The chronic myeloproliferative diseases, polycythemia vera (PV), essential thrombocythemia (ET), chronic idiopathic myelofibrosis (IMF), and chronic myelogenous leukemia (CML), were grouped into a spectrum of pathogenetically related disorders of varying clinical manifestations by Dameshek in 1951.1 Recently, the World Health Organization has included the chronic eosinophilic leukemia/hypereosinophilic syndrome, chronic neutrophilic leukemia, and systemic mast cell disease (SMCD) within this grouping. Although the origin of CML has been traced to a dysregulated protein kinase, the product of the BCR/ABL oncogene,2 and that of SMCD to 1 of 2 dysregulated receptor tyrosine kinases, c-kit, or the platelet-derived growth factor (PDGF) A receptor,3 the molecular basis of the 3 classic disorders, PV, ET, and IMF, which as a group share more similarities with each other than with CML, has been more recalcitrant to solution. However, a major insight into the molecular basis for the enhanced myeloproliferation and clonal dominance that characterizes these disorders has now been reported; the answer is another kinase, and, best of all, it all makes sense! In recent issues of the Lancet,4 Cancer Cell,5 Nature,6 and the New England Journal of Medicine,45 a single, somatic mutation in the protein tyrosine kinase Janus kinase 2 (JAK2) appears responsible for many of the features of PV, ET, and IMF, a finding that promises to impact the diagnosis and treatment of patients with these disorders and to spur additional research into the origins of dysregulated cell growth and function.
The chronic myeloproliferative disorders share many features: the marrow is hypercellular and overproduces one or more of the formed elements of the blood in the absence of any apparent appropriate or pathologic stimulus; the exuberant hematopoiesis often also extends to one or more extramedullary sites. Based on studies using polymorphic genes, these disorders were shown to be clonal,7 thought to arise in a single, multipotent hematopoietic progenitor or stem cell, which comes to dominate the marrow and blood. Hematopoietic progenitor cells from the marrow or peripheral blood display altered growth properties, proliferating in serum containing cultures in the absence of exogenous hematopoietic growth factors.8 Marrow megakaryocytes are hyperplastic and variably dysplasia and are responsible for the myelofibrosis of these disorders, patients often display a tendency toward thrombotic and hemorrhagic complications in addition to their signs and symptoms related to expansion of each hematopoietic lineage. However, compared with patients with CML, transformation to acute leukemia is far less common, especially in the absence of therapy with known mutagenic agents. But despite these clinical and pathologic features, the diagnosis of an individual patient with isolated erythrocytosis or thrombocytosis is often difficult, and their treatment is usually a nonspecific suppression of hematopoiesis.
Although consistent chromosomal rearrangements have been the vital roadmap pointing investigators toward the molecular pathogenesis of CML and multiple types of acute myelogenous and lymphoid leukemia, these same clues have been neither abundant nor particularly informative in the study of chronic myeloproliferative disorders; without such signs, progress in understanding PV, ET, and IMF has been slow. Nevertheless, some successes have been reported. Instances of inherited, isolated erythrocytosis and pure thrombocytosis have been solved, traced to the loss of a negative regulatory domain of the erythropoietin (EPO) receptor,9 to an abnormality in an inhibitory regulator (von Hippel Lindau protein) of the key transcription factor for EPO, hypoxia inducible factor 1α,10 to abnormal splicing of thrombopoietin (TPO) mRNA resulting in enhanced translational efficiency and high levels of the primary humoral regulator of platelet production,11 or to 2 separate point mutations in the TPO receptor, c-Mpl.12,13 Nevertheless, none of these clinical instances are characterized by the clonal, pan-myeloid myeloproliferation that characterizes patients with chronic myeloproliferative disorders.
One of the most intriguing characteristics of PV has been endogenous erythroid colony (EEC) formation, in which erythroid progenitor cells obtained from the marrow or peripheral blood proliferate in semisolid, serum-containing cultures in the absence of exogenous EPO. Although initially described for patients with PV,8 similar results can be demonstrated in a significant proportion of patients with ET and IMF, but never in healthy individuals. Thus, EEC formation has become a relatively useful, albeit cumbersome diagnostic test for myeloproliferative disorders. Unfortunately, the assay is technically demanding and is positive in only about 35% to 80% of patients with these diseases.
Although initially believed to represent “EPO-independent” growth, subsequent studies revealed the phenomenon of EEC to represent hypersensitivity to the EPO present in the serum used in the cultures.14 These findings suggested that abnormalities of the EPO receptor might underlie PV. Unfortunately, except for the description of familial erythrocytosis noted earlier, the EPO receptor has been found to be completely normal in all patients with bona fide PV.15 Moreover, subsequent studies revealed that marrow and blood cells from patients with PV were hypersensitive not only to EPO but also to several other hematopoietic growth factors, including interleukin 3 (IL-3), stem cell factor (SCF), granulocyte-macrophage colony-stimulating factor (GM-CSF), insulin-like growth factor-1 (IGF-1), and TPO.16-19 These findings suggested that events downstream from receptor engagement might be responsible for EEC formation.
Protein tyrosine phosphorylation is vital for transduction of growth-promoting signals for a wide range of cellular mitogens. However, with the cloning of the EPO receptor in 1989,20 a conundrum emerged; unlike the receptors for well-characterized mitogens such as epidermal growth factor, insulin, and PDGF, which all contain cytoplasmic tyrosine kinase domains, the cloned EPO receptor failed to display any recognizable intracellular signaling motifs. With the cloning of the GM-CSF, granulocyte colony-stimulating factor, TPO, and multiple interleukin receptors, a family of receptors, termed the type I cytokine receptors, was born, and a mechanism for signaling was soon identified. Based on similarities with the interferon receptors, which use a novel kinase/signal transduction system termed the JAK/signal transducers and activators of transcription (STAT) pathway,21 it was quickly realized that the type I hematopoietic cytokine receptors also used JAKs and STATs to initiate signaling.22
First cloned (1989) based on homology to other kinase domains23 and playfully named JAK for “just another kinase,” within 5 years 60 publications appeared on the 4 members of the JAK family, a number which has grown to more than 2000 at present, attesting to the importance of these kinases in cytokine and growth factor signaling pathways. Certainly, proteins of such importance must have equally important names, leading to their being termed Janus kinases, for the Roman God of gates and passages. Structurally, JAKs are characterized by 3 critical domains, JH1, the kinase domain, resides near the carboxyl terminus of the protein. Immediately upstream of this lies the JH2 domain, which resembles the JH1 domain but is not an active kinase. Toward the amino terminus of JAKs lies a FERM (4-point-one, Ezrin, Radixin, Moesin) homology domain, which is responsible for noncovalent binding to the “box1/2 motifs” present in the juxtamembrane cytoplasmic region of type I cytokine receptors. It is not hyperbole to state that for this type of cell surface receptor, all signaling begins with JAK. The first target of kinase action is the initiating receptor itself, which once tyrosine phosphorylated provides a docking site for src homology 2 (SH2)–bearing secondary signaling molecules, such as nascent transcription factors (STATs), adaptor molecules (Shc, Gab/IRS [Grb2-associated binder/insulin receptor substrate]), and kinase regulatory subunits (p85 phosphoinositol-3-kinase), among others.
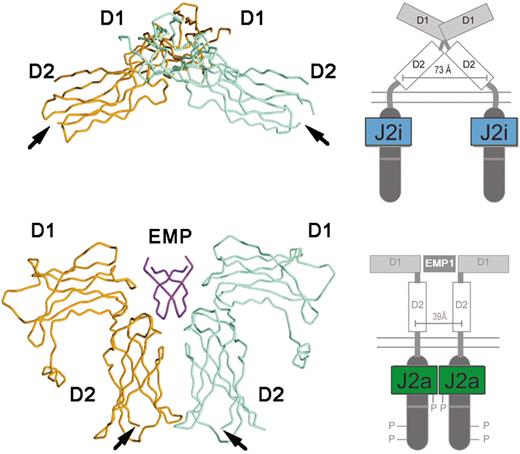
Much is known of the mechanism of JAK activation and regulation. Important insights into the effects of binding of cognate ligand to a type I cytokine receptor came with elucidation of the tertiary structure of the EPO receptor in the absence and presence of an activating ligand.24 In the unliganded state, the EPO receptor exists as a dimer, with the transmembrane domains separated by 73 Å. Upon EPO binding, or that of an EPO mimetic peptide, a large conformational shift ensues, bringing the cytoplasmic domains into closer juxtaposition, separated by 39 Å (Figure 1). It is believed that this close apposition of 2 prebound JAK2 molecules is responsible for their mutual activation by cross-phosphorylation.
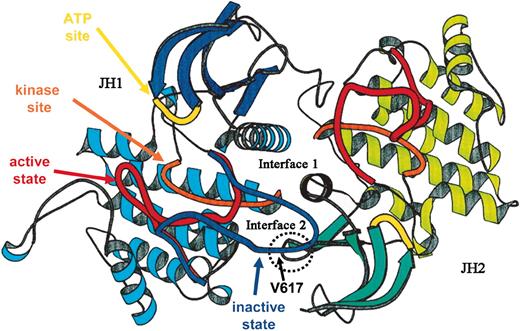
Numerous molecular mechanisms have been identified that regulate the activity of JAK kinases. In the unliganded state, perhaps the most important is the presence of the adjacent JH2 domain of the kinase. Expression of an isolated JAK2 JH1 kinase domain leads to its constitutive activity; addition of the pseudokinase JH2 domain greatly reduces the level of autoactivation.25 Molecular modeling of the JH1 and JH2 domains of JAK2, based on the dimeric structure of the fibroblast growth factor receptor kinase, indicates that a region of JH2 interacts with the activation loop of the kinase domain (Figure 226 ). Subsequent mutagenesis experiments have more directly shown that the region of amino acids 619 to 670 is required for JH2-induced inhibition of JH1 domain kinase activity.27 Moreover, mutation of 2 separate residues in this region of the JH2 domain, Glu665 to Lys and Tyr570 to Phe, results in constitutive activity of the kinase.28,29
Additional levels of regulation of cytokine receptor activation exist, all of which operate at the postligand binding stage. Upon ligand binding, growth factor/receptor complexes are rapidly internalized through clathrin- and ubiquitin-mediated processes30,31 ; down-modulation of receptor expression thus reduces additional signaling. Another mechanism of reduced receptor signaling is removal of critical phosphotyrosine residues, mediated by receptor-associated phosphatases.32 Of considerable interest for the field, genetic elimination of one such phosphatase, the hematopoietic cell phosphatase, now termed SHP1, results in a myeloproliferative disorder in the mouse, termed motheaten.33 Finally, activation of receptor-bound JAKs invariably leads to induction of STAT activation; one group of genes rapidly induced by the transcriptional activity of STATs is the suppressor of cytokine signaling (SOCS) family. Upon transcription and translation, SOCS proteins bind to either JAKs or to the phosphotyrosine residues of the activated cytokine receptor, blocking further signaling.34
The structure of EPO-R in the absence and presence of an activating ligand. The α-carbon trace of the extracellular domain of EPO receptor in the absence (A) and presence (B) of an activating EPO mimetic peptide (EMP) is shown, along with a cartoon representation of the intracytoplasmic events that ensue upon ligand binding. J2i indicates inactive JAK2; J2a, active JAK2. Adapted from Livnah et al24 and reproduced with permission. Copyright 1999 AAAS.
The structure of EPO-R in the absence and presence of an activating ligand. The α-carbon trace of the extracellular domain of EPO receptor in the absence (A) and presence (B) of an activating EPO mimetic peptide (EMP) is shown, along with a cartoon representation of the intracytoplasmic events that ensue upon ligand binding. J2i indicates inactive JAK2; J2a, active JAK2. Adapted from Livnah et al24 and reproduced with permission. Copyright 1999 AAAS.
Based on a growing understanding of the molecular mechanisms that initiate and regulate cytokine action, multiple investigators hypothesized that the chronic myeloproliferative diseases might represent disorders of cytokine signal transduction. Several molecular observations in patients support this notion: blood cells of patients with chronic myeloproliferative disorders display constitutive activation of STAT3,35 up-regulation of BclXL,36 and increased Akt activity.37 Moreover, a strong link exists between activated signaling kinases and hematologic malignancies; Bcr-abl is a dysregulated hematopoietic kinase wholly responsible for CML,38 an internal tandem duplication in the flt-3 receptor tyrosine kinase is seen in approximately 30% of blasts from patients with AML and contributes to the disease,39 JAK2 contributes to the pathogenesis of the acute leukemia associated with t(9;12)(p24; p13), in which the transcription factor Tel is linked to JAK2, leading to its constitutive activation,40 and the same partner is linked to the receptor tyrosine kinase PDGF-Rβ in t(5;12)–associated chronic myelomonocytic leukemia.41
Because so many clues pointed toward a signaling defect in the chronic myeloproliferative disorders, and altered kinases contribute to a growing number of other myeloproliferative diseases, JAK2 became an attractive candidate gene for PV, ET, and/or IMF. In a recent issue of the Lancet a group headed by Anthony Green identified a somatic missense mutation in the JAK2 gene in 97% of patients with PV, 57% of patients with ET, and 50% of those with IMF, but in none of their 90 normal control samples.4 Agroup headed by Gary Gilliland identified the same point mutation in 74% of patients with PV, 33% of patients with ET, and 35% of patients with IMF.5 Moreover, those researchers failed to find any mutations in the activation loops or autoinhibitory domains of 85 other kinases in a Herculean sequencing effort. Likewise, a group headed by William Vainchenker found that 89% of patients with PV, 43% of those with ET, and 43% of patients with IMF bear the JAK2 mutation, but in none of their controls or in patients with secondary erythrocytosis.6 And using genetic loss of heterozygosity to home in on the short arm of chromosome 9, Kralovics et al found that 83 of 128 patients with PV (65%), 13 of 23 with IMF (57%), and 21 of 93 patients with ET (23%) harbored the same JAK2 mutation.45 Importantly, this group of investigators also found that patients who bore the mutation displayed a poorer prognosis than those not carrying it, having a statistically greater likelihood of developing myelofibrosis, hemorrhage, and thrombosis. The majority of individuals with the Val617Phe mutation in all studies carry one copy of the gene, although approximately one third were reported as homozygous in each of the studies, the result of a mitotic cross-over and clonal expansion.
A proposed structure of JAK2. The ribbon diagrams of the JH1 (predominant color blue) and JH2 (predominant color green) domains of JAK2 are shown, modeled on the structure of the dimeric fibroblast growth factor receptor structure. The activation loop of JH1 is shown in 2 possible conformations, active (red) and inactive (blue). The kinase site is shown in orange and the adenosine triphosphate (ATP) binding site in yellow. The site of interaction of JH2 and the activation domain of JH1 is shown encircled, along with the location of Val617. Adapted from Lindauer et al26 and reproduced with permission of Oxford University Press.
A proposed structure of JAK2. The ribbon diagrams of the JH1 (predominant color blue) and JH2 (predominant color green) domains of JAK2 are shown, modeled on the structure of the dimeric fibroblast growth factor receptor structure. The activation loop of JH1 is shown in 2 possible conformations, active (red) and inactive (blue). The kinase site is shown in orange and the adenosine triphosphate (ATP) binding site in yellow. The site of interaction of JH2 and the activation domain of JH1 is shown encircled, along with the location of Val617. Adapted from Lindauer et al26 and reproduced with permission of Oxford University Press.
Obviously, these findings are of great interest on several levels. Of note, of the growth factors to which hematopoietic progenitors are hypersensitive in myeloproliferative disorders, EPO, SCF, GM-CSF, IL-3, TPO and IGF-1, all use JAK2 for signaling. More remarkable, precisely the same mutation was identified in all of the patients studied, despite their being very few related individuals in the study cohorts. The mutation, Val617Phe, was acquired, as it was identified only in short-lived blood cells, not in T lymphocytes or in buccal mucosal cells. As noted earlier, this site in JAK2 lies immediately adjacent to a region of the pseudokinase domain found necessary for inhibition of kinase activity, and, in a previously reported model structure of JH1-JH2 interaction, Val617 appears to make close contact with the activation loop of the kinase domain (presumably stabilizing it) in the inactive conformation.26
That this mutation contributes to the myeloproliferative state is virtually certain, based on several reported findings. First, in patients with mixed clonal and polyclonal progenitors, only erythroid progenitors carrying the mutation were able to grow in the absence of exogenous EPO.4-6 Second, a small interfering RNA (siRNA) reagent that reduces JAK2 expression by 90% greatly reduced EEC formation,6 a hallmark of these disorders. Third, expression of the mutant kinase in human kidney (293T) cells leads to its autophosphorylation (activation), and, when expressed in a factor-dependent cell line, BaF3/EPO, expression of the mutant, but not the wild-type, JAK2 kinase leads to EPO hypersensitivity and growth factor–independent cell survival.5,6,45 Fourth, the occurrence of individuals homozygous for the mutation further suggests that the cellular cross-over events that lead to loss of heterozygosity result in a proliferative advantage, allowing their replacement of the clone of cells carrying a single mutant JAK2 locus. This supposition was confirmed by finding that, compared with cells expressing only the mutant JAK2, coexpression of both wild-type and mutant JAK2 reduced the total cellular autonomous JAK2 activity and led to reduced growth factor–independent behavior.6 Fifth, expression of Val617Phe JAK2, but not the wild-type kinase, along with the EPO-R in a cell line lead to EPO-independent STAT5 activation.6 Finally, and most compelling, introduction of Val617Phe JAK2 into murine marrow cells, but not the wild-type kinase, leads to substantial erythrocytosis (hematocrit [Hct], ∼60%) following their transplantation into lethally irradiated recipients.6
Just as these reports are of great pathophysiologic interest, they carry tremendous potential for the clinical assessment and management of patients with chronic myeloproliferative disorders. Two initial questions that arise include the following: (1) do patients with the Val617Phe JAK2 mutation differ from those without the mutation, and (2) do patients with homozygous expression of the mutant kinase display more aggressive disease? Although in the 3 cited studies4-6 patients with Val617Phe were not reported to differ from patients with PV, ET, or IMF not having the mutation, as the number of such patients in those studies was relatively small, the presence or absence of EEC, or additional chromosomal abnormalities, or whether complications of the disease such as marrow fibrosis, bleeding or thrombosis, or transformation to acute leukemia are more common in patients with Val617Phe, or in patients with 2 copies of the mutant gene, must await larger studies.
The finding of a consistent activating mutation in JAK2 in patients with myeloproliferative disorders raises a number of additional questions. For example, how can one mutation give rise to 3 disorders? Perhaps this reflects the accumulation of additional genetic alterations not yet detected, or is due to the mutation arising in different subpopulations of primitive hematopoietic cells or to a particular biochemical property of the mutant kinase that only allows its activity in cells of a specific lineage(s). Does this finding presage explanations for other disorders whereby JAK kinases are of central signaling importance? Disorders of fat metabolism (the leptin receptor uses JAK2), of body growth (the human growth hormone receptor uses JAK2), or of the immune system (numerous interleukin receptors use JAK1 and JAK3) are candidate diseases that should garner future research attention in this context. What is the cause of familial myeloproliferative syndromes, or the cases of the disorder that do not carry the Val617Phe mutation? Is the thrombotic diathesis in ET and the other disorders related to JAK2 activation? Recent evidence suggests that TPO, a hormone that promotes the activiation of JAK2,44 acts to increase platelet aggregation and adhesion.42,43 And why do the counterregulatory measures that normally shut down signaling from hematopoietic growth factor receptors not operate in JAK2 mutant-bearing patients? Does this observation provide important insights into the molecular mechanism of SOCS inhibition of JAK signaling, or of the phosphatases that inactivate JAK?
Although these and many additional questions arise from the finding of a single genetic alteration in the JAK2 kinase in patients with PV, ET, and IMF, the finding nevertheless represents a remarkable step forward in both the molecular pathogenesis and clinical evaluation of patients with the myeloproliferative disorders. Two salient features stand out. First, the results have important implications for the diagnosis of patients with PV, ET, and IMF, allowing for a rapid, reliable, and unambiguous diagnosis to be made in a large number of patients. Moreover, the new information should spur development of targeted therapy against the mutant form of the kinase. Second, it is gratifying that a large number of observations, beginning with Dameshek1 in 1951, expanded upon by Prchal and Axelrad8 in 1974, by Adamson et al7 in 1976, by Zanjani et al14 in 1977, and by Krantz (Dai et al16,17 ) and by Axelrad (Correa et al18 ) in the 1990s are now explicable at the molecular level. Simply put, it all makes sense, and the results of future investigation in the field almost certainly holds many satisfying clinical and pathogenetic insights into the chronic myeloproliferative disorders.
Prepublished online as Blood First Edition Paper, April 7, 2005; DOI 10.1182/blood-2005-03-1287.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal