Although these observations are likely to discourage the consideration of aggressive chemotherapy in the majority of the patients, such therapy should not be discounted in the presence of either “favorable in AML” cytogenetic lesions (2 patients developed core-binding factor AML) or the possibility to offer allogenic stem cell transplantation for selected patients (41% reverted into chronicphase disease). Otherwise, participation in experimental treatment protocols is strongly advised. Ultimately, the best chance for overall success might reside in the development of effective therapy for chronic-phase disease.
Prepublished online as Blood First Edition Paper, September 23, 2004; DOI 10.1182/blood-2004-07-2864.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal