Abstract
Graft-versus-host disease (GVHD) remains a significant complication of allogeneic bone marrow transplantation (allo-BMT). Acute GVHD is mediated by immunocompetent donor T cells, which migrate to lymphoid tissues soon after infusion, recognize host alloantigens, and become activated upon interaction with host antigen-presenting cells (APCs). Recent work from our group and others suggests that activated effector T cells exit lymphoid tissues and traffic to mucosal sites and parenchymal target organs such as the gastrointestinal (GI) tract, liver, lung, and skin where they cause tissue damage. The molecular interactions necessary for effector cell migration during GVHD have become the focus of a growing body of research, as these interactions represent potential therapeutic targets. In this review we discuss chemokine and chemokine receptor interactions and adhesion molecules that have been shown to play roles in effector cell migration in experimental GVHD models, and we discuss a potential model for the role of chemokines during the activation phase of GVHD.
Introduction
Graft-versus-host disease (GVHD) remains a significant complication of allogeneic bone marrow transplantation (allo-BMT) and limits the clinical applicability of transplantation. Three phases have been described in acute GVHD.1,2 In the conditioning phase, host tissues are damaged by the pretransplantation conditioning regimen. Conditioning regimens up-regulate inflammatory mediators, including interleukin 1 (IL-1) and tumor necrosis factor-α (TNFα), and adhesion molecules and enhance the expression of major histocompatibility complex (MHC) and costimulatory molecules by tissue antigen-presenting cells (APCs). In the activation phase, donor T cells interact with host APCs, leading to activation and differentiation toward a T helper 1–T cytotoxic 1 (TH1/TC1) effector pathway and migration of these cells to target tissues affected during acute GVHD. The effector phase involves target organ damage by cytolytic effector mechanisms such as TNFα, perforin and granzyme, Fas/FasL (CD95/CD95L), and reactive oxygen species. This review focuses on the migration of donor T cells during the activation phase of GVHD and the proteins that are important for this.
Migration of donor cells during experimental GVHD
Our group tracked the migration of enhanced green fluorescent protein (eGFP) transgenic donor cells during the first week after transplantation in a fully MHC-mismatched murine allo-BMT model.3 Interestingly, donor T cells partitioned to lymphoid tissues within hours after transplantation, independently of recipient conditioning and allogeneic disparity. Within 2 to 3 days after transplantation, allogeneic T cells expanded in lymphoid tissues. Between 3 and 7 days after transplantation, allogeneic T-cell numbers increased in GVHD target organs, including the gastrointestinal (GI) tract, liver, lung, skin, and bone marrow, and tissues not considered to be common GVHD target organs such as the CNS, gingiva, and nasal mucosa. These data suggested that donor T cells had seeded target organs at a level below the detection limits of the imaging system, and required 3 to 7 days to expand to detectable levels in situ, or that donor T cells had been activated in lymphoid tissues during the first days after transplantation and subsequently migrated into target organs. The latter notion is supported by data described in “Molecular interactions directing the migration of effector cells during GVHD,” demonstrating the presence, early after transplantation, of T-cell effector cytokine expression in spleen but not target organs such as liver and skin.4,5 Additionally, the sphingosine-1–phosphate receptor inhibitor FTY720, which prevents lymphocyte egress from lymphoid organs,6 inhibited target organ infiltration and GVHD lethality when administered, starting at the time of transplantation, in a murine model.7 The efficacy of FTY720 in reducing target organ infiltration suggests that T cells do not arise de novo from direct donor cell expansion in target organs, but from the migration of donor cells previously activated in lymphoid tissues.
Leukocyte migration paradigm
Lymphocytes migrate into secondary lymphoid tissues by a well-characterized, multistep process8 (Supplemental Video S1, available at the Blood website; see the Supplemental Video link at the top of the online article). The initial step involves reversible tethering and rolling of lymphocytes on the surface of specialized high endothelial venules (HEVs), primarily by the interaction of selectins and their carbohydrate ligands. Next, the rolling lymphocyte encounters chemokines linked to the lumenal surface of the HEVs by proteoglycans. Signaling through lymphocyte chemokine receptors leads to firm arrest of the lymphocyte on the HEV surface.9 Transmigration through the HEV wall and into the node is currently not well understood, but it may involve interactions between lymphocyte integrins and junctional adhesion molecules, as well as CD31 and CD99, which localize to the intercellular junctions between high endothelial cells (reviewed in Johnson-Leger and Imhof10 ).
While the migration of T cells by HEVs has been well characterized, the migration of leukocytes into parenchymal organs during inflammation is less well understood. This process likely involves changes in vascular permeability and, in certain systems, has been shown to require specific selectin-ligand, chemokine-receptor, and integrin-ligand interactions.
Introduction to leukocyte migration
Selectins
The selectins, L-, P-, and E-selectin, are a family of C-type lectins expressed primarily by leukocytes and endothelial cells, which bind specific carbohydrate epitopes (reviewed in Kansas11 ). The interaction of selectins with their ligands is characterized by a rapid on-off rate, leading to low-affinity adhesion of leukocytes to endothelial cells and rolling during blood flow.12 L-selectin is constitutively expressed on myeloid cells, naive lymphocytes, and central memory T cells. Carbohydrate ligands for L-selectin are the peripheral node addressins (PNAds). PNAds are constitutively present on HEVs and inducibly present at sites of chronic inflammation.13 E- and P-selectins are expressed on endothelial cells (as well as platelets in the case of P-selectin). P-selectin was previously shown to be constitutively expressed on lung endothelium14 and E-selectin on cutaneous endothelium.15 However, in response to inflammation, both are up-regulated on endothelial cells regardless of tissue location.13 The carbohydrate ligands for E- and P-selectins are expressed on various leukocyte subsets, including activated T cells.13,16,17 P- and E-selectins have been shown to play crucial roles in T-cell recruitment to inflamed skin and synovium,18 but their role in recruiting effector cells to GVHD target organs is not yet clear.
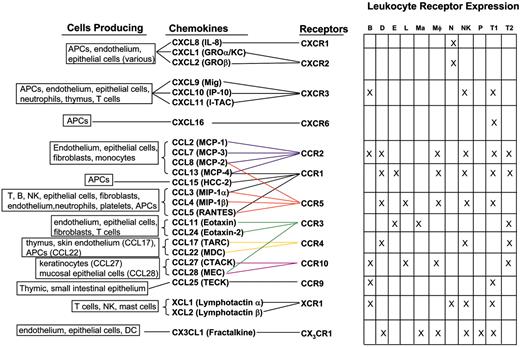
Inflammatory chemokines and receptors.20,43-93 APC indicates antigen-presenting cell; B, B cell; D, dendritic cell; E, eosinophil; L, Langerhan cell; Ma, mast cell; Mφ, macrophage; N, neutrophil; NK, natural killer cell; P, platelet; T1, TH1/TC1 cell; and T2, TH2/TC2 cell. DC indicates dendritic cell; GRO, growth-related oncogene; I-TAC, inducible T cell alpha chemoattractant; MCP, macrophage chemotactic protein; HCC, hemofiltrate CC chemokine; MIP, macrophage inflammatory protein; RANTES, regulated on activation normal T cell expressed and secreted; TARC, thymus and activation regulated chemokine; MDC, macrophage-derived chemokine; CTACK, cutaneous T-cell–attracting chemokine; MEC, mucosae-associated epithelial chemokine; and TECK, thymus-expressed chemokine.
Inflammatory chemokines and receptors.20,43-93 APC indicates antigen-presenting cell; B, B cell; D, dendritic cell; E, eosinophil; L, Langerhan cell; Ma, mast cell; Mφ, macrophage; N, neutrophil; NK, natural killer cell; P, platelet; T1, TH1/TC1 cell; and T2, TH2/TC2 cell. DC indicates dendritic cell; GRO, growth-related oncogene; I-TAC, inducible T cell alpha chemoattractant; MCP, macrophage chemotactic protein; HCC, hemofiltrate CC chemokine; MIP, macrophage inflammatory protein; RANTES, regulated on activation normal T cell expressed and secreted; TARC, thymus and activation regulated chemokine; MDC, macrophage-derived chemokine; CTACK, cutaneous T-cell–attracting chemokine; MEC, mucosae-associated epithelial chemokine; and TECK, thymus-expressed chemokine.
Chemokines and their receptors
Chemokines are a large family of predominantly 8- to 12-kDa proteins which primarily function as leukocyte chemoattractants.19,20 These proteins have additional functions in processes such as angiogenesis (reviewed in Belperio et al21 ), hematopoiesis (reviewed in Youn et al22 ), and immune cell activation (reviewed in Luther and Cyster23 ). The family is subdivided according to the number and position of NH2-terminal cysteine (C) residues. The majority of chemokines fall into the CC (CCL1-28) and CXC (CXCL1-16) subfamilies, while the C family contains only 2 members (XCL1 and XCL2) and CX3C only 1 member (CX3CL1).24,25 These proteins exert their effects through interactions with a family of 7 transmembrane domain-containing G-protein coupled receptors (GPCRs). Signaling by chemokine receptors is mediated by heterotrimeric G-proteins containing Gαi. These activate protein and lipid kinases such as mitogen-activated protein (MAP), Janus kinase–signal transducer and activator of transcription (JAK-STAT), and phosphatidyl inositol-3-kinase (PI3K), which mediate actin cytoskeleton rearrangement, changes in integrin affinity and avidity, leukocyte proliferation, differentiation, and apoptosis (reviewed in Mellado et al26 ). There is significant redundancy in the chemokine system as shown by the binding of multiple chemokines to a particular receptor and multiple receptors interacting with a particular chemokine. There are currently 10 identified CC chemokine receptors (CCR1-10), 6 CXC receptors (CXCR1-6), 1 C receptor (XCR1), and 1 CX3C receptor (CX3CR1).24,25
Three functional classes of chemokines and receptors have been defined.19 The homeostatic family includes those molecules functioning specifically in the migration of leukocytes to and within lymphoid tissues, as well as those functioning in hematopoiesis, including chemokines such as CCL19 and CCL21, which bind to CCR7,27-33 CXCL12 and its receptor CXCR4,34-39 and CXCL13 and its receptor CXCR5.40-42 The inflammatory family includes a wide array of molecules involved in the migration of innate and adaptive immune effector cells to sites of inflammation (Figure 1). Chemokines in this group are expressed in response to a host of inflammatory stimuli, including pathogen-associated molecular products, and inflammatory cytokines such as TNFα and type I and type II interferons.19 These chemokines are expressed by tissue cells, fibroblasts, endothelial cells, dendritic cells, monocytes, NK cells, and T cells. The dual function family includes CCL1, CCL17, CCL25 (and receptors CCR8, CCR4, and CCR9, respectively), CXCL9 to CXCL11 (interacting with CXCR3), and CXCL16 (interacting with CXCR6) which function both in inflammation and in the migration of T cells within the thymus.19 Several chemokines within this group are involved in the recirculation of memory T cells restricted to specific tissues such as the small intestine (CCL25/CCR9)43,44,94 and skin (CCL17, CCL22/CCR4).46,47
The cellular makeup of infiltrates at a site of inflammation is determined both by the chemokines induced at the site and the responsiveness of various cell types to those chemokines. After activation within secondary lymphoid tissues, T cells down-regulate CCR7 and become less responsive to chemokines involved in trafficking of naive cells to lymphoid tissues. Activated T-effector cells up-regulate different sets of receptors for inflammatory chemokines.95 TH1/TC1 cells preferentially express CCR1, CCR2, CCR5, CXCR3, and CXCR6, whereas TH2/TC2 cells preferentially up-regulate CCR4 and some studies indicate CCR3 (Figure 1).
Responsiveness to chemokines is further regulated by desensitization of receptors after chemokine binding. Homologous desensitization occurs in a ligand-dependent manner and involves phosphorylation of the receptor by specific G-protein–coupled receptor kinases (GRKs), followed by β-arrestin–mediated targeting for endocytosis.96 Ligand-independent desensitization also occurs, when stimulation of a heterologous GPCR leads to phosphorylation of others through second messenger-dependent kinases such as protein kinase C, and subsequent G-protein decoupling.97 In this manner, cross talk between chemokine receptors has been demonstrated.98,99
Integrins
The integrins are a family of heterodimeric transmembrane proteins functioning in cell-cell and cell–extracellular matrix (ECM) adhesion. There are currently 18 known α subunits and 8 β subunits in mammals.100 The integrins relevant to the immune system include those in the β1, β2, and β7 families (Table 1). The β2 family is restricted to leukocytes102 and includes αLβ2 (CD11a/CD18, LFA-1 [lymphocyte function antigen-1]), αMβ2 (CD11b/CD18, Mac-1), αXβ2 (CD11c/CD18), and αDβ2 (CD11d/CD18). The αLβ2 integrin is expressed constitutively on all leukocytes, whereas the other members of this family are restricted to various subsets. The β2 integrins bind ligands in the immunoglobulin superfamily, expressed by leukocytes and endothelial and epithelial cells, called intracellular adhesion molecules, (ICAM-1 to -3 [CD54, CD102, CD50]).101 The β1 integrins are expressed on a wide array of cells, including immune and inflammatory cells, and they primarily bind ECM components such as collagen and fibronectin, as well as the vascular cell adhesion molecule, VCAM-1 (CD106).101,103 The β7 integrins are expressed on lymphocytes residing in the intestinal mucosa, including lamina propria and intraepithelial lymphocytes. These integrins bind ligands such as mucosal addressin cell adhesion molecule-1 (MadCAM-1) and E-cadherin, expressed on mucosal endothelium and intestinal epithelial cells, respectively.8,101
Integrins function in both tethering and rolling of leukocytes on endothelium through low-affinity and avidity interactions and in the firm arrest and transmigration of leukocytes through the vascular wall.104 Most integrins expressed on circulating leukocytes exist in low-affinity states. Stimuli such as chemokine-receptor interactions, initiate intracellular signaling events (“inside-out” signaling), which rapidly and transiently induce both conformational changes and clustering of integrins at the site of cell-cell or cell-ECM contact, resulting in increased affinity and avidity.105 This results in rapid and transient adhesion of rolling leukocytes to the ligand-expressing vascular endothelium.9 Signaling leading to integrin clustering involves activation of PI3K,105 the guanosine triphosphatase (GTPase) Rap-1,106,107 the Rac-1 guanine nucleotide exchange factor Vav-1 (in T cells),108 and the Ca++-sensitive protease, calpain.105 The net effect of these molecular events is the release of integrins from their attachments to the cytoskeleton and increased lateral mobility in the plasma membrane. Inside-out signaling events leading to conformational changes that enhance integrin affinity are not yet clear. Upon ligand binding, integrins activate phospholipase Cγ and tyrosine kinases, including Lck, FAK (focal adhesion kinase), Pyk2, and ZAP70 (ζ-associated protein 70), ultimately leading to activation of Rap1, Rho-family GTPases, and myosin light chain kinase, which play roles in cytoskeletal rearrangements important in cell adhesion and migration (reviewed in Hogg et al104 ).
The involvement of specific integrins in cell migration is determined by their expression on specific cell types, the regulation of their ligand affinity or avidity, and the site-specific expression or deposition of their ligands. For example, αLβ2 is constitutively expressed on naive and activated lymphocytes, and it functions primarily in migration into lymphoid tissues by HEVs, which express high levels of ICAM-1.100 Certain β1 integrins (the VLAs, or very late activation antigens), are expressed after T-cell activation and function in migration on ECM components such as collagen and fibronectin, which are deposited during inflammatory processes.100,101
In addition to their role in leukocyte migration, integrins function in T-cell activation by stabilizing the interaction of T cells with APCs. The interaction of αLβ2 integrin on T cells with ICAM-1 expressed on APCs is a critical component of the immunologic synapse (reviewed in Sims and Dustin109 ). Furthermore, integrins can provide costimulatory signals to T cells during activation, facilitating proliferation and acquisition of effector functions.100
Molecular interactions directing the migration of effector cells during GVHD
Chemokines and chemokine receptors
Lymphoid tissues. Our group has shown that T cells infiltrate into lymphoid tissues within hours of administration. The proteins involved in this early phase of migration have not yet been determined.3 Within 3 days after transplantation, however, several studies have demonstrated up-regulation of proinflammatory chemokines such as CCL2, CCL3, CCL4, CCL5 and CXCL9, CXCL10, and CXCL11 in lymphoid tissues, suggesting that after activation, alloreactive T cells may respond to proinflammatory chemokines to recirculate to these sites during GVHD.110-112 Expression of CXCL9, CXCL10, and CXCL11 preceded that of other chemokines.111,112 Expression of all chemokines increased earlier in spleen than in liver and lung,111-113 consistent with earlier expansion of donor cells at this site. Interestingly, production of CCL3 in spleen required expression by donor T cells themselves.111
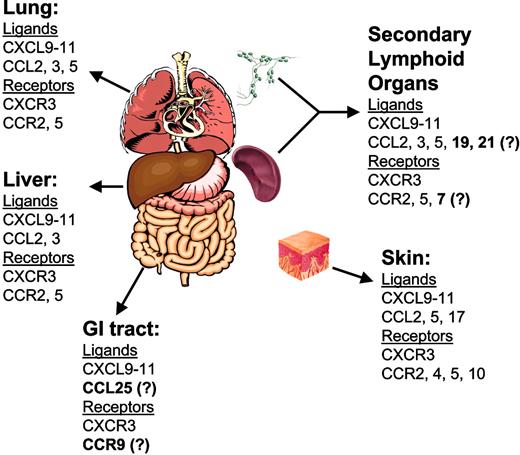
Chemokines and receptors in GVHD target organs. Boldface text denotes chemokines and receptors whose role in acute GVHD has not been demonstrated experimentally.
Chemokines and receptors in GVHD target organs. Boldface text denotes chemokines and receptors whose role in acute GVHD has not been demonstrated experimentally.
Specific proinflammatory chemokine-receptor interactions have been examined with regard to lymphoid homing during GVHD (Figure 2). CCR5 expression on donor T cells was shown to play a critical role in their accumulation in lymphoid tissues, including the spleen and Peyer patch (PP), at day 14 during GVHD in nonconditioned recipients.112,114 Similarly, eliminating the expression of a CCR5 ligand, CCL3, from donor T cells resulted in reduced CD8+ T-cell accumulation in spleen in a sublethally conditioned GVHD model.111 However, this was not the case in lethally conditioned recipients, because eliminating CCR5 expression on or CCL3 production by donor T cells did not significantly affect their accumulation in the PP and actually modestly increased their accumulation in the spleen.112,115,116
Elimination of CXCR3 expression from donor T cells in conditioned models resulted in increased accumulation of T cells in the spleen, with a concomitant reduction of accumulation in small bowel and lung.117,118 The overall graft-versus-host response was not different in recipients of wild-type versus CXCR3-/- T cells. Therefore, CXCR3 was not required for recruitment and expansion of T cells in lymphoid tissues, but it was required for migration from those sites to these parenchymal target organs.
CCR2 expression did not affect the accumulation of CD4+ T cells in the spleen, as determined by the transfer of allogeneic CCR2-deficient CD4+ T cells to sublethally conditioned recipients in a GVHD model. However, when CCR2-deficient whole splenocytes were transferred, a significant increase in activated CD4+ T cells was observed in the spleen, suggesting that CCR2 affects the recruitment or function of a regulatory population that does not express CD4.119
Liver. Several studies have demonstrated expression of the chemokine ligands CCL2, CCL3, CCL4, CCL5, CXCL9, CXCL10, and CXCL11 in the liver during experimental GVHD.4,110-112,120 In studies using conditioned murine GVHD models, a consistent pattern in the kinetics of expression of these ligands was observed. Ligands, including CCL2 and CXCL9 to CXCL11, were expressed early after transplantation. CXCL9, CXCL10, and CXCL11 are interferon-γ (IFN-γ) inducible, yet IFN-γ protein was not expressed in liver during the time frame in which these ligands were expressed. IFN-γ protein was detected in the spleen during this time frame, suggesting that the expression of chemokines could be induced in the liver by endocrine effects of IFN-γ.4 The roles of CXCL9, CXCL10, and CXCL11 in recruitment of alloreactive T cells to liver are not yet clear, although elimination of CXCR3, the receptor that binds these ligands, from donor T cells resulted in reduced liver pathology in a murine model of GVHD induced by minor histocompatibility antigen (mHA) mismatch.117 The roles of CCL2 and its primary receptor CCR2 in liver GVHD are similarly unclear. Elimination of CCR2 from donor T cells did not affect liver pathology, although pathology was assessed at a late time point after transplantation.121 As CCL2 expression in liver was demonstrated early after transplantation,4 its effect on T-cell infiltration may occur prior to the time point assessed in this study.
CCL3 and its primary receptor on TH1/TC1 T cells, CCR5, have been shown to play critical roles in liver GVHD in nonconditioned and reduced-intensity conditioned murine GVHD models. CCL3 production in liver during GVHD was shown to be dependent on expression by donor T cells.111 Expression of CCL3 occurred later compared with the expression of CXCL9, CXCL10, and CXCL11 and correlated with donor T-cell infiltration.111,112,120 Studies using either antibody-mediated neutralization of CCL3 or transfer of CCL3-/- donor T cells resulted in reduced CD8+ T-cell infiltration into liver.111,120 This correlated with lower serum alanine aminotransferase and GVHD-specific liver pathology, respectively, demonstrating that a feedback mechanism exists in which CCL3 production by liver-infiltrating T cells serves to recruit additional CD8+ cytotoxic T cells. Interestingly, CD4+ T-cell infiltrates were not significantly affected by treatment of recipient mice with anti-CCL3 mAb and were increased when CCL3-/- donor cells were used, indicating that CD4+ and CD8+ T cells may use different chemokines and receptors during recruitment to the liver.
The expression of several receptors found on T cells, NK cells, and macrophages, including CXCR3, CCR1, CCR4, and CCR5, were shown in the liver during the first 2 weeks after donor cell transfer. The kinetics of CCR5 expression correlated with donor T-cell infiltration in this model. Antibody-mediated blocking of CCR5 resulted in selective inhibition of CD8+ T-cell infiltration in the nonconditioned model. This finding was confirmed by using CCR5-/- (eGFP+) donor cells, although in the latter study differences in CD4+ and CD8+ T-cell infiltrates were not addressed.114 The clear role of CCR5 in directing the migration of donor T cells to the liver during GVHD in nonconditioned mice correlated with high-level expression of the CCR5 ligands CCL3 and CCL4 in the livers of these mice.112 Interestingly, when recipients in this model were conditioned with a myeloablative dose of total body irradiation prior to transplantation, mice receiving CCR5-/- donor T cells suffered exacerbated GVHD112,116 and had significantly increased donor CD4+ and CD8+ T-cell infiltration in liver.112 Therefore, in the conditioned host, CCR5 expression on alloreactive T cells may function to down-modulate accumulation of alloreactive T cells in the liver and other sites of inflammation. Importantly, expression of CCR5 ligands in conditioned recipients did not achieve the high levels observed in nonconditioned recipients.112 Activated T cells from CCR5-/- mice had an increased migratory response as compared with wild-type cells to CXCL10 (a CXCR3 ligand).112 These data suggested that stimulation of CCR5 may cause heterologous desensitization of the CXCR3 receptor, and eliminating this receptor cross talk could affect effector cell recruitment to GVHD target organs. Thus, current data suggest that, in our model, migration of T cells to the liver is influenced early by the expression of the chemokine ligands CXCL9, CXCL10, and CXCL11, which are produced by host cells, but dependent on the receipt of alloreactive donor T cells. More than 1 week after transplantation, the chemokine ligands CCL3, CLL4, and CCL5, which are generated by donor T cells, appear to play a significant role in the recruitment of alloreactive T cells expressing CCR5.
Gastrointestinal tract. A comprehensive analysis of chemokine expression in the small intestine and colon during GVHD has not yet been performed. A recent study identified an important role for CXCR3 in infiltration of the small intestine during GVHD in a conditioned, mHA-mismatched GVHD model.117 Eliminating CXCR3 expression from donor T cells resulted in reduced pathology and infiltration of CD8+ T cells into the lamina propria and epithelium at this site.
The cellular sources of chemokines in the GI tract may be different than in other target organs. For example, production of CCL3 was observed in the colon in murine allogeneic transplant recipients, but this was not dependent on expression by donor T cells.111 This suggests that the major sources of CCL3 in the colon during GVHD are the cells of the colonic mucosa itself, and that the CCL3-mediated feedback mechanism described in “Liver” may not play a significant role in recruitment of donor T cells to this site. As high levels of CCL3 were produced in the colon after allogeneic T-cell transfer, eliminating CCR5 expression from donor T cells was expected to significantly impair GI tract infiltration. In nonconditioned recipients, eliminating CCR5 expression from donor splenocytes did result in significantly reduced accumulation of donor cells in PPs and small intestinal epithelium.114 In conditioned recipients, eliminating CCR5 expression from donor T cells had no effect on the infiltration of donor T cells into the PPs or colon,112 again showing that the function of chemokines and chemokine receptors is different in conditioned compared with nonconditioned recipients. The expression of CXCL9, CXCL10, and CXCL11 and recruitment of CXCR3-expressing T cells may compensate for the loss of CCR5 in migration of T cells to the GI tract.
Other chemokines expressed in the GI tract may also play important roles in conditioned recipients. The chemokine CCL25 is expressed by small intestinal epithelium.43,44 This molecule interacts with CCR9, expressed on T cells activated within mucosal tissues such as PPs and the mesenteric lymph node (LN), to direct the specific migration of effector memory cells to the small intestine.122,123 Whether CCL25/CCR9 interaction plays a role in directing GI tract–specific T-cell recruitment during GVHD is an area of current research, but a central role for PPs in initiating GVHD has been proposed.114
Skin. A comprehensive analysis of chemokine and chemokine receptor expression in the skin in a lethally conditioned model of GVHD caused by mHA mismatch was recently performed.5 As in the liver, the IFN-γ–inducible chemokines CXCL9 and CXCL10 were expressed at high levels within the first week after allogeneic transplantation, although IFN-γ itself was not. CCL2 expression was up-regulated early after transplantation, as were CCL6, CCL7, CCL9, CCL11, and CXCL1. Also consistent with data in the liver, CCL5 was up-regulated later after transplantation (week 2), and its expression correlated with 2 of its receptors, CCR1 and CCR5. CCL17, which binds to CCR4 and has been shown, in conjunction with CCL27/CCR10, to play an important role in the recruitment of memory T cells to inflamed skin, was significantly up-regulated during the first week after transplantation.47 This finding raises the possibility that interactions such as CCL17/CCR4 and CCL27/CCR10 may participate in the tissue-specific migration of alloreactive T cells during GVHD. No definitive roles have yet been identified for specific chemokine and receptor interactions in the recruitment of GVHD effector cells to skin, although a requirement for CCR6 in the recruitment of donor Langerhan cell precursors to the epidermis after allogeneic BMT was recently demonstrated.124
Lung. Several studies have examined chemokine-receptor interactions involved in the recruitment of effector cells to the lung during GVHD and idiopathic pneumonia syndrome (IPS). Chemokines expressed in lung after allo-BMT include CXCL9, CXCL10, and CXCL11, CCL2, CCL3, CCL4, and CCL5, and CCL11.111-113 TNF-α production by infiltrating donor T cells was shown to be critical for proinflammatory chemokine expression in the lung after allo-BMT.125 The expression of CCL2, CXCL10, and CXCL11 were found prior to the expression of other chemokines such as CCL3 and CCL4.112,113,125 Antibody-mediated neutralization of CXCL9 or CXCL10 reduced IPS severity and CD8+ T-cell infiltrates. The effect of inhibiting these ligands was additive, and further inhibition was possible by eliminating expression of CXCR3 from donor T cells. Thus, as demonstrated in the liver, CXCR3 was critical for donor T-cell recruitment to lung after allo-BMT.118
CCL3 production in the lung was dependent on expression by allogeneic donor T cells.111 Significantly reduced recruitment of CCL3-/- CD8+ donor T cells to the lung was observed in sublethally conditioned recipients. Additionally, recruitment of CCR5-/- CD4+ and CD8+ donor T cells to lung was impaired in a nonconditioned model, demonstrating that lung-infiltrating donor T cells recruit additional donor T cells to this site through a CCL3/CCR5-mediated feedback mechanism.111,112 As in the liver, however, recruitment of donor T cells to lung was enhanced in lethally conditioned transplant recipients, receiving CCL3- or CCR5-deficient donor T cells.112,115 That this occurred only in conditioned recipients, likely related to differences in the relative expression of CXCR3 and CCR5 ligands in the lung after allo-BMT in conditioned versus nonconditioned recipients.112 Interestingly, eliminating CCL5 expression from donor T cells impaired their recruitment to the lung at late time points (4-6 weeks) after transplantation in a lethally conditioned model.126 In this study, CCR5 expression was reduced at late time points, while CCR1, which also binds CCL5, remained high.
The pathogenesis of IPS involves recruitment of host macrophages to the lung during the peritransplantation period, which is believed to contribute to alloantigen presentation to donor T cells infiltrating the lung, and to lung damage through cytokine production.127 CCL2 production in lung correlated with the early influx of host macrophages.113 However, eliminating CCL2 did not significantly affect host macrophage influx into lung or IPS severity early after transplantation.128 Interestingly, another study assessed the role of CCR2, the receptor for CCL2, on donor T cells and found that eliminating it reduced the severity of late-onset IPS.121 IPS was assessed at weeks 4 and 6, time points at which the investigators had demonstrated full donor chimerism in lung lymphocytes and macrophages. IPS at these time points was significantly reduced in recipients of CCR2-deficient T cells, correlating with reduced donor T-cell accumulation in the lung. Thus, although CCL2/CCR2 interaction does not play a role in early recruitment of host macrophages to lung after allo-BMT, this interaction has clear significance in the recruitment of donor T cells later. Thus, the roles of chemokines and chemokine receptors may differ, depending on when they are evaluated after allo-BMT.
Selectins and adhesion molecules
Lymphoid tissues. The complexity of the proteins involved in migration was underscored by Li et al129 who evaluated the functions of L-selectin (CD62L) and α4 integrins during GVHD. They did not find an effect on the occurrence of GVHD by targeting CD62L or α4 integrins alone. However, treating donor cells with anti-CD62L and anti–α4 integrin mAbs prior to infusion delayed GVHD, predominantly by delaying the homing of donor T cells to the mesenteric LN.129 This work underscores the important role of donor T-cell migration to secondary lymphoid tissue in the initiation of GVHD.
Liver. Very early up-regulation of ICAM-1 on endothelium was demonstrated in a mouse model of BMT across mHA barriers and was associated with increased numbers of infiltrating cells expressing αLβ2 integrin.130 Harning et al131 demonstrated in a parent-into-F1 (first filial generation) mouse model that a combination of anti-αLβ2 and anti–ICAM-1 antibodies prevented GVHD. This therapy decreased the number of T cells homing to the liver with a consequent decrease in the generation of IL-2 and IL-12 in the liver. Our group confirmed a role for αLβ2 by using an anti-αLβ2 mAb, along with cytotoxic T lymphocyte antigen 4 (CTLA4)–Ig, to prevent GVHD across fully mismatched MHC BMT in mice.132 Interestingly, our work using ICAM-1-/- recipients in a lethally conditioned MHC-mismatched GVHD model indicated that there is not a specific requirement for ICAM-1 in liver inflammation.133 Thus, the interaction of αLβ2 integrin with other ligands, such as ICAM-2 or ICAM-3, may contribute to the homing of alloreactive effector cells to liver during GVHD. The interaction of α4β1 integrin with VCAM-1 has also been shown to play a role in liver inflammation during GVHD.134
GI tract. The α4β7 integrin (lymphocyte PP adhesion molecule, or LPAM) and its ligand, MadCAM-1, expressed on HEVs in PPs and lamina propria, have been shown to be important in the homing of alloreactive T cells to the intestine during GVHD. Treatment of lethally irradiated recipients with anti-α1 and -α4 integrin mAbs, inhibited mucosal GVHD in a parent-into-F1 model.135 When allogeneic recipient mice, mismatched at minor or major antigens, were given
Lung. While the absence of ICAM-1 expression in recipient mice did not affect the incidence of GVHD, or the migration of alloreactive donor T cells to the liver, we did observe a marked decrease in IPS in ICAM-1-/- recipients.133 The influx of T cells, macrophages, and neutrophils and the production of inflammatory cytokines were dramatically decreased in the lungs of ICAM-1-/- recipients compared with wild-type recipients. In contrast, systemic levels of these cytokines were unaffected, and GVHD-related mortality was accelerated in ICAM-1-/- recipients. This implies that ICAM-1 plays a critical role in the generation of postallogeneic BMT inflammation in the lung, but it may be redundant in its function with other adhesion molecules in inducing GVHD in the liver and GI tract.
Discussion and conclusions
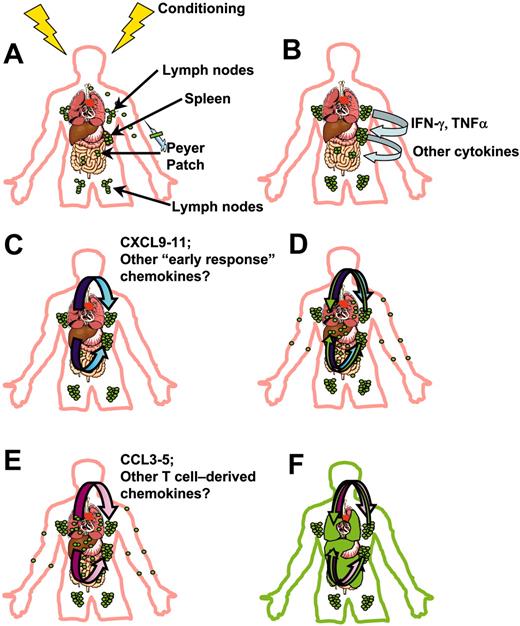
On the basis of our demonstration of the early events in donor cell migration during GVHD and quantitative and kinetic analyses of inflammatory chemokine and adhesion molecule expression in target organs, we propose a general model for the migration of effector cells during GVHD (Figure 3). Donor T cells initially migrate to spleen and peripheral lymphoid tissues within hours after transplantation, where they encounter host APCs matured by the effects of the conditioning regimen (Figure 3A). This initial migration may depend on homeostatic chemokines and adhesion molecules such as L-selectin and αLβ2 integrin. Interaction with those APCs leads to activation of alloantigen-specific donor T cells and their differentiation into TH1/TC1 effector cell types, expressing CCR2, CCR5, CXCR3, and CXCR6. During the first 2 to 3 days, activated, proliferating T cells produce inflammatory cytokines such as IFN-γ and TNFα within lymphoid tissues (Figure 3B), which enter the circulation and induce the production of chemokines in target organs by host cells. The inflammatory milieu that occurs after myeloablative conditioning appears to be quite important in early donor T-cell migration. The interferon-inducible chemokines, CXCL9, CXCL10, and CXCL11, produced in target organs such as liver, lung, and skin (Figure 3C), play primary roles in directing the early migration of activated, CXCR3-expressing T cells after egress from lymphoid tissues (Figure 3D). These chemokines likely direct the migration of effector cells until the host APCs producing them are eliminated.
Model for early donor T-cell migration during acute GVHD.3-5,111-113 (A) Recipients of allo-BM transplant receive conditioning therapy, followed by intravenous infusion of bone marrow or peripheral blood stem cells (containing mature donor T cells). Within the first hours after transplantation, donor T cells (green) accumulate in peripheral lymphoid tissues such as LNs, Peyer patches, and spleen. (B) Alloreactive donor T cells expand in peripheral lymphoid tissues, differentiating into TH1/TC1 effectors, and producing IFN-γ, TNFα, and other cytokines (light blue arrows). (C) Circulating IFN-γ synergizes with inflammatory mediators and bacterial products released into circulation by the conditioning regimen to induce production of interferon-inducible chemokines (blue gradient arrows) by APCs and endothelial and epithelial cells in target organs. (D) Activated effector T cells follow gradients of these chemokines during early target organ infiltration (green arrows). (E) Amplification of T-cell infiltrates: donor T cells having infiltrated target organs produce T-cell–tropic chemokines CCL3, CCL4, and CCL5 (and possibly others) (pink gradient arrows). (F) Effector cells continue to follow gradients of these chemokines to infiltrate target organs (green arrows), leading to tissue pathology and clinical manifestations of GVHD, represented by green infiltrates in GI tract, liver, lung, and skin.
Model for early donor T-cell migration during acute GVHD.3-5,111-113 (A) Recipients of allo-BM transplant receive conditioning therapy, followed by intravenous infusion of bone marrow or peripheral blood stem cells (containing mature donor T cells). Within the first hours after transplantation, donor T cells (green) accumulate in peripheral lymphoid tissues such as LNs, Peyer patches, and spleen. (B) Alloreactive donor T cells expand in peripheral lymphoid tissues, differentiating into TH1/TC1 effectors, and producing IFN-γ, TNFα, and other cytokines (light blue arrows). (C) Circulating IFN-γ synergizes with inflammatory mediators and bacterial products released into circulation by the conditioning regimen to induce production of interferon-inducible chemokines (blue gradient arrows) by APCs and endothelial and epithelial cells in target organs. (D) Activated effector T cells follow gradients of these chemokines during early target organ infiltration (green arrows). (E) Amplification of T-cell infiltrates: donor T cells having infiltrated target organs produce T-cell–tropic chemokines CCL3, CCL4, and CCL5 (and possibly others) (pink gradient arrows). (F) Effector cells continue to follow gradients of these chemokines to infiltrate target organs (green arrows), leading to tissue pathology and clinical manifestations of GVHD, represented by green infiltrates in GI tract, liver, lung, and skin.
Following this initial phase of production of chemokine ligands, a second wave of production is induced from recruited donor T cells. These T cells produce CCR5 ligands such as CCL3, CCL4, and CCL5, which serve to propagate target organ infiltration after CXCL9, CXCL10, and CXCL11 production subsides (Figure 3E-F). Initially, however, production of chemokines such as CCR5 ligands by donor T cells appears to desensitize CXCR3 and limits inflammation by limiting effector cell responsiveness to CXCL9, CXCL10, and CXCL11. The reverse may also be true, and CXCL9, CXCL10, and CXCL11 may at some point serve to limit responsiveness to chemokines such as the CCR5 ligands. The net effects may be dependent on the inflammatory conditions created by the conditioning regimen and the degree of antigen mismatch, which likely influence the chemokines and receptors that dominate the GVHD response.
Entry of effector T cells into the lung involves interactions between αLβ2/ICAM-1 and into the liver involves αLβ2/ICAM-1, -2, and -3 and α4β1/VCAM-1. Entry into the intestinal mucosa requires α4β7/MadCAM. As disease progresses in animals surviving the early posttransplantation period, changes may occur in the magnitude and types of cytokines, chemokines, chemokine receptors, and adhesion molecules expressed in target tissues and on effector cells. Thus, several weeks after transplantation, the molecular interactions directing the recruitment of effector cells to target organs may be quite different from those playing primary roles during the first weeks after transplantation. This model is based on findings in a parent-into-F1 transplantation model. Other chemokines and chemokine receptors may be critical for migration in other models.
While there has been increasing interest in the roles of various proteins in the migration of T cells during GVHD, the results of animal studies have often been confusing and in specific instances have yielded conflicting data. We believe that this model begins to address some of those contradictions. Our work shows that chemokine receptors have different functions in both limiting and exacerbating GVHD, depending on the use of conditioning therapy. Thus, comparisons between studies need to take into account the use and intensity of conditioning therapy. Additionally, in recipients of myeloablative conditioning, simultaneous targeting of multiple different chemokine ligands and receptors may be required to ameliorate GVHD target organ infiltration in recipient animals. As a corollary to this, preferential roles for specific chemokine ligand–receptor interactions may only be found in the absence of conditioning, or in the setting of reduced-intensity conditioning. Additionally the degree of MHC disparity may play a role in the production of chemokines by alloreactive donor T cells, confounding results found using different models. Finally, the current data suggest that certain chemokine-receptor and integrin-ligand interactions direct the recruitment of alloreactive donor T cells to a specific or selective set of target organs. Thus, the failure to find a broad effect on GVHD incidence in experimental systems does not preclude the involvement of a specific ligand or receptor in organ-specific T-cell infiltration.
Small-molecule chemokine receptor inhibitors are currently available or in development for the treatment of inflammatory disorders and as HIV coreceptor inhibitors (reviewed in Shaheen and Collman137 ). Anti–LFA-1 and -α4 mAbs are or should be available in the next year for the treatment of psoriasis and multiple sclerosis. These therapies may be evaluated in clinical trials for the treatment of GVHD. However, our studies suggest that a thorough knowledge of chemokine-receptor and adhesion molecule–integrin interactions important in target organ inflammation in multiple specific transplantation settings is critical prior to initiating these clinical trials. For example, targeting receptors important in migration after reduced-intensity allo-BMT may exacerbate GVHD after a fully myeloablative transplantation. Thus, the tools with which to prevent target organ inflammation through inhibition of chemotactic and adhesion molecules may already be in place, but they currently await a more thorough understanding of the function of this complex system during GVHD.
Prepublished online as Blood First Edition Paper, February 8, 2005; DOI 10.1182/blood-2004-12-4726.
Supported by from the National Institutes of Health (grants CA 58233 and CA 102052, J.S.S.; grants AI 34495, HL 56067, HL 55209, HL 63452, and HL 66308, B.R.B.).
The online version of the article contains a data supplement.