Abstract
Notch transmembrane receptors are known to play a critical role in cell-fate decisions, with Notch1 shown to enhance self-renewal of hematopoietic stem cells and cause T-cell leukemia. Four Notch receptors exist, and the extent of redundancy and overlap in their function is unknown. Notch4 is structurally distinct from Notch1 through Notch3 and has not been extensively studied in hematopoiesis. By polymerase chain reaction (PCR) we find Notch4 transcript expression in human marrow cells and in both CD34+ and CD34– populations. When constitutively active Notch1 or Notch4 was overexpressed in normal human marrow or cord cells, we found reduced colony-forming and short-term proliferative ability while the primitive progenitor content of myeloid long-term cultures was significantly increased. Notch4–intracellular domain (Notch4-IC)–transduced cord cells transplanted into β2-microglobulin–/– nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice resulted in significantly higher levels of engraftment of both green fluorescent protein–positive (GFP+) and GFP– populations as compared with controls. GFP+ cells in bone marrow and spleen of animals that had received transplants gave rise to an immature CD4+CD8+ T-cell population, whereas B-cell development was blocked. These results indicate that activation of Notch4 results in enhanced stem cell activity, reduced differentiation, and altered lymphoid development, suggesting it may influence both stem cells and the fate of the common lymphoid progenitor.
Introduction
The highly conserved Notch gene family plays an important role in cell-fate decisions in a wide range of lineages in invertebrates and vertebrates.1,2 In mammals, 4 Notch receptors have been identified (Notch1 through Notch4) and 5 ligands Jagged1, Jagged2, and Delta1, Delta3, and Delta4. Notch activation in mammals occurs through binding to one of its ligands, which results in the translocation of the intracellular domain (IC) to the nucleus.3,4 Notch receptors have an extracellular domain, containing tandem epidermal growth factor (EGF) repeats and 3 Notch repeats, a single transmembrane domain and an IC, containing 6 cdc10/ankyrin repeats, putative nuclear localization signals, and a C-terminal region rich in glutamine (OPA), and a region rich in glutamide seride and threonin (PEST) (OPA/PEST region).1 Although the 4 Notch receptors are similar in structure, Notch4 appears to be evolutionary: the most distant from other Notch members. Notch4 has fewer EGF repeats, a significantly shorter IC, no transactivating domain, and no cytokine-responsive elements as compared to other Notch receptors.5 Notch4 also differs from the other Notch receptors by its expression pattern in the mouse, which is strongest in endothelial cells and male germ cells.6 Notch4 has known effects on embryonic endothelial development,7 and active forms of Notch4 inhibit angiogenesis.8 Furthermore, the constitutively active IC of Notch4 (Int-3) has oncogenic activity in the mouse mammary gland9 and is detected in human breast, lung, and colon carcinoma cell lines.10
The hematopoietic system is a complex and tightly regulated process in which pluripotent stem cells undergo proliferation and differentiation to produce mature blood cells of the various lineages, while maintaining a compartment of uncommitted cells. A role for Notch receptors in hematopoiesis was first proposed after a translocation in human T-cell leukemia involving the Notch1 gene was identified.11 Overexpression of the IC of Notch1 in murine bone marrow resulted in T-cell leukemia and thymoma in mice receiving transduced marrow.12 Furthermore, Notch1-IC overexpression has been shown to induce thymic-independent accumulation of CD4+CD8+ T cells at the expense of B cells in the marrow of mice.13 Activated forms of Notch2 and Notch3 have been implicated in thymic lymphoma.14-16 Notch signaling also plays a role in the maintenance of hematopoietic stem cells17-21 and in the inhibition of differentiation.22-24 The role of Notch4 in the hematopoietic system has not been studied. However, Notch4 has GATA recognition sites in its promoter region, transcription factors that play a role in lineage commitment in hematopoiesis,25,26 suggesting that Notch4 could play a role in hematopoiesis.5 Notch4 expression in hematopoiesis has so far been reported only in maturing macrophages with the use of single-cell polymerase chain reaction (PCR).27
To study a potential role for Notch4 in human hematopoiesis, we determined expression of Notch4 in subpopulations of bone marrow cells and evaluated the functional activity of a constitutively active form of Notch4 in human cord and marrow cells as compared with controls and Notch1-IC. Our studies indicate that the Notch4 transcript is expressed in normal marrow as well as in its CD34+ and CD34– populations. In addition, Notch1-IC and Notch4-IC overexpression results in an inhibition of short-term proliferation, with a decrease in monocytic and erythroid differentiation, but an increased long-term proliferative ability is measured in long-term cultures. Furthermore, transplantation of Notch4-IC–transduced cord cells results in higher levels of human engraftment in the bone marrow of immunodeficient mice. The mice also developed an increase of CD4+CD8+ immature T cells in their bone marrow. Notch4-IC–transduced cells did not contribute to the B-cell population in the spleen whereas they did in controls. These results suggest that Notch4 activation in primary human hematopoietic cells results in a decrease of differentiation while stem cell activity is enhanced. Furthermore, a proliferation of immature T cells is seen with reduced B cells, suggesting Notch4 may influence common lymphoid progenitor fate.
Materials and methods
Virus production
The cDNA of the IC of Notch1 (kindly provided by S. Artavanis-Tsakonas) and hemagglutinin (HA)–tagged Notch4-IC (kindly provided by L. Li)5 were cloned into a murine stem cell virus (MSCV)–internal ribosome entry sequence–green fluorescent protein (MSCV-IRES-GFP) vector (MIG) (from R. K. Humphries, Terry Fox Laboratory, B.C. Cancer Agency, Vancouver, BC, Canada) to generate MSCV–Notch1-IC–IRES–GFP and MSCV–Notch4-IC–IRES–GFP vectors and were verified by sequencing. Helper-free virus was obtained by transfecting amphotrophic Phoenix packaging cells28 cultured in Dulbecco Modified Eagle Media (DMEM) plus 10% fetal calf serum (FCS) (StemCell Technologies, Vancouver, BC, Canada) with the use of CaPO4 and the harvesting of the virus-containing medium (CM) 24 hours later as described.29 Virus-CM was filtered through a 0.45-μm filter and used freshly each time.
Cells
Bone marrow cells from healthy donors were obtained after informed consent and with approval of the Clinical Research Ethics Board of the University of British Columbia (Vancouver, BC, Canada). Low-density (fewer than 1.077 g/mL) cells were isolated by centrifugation on Ficoll-Hypaque (Amersham Pharmacia, Piscataway, NJ) and fractionated directly, or later, after being cryopreserved in 10% dimethyl sulfoxide (DMSO) with 90% FCS.
Cord blood cells were obtained with informed consent from mothers undergoing cesarean delivery of healthy full-term infants. Low-density (fewer than 1.077 g/mL) cells were isolated by means of Ficoll-Hypaque (Amersham Pharmacia). Primary lineage (CD2, CD3, CD14, CD16, CD19, CD24, CD56, CD66b, glycophorin A)–depleted cord cells, which are highly enriched for CD34+ cells (approximately 70%), were obtained by means of StemSep columns (StemCell Technologies).
Retroviral transduction
Cells to be transduced were first cultured at 1 to 2 × 105/mL for 48 hours in complete serum-free media (SFM) consisting of Iscove medium supplemented with bovine serum albumin (BSA), insulin, and transferrin (BIT) (StemCell Technologies), 40 μg/mL low-density lipoproteins, and 10–4 M 2-mercaptoethanol (both from Sigma Chemical, St Louis, MO) to which the following 5 purified recombinant human growth factors (GFs) were added: Flt-3 ligand (FL), 100 ng/mL (Immunex, Seattle, WA); Steel factor (SF), 100 ng/mL (Terry Fox Laboratory, Vancouver, BC, Canada); interleukin-3 (IL-3), 20 ng/mL (Novartis, Basel, Switzerland); IL-6 (20 ng/mL; Cangene, Mississauga, ON, Canada); and granulocyte colony-stimulating factor (G-CSF; 20 ng/mL; StemCell Technologies) as previously described.30 At the end of this 2-day prestimulation period, the cells were resuspended in filtered virus-CM supplemented with the same 5 GFs. Protamine sulfate (5 μg/mL; Sigma Chemical) was added, and the cells were placed in fibronectin-coated Petri dishes previously loaded twice with virus-CM (30 minutes each). After 12 hours, the cells were harvested and resuspended in fresh virus-CM containing the 5 GFs and protamine sulfate. This infection procedure was performed 3 times. After 36 hours, the cells were transferred to fresh SFM. Cells for in vivo studies were used at this point, whereas cells for in vitro studies were incubated for a further 48 hours prior to harvesting and further analysis. Expression of the Notch4-IC construct was confirmed in 3 cord samples with real-time PCR with primers to the IC of Notch4. Notch4-IC expression was about 1000-fold higher in the Notch4-IC–transduced cord cells than in the MIG-transduced cord cells in all 3 samples.
Flow cytometry
Viable (propridium iodine–negative) GFP+ cord or normal marrow cells were isolated at a purity of greater than 99% by means of a 3-laser FACStarplus (Becton Dickinson, San Jose, CA) as described previously.31 Cells were sorted into SFM in microcentrifuge tubes at 4° C, and known cell numbers were used to initiate colony-forming unit (CFU) assays, suspension culture (SC) assays, and long-term cultures. For the phenotype analyses, cells were stained with anti-CD34 (8G12)–cyanin 5 (Cy5) (Dr P. Lansdorp, Terry Fox Laboratory); glycophorin A (10F7)–phycoerythrin (PE); anti-CD71 (OKT-9)–PE (both from Dr P. Lansdorp); anti-CD41a–PE (Pharmingen, Mississauga, ON, Canada); anti-CD38–PE; anti-CD13–PE; anti-CD33–PE; anti-CD11b–PE; anti-CD20–PE; or anti-CD3–PE (all from Becton Dickinson). Cells were then analyzed on a FACScalibur by means of CellQuest software (Becton Dickinson) with gates set to exclude at least 99.9% of the cells in the matched isotype control.
RT-PCR analyses
The cDNA from 2 human marrow samples and the fluorescence-activated cell sorter (FACS)–sorted CD34+ or CD34– subpopulations was kindly provided by Dr C. Eaves. Aliquots of 1 to 5 μL of the first round of PCR generated from the initial amplification of total cDNA were subjected to a second round of PCR amplification in 50-μL volumes of 1 × Pfx amplification buffer (Gibco BRL, Gaithersburg, MD); 0.3 mM each deoxynucleoside triphosphate (dNTP; Amersham Pharmacia); 1 mM MgSO4; 1 unit platinum Pfx DNA polymerase; and 10 pmol specific primers for the extracellular domain of Notch4 (5′-TAGGGCTCCCCAGCTCTC-3′ and 5′-GGCAGGTGCCCCCATT-3′) to give a DNA fragment of 486 bp (kindly provided by Dr A. Karsan, B.C. Cancer Agency, Vancouver, BC, Canada). The primers started in different exons, to make sure that genomic DNA contamination did not interfere with the results. As a positive control, cDNA of human aortic endothelial cells (kindly provided by Dr A. Karsan), known to express human Notch4, was used. As negative controls, a PCR reaction without primers and a PCR reaction without cDNA were performed. Forty cycles (94° C for 30 seconds, 60° C for 1 minute, 72° C for 30 seconds) were then performed.
Real-time PCR
Total RNA from unsorted and from CD34+CD38–, CD34+CD38+, and CD34– sorted cells was extracted by means of TRIzol (Life Technologies, Burlington, ON, Canada). To remove any contaminating DNA, RNA was DNAse I treated following the manufacturer's instructions (Life Technologies). Synthesis of first-strand cDNA was carried out with Superscript II (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions with the use of random hexamer oligonucleotides (Amersham Pharmacia). As a negative control, a reverse-transcription PCR (RT-PCR) without Superscript II was performed.
Primers for Notch1, Notch2, Notch3, Notch4, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) specific amplification were designed by means of Primer Express software (PE Applied Biosystems, Foster City, CA). For Notch1, the sense primer CGGGTCCACCAGTTTGAATG and antisense primer GTTGTATTGGTTCGGCACCAT were used; for Notch2, sense primer TTTGGCAACTAACGTAGAAACTCAAC and antisense primer TGCCAAGAGCATGAATACAGAGA; for Notch3, the sense primer ATGCAGGATAGCAAGGAGGA and the antisense primer AAGTGGTCCAACAGCAGCTT; for Notch4, the sense primer CCCAGGAATCTGAGATGGAA and antisense primer CCACAGCAAACTGCTGACAT; and for GAPDH, the sense primer CGACAGTCAGCCGCATCTT and the antisense primer ACCTTCCCCATGGTGTCTCA. One-step real-time PCR was performed with the use of the GeneAmp 5700 Sequence Detection System Mix (PE Applied Biosystems). Reactions were performed in triplicate in a 50-μL total volume containing 25 μL SYBR Green Master Mix (PE Applied Biosystems), 1 to 10 ng cDNA, sense and antisense primers, and ddH20. The primer concentrations used were the minimum primer concentrations giving the lowest threshold cycle (Ct) and maximum change in resistance (ΔRn), while minimizing nonspecific amplification. The optimal concentration for both the sense and antisense primer for Notch1 and Notch2 was 100 nM, and for Notch3, Notch4, and GAPDH was 300 nM in a 50-μL reaction. As negative controls, a PCR reaction without cDNA was performed, and a reaction containing 3 μL of the negative control from the PCR reaction instead of cDNA was performed. Real-time PCR was also subjected to melt-curve analysis for verification of true target amplicons and absence of primer dimers. To relatively quantitate Notch expression, the ΔΔCt method was performed according to the manufacturer's instructions.
The concentration of cDNA used for the real-time PCR was standardized by comparison with a K562 cDNA control curve. K562 cDNA was prepared, quantitated by means of spectometry, and diluted to obtain 0.01 to 100 ng cDNA. These serial dilutions, along with 3 dilutions of experimental cDNA, were used to perform a PCR reaction using GAPDH primers. Concentrations of experimental cDNA were determined by comparison with the logarithmic section of the standard curve generated.
Notch1, Notch2, Notch3, and Notch4 PCR products obtained from real-time PCR reactions were purified on agarose gels and cloned in a pGEM-T vector with T7 and SP6 promoters (Promega, Madison, WI). The plasmids were isolated with the Maxi Prep Kit (Qiagen, Hilden, Germany) and verified by sequencing by means of an ABI PRISM 377 sequencer.
Cultures
Suspension cultures were initiated with a known number of transduced cord cells (5 to 10 × 104) cultured in complete SFM with the same 5 GFs described above and maintained with weekly media changes at 37° C in a 5% CO2 humidified incubator. Cell counts were performed at days 3, 7, 14, and 21 by means of trypan blue staining. At days 7 and 21, cells were also harvested for FACS analyses. To detect long-term culture initiating cells (LTC-ICs), 1 × 105 transduced cord cells were placed in 2.7 mL Myelocult media (StemCell Technologies) with 10–6 M hydrocortisone sodium succinate and seeded onto a confluent layer of irradiated (80 cGy) mouse embryonic fibroblasts engineered to produce human SF, human IL-3, and human G-CSF.32 Cultures were incubated at 37° C in a 5% CO2 humidified incubator and received a weekly half-media change. After 5 weeks, both the adherent and the nonadherent cells were harvested, pooled, counted, and plated into methylcellulose-based medium (StemCell Technologies) supplemented with 20 ng/mL recombinant human IL-3 (rhIL-3) (Sandoz, Princeton, NJ), 20 ng/mL rhIL-6 (Terry Fox Laboratory), 20 ng/mL rhG-CSF (Amgen Canada, Mississauga, ON, Canada), 20 ng/mL recombinant human granulocyte-macrophage CSF (rhGM-CSF) (Sandoz), 50 ng/mL rhSF (Terry Fox Laboratory), and 3 U/mL erythropoietin. Colonies were counted after 21 days of culture. To detect primary colony-forming cells, 500 transduced cord or marrow cells were plated in SFM-containing methylcellulose cultures (StemCell Technologies) with human SF (50 ng/mL) and 20 ng/mL each of the following: IL-3, IL-6, G-CSF, and GM-CSF. After 10 days, colonies were counted.
Transplantation of Notch-transduced cord blood cells into β2-microglobulin–/– NOD/SCID mice
The β2-microglobulin–/– nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice (Jackson Laboratory, Bar Harbor, ME) were bred and maintained in the animal facility of the British Columbia Cancer Research Centre (Vancouver, BC, Canada) under sterile conditions in sterile microisolator cages. The animals were provided exclusively with autoclaved food and water containing 100 mg/L ciprofloxacin and HCl. At 24 hours prior to undergoing transplantation, mice were irradiated with 3.5 Gy γ-irradiation from a 137Cs source at a dose of 1.22 cGy/min. A known number of unsorted cord cells transduced with MIG or MIG–Notch4-IC were resuspended in 0.3 mL Alpha minimal essential medium (Alpha MEM) (StemCell Technologies) with 5% FCS and injected intravenously into the lateral tail vein of 6- to 8-week-old β2-microglobulin–/– NOD/SCID mice. Marrow aspirations were performed every 3 weeks on anesthetized mice. At 12 weeks after injection, the mice were killed by CO2 inhalation. Marrow was removed from the femurs by flushing with Alpha MEM with 5% FCS. Spleen and thymus were removed, and single-cell suspensions were made for FACS analysis.
Flow cytometry analysis of murine tissues
Cell suspensions from femoral marrow and spleens were lysed in ammonium chloride (StemCell Technologies) for 20 minutes and then washed in Hanks medium (StemCell Technologies) with 5% human serum for blocking of human Fc receptors and prepared for flow cytometric analysis. Thymus cells were also incubated with 5% human serum in Hanks medium.33 Cells were then stained with a Cy5-labeled antihuman CD45 antibody (kind gift from Dr P. Lansdorp) in combination with CD71-PE, CD34-PE, CD33-PE, glycophorin A–PE, CD41a-PE, CD11b-PE, CD20-PE, CD3-PE, CD4-PE, or CD8-PE for 30 minutes on ice. Unstained cells were used as control. Samples were analyzed by means of a FACScan (Becton Dickinson); gate settings excluding at least 99.9% of the cells in the unstained control were used to determine lineage markers on CD45+GFP+ cells in each sample. We defined human engraftment as expression of at least 0.1% CD45+ cells in a sample.
Statistical analysis
Results are shown as the mean ± standard deviation (SD) of values obtained in independent experiments. Differences between groups were assessed by means of the 2-tailed Student t test with the use of pairing when appropriate. For the in vivo data, a paired t test was performed with the mean values of the marrow aspirations at weeks 9 and 12. A significance level of 0.05 was chosen.
Results
Notch4 transcript is expressed in human marrow cells with highest levels in primitive progenitors
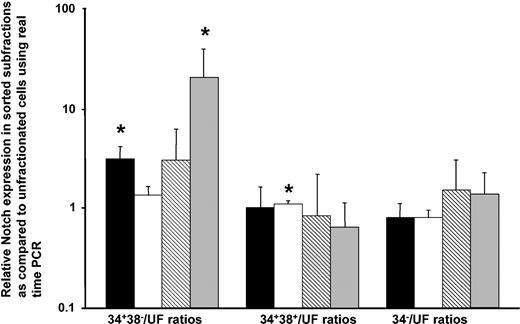
To investigate the expression of Notch4 in human hematopoietic cells, RT-PCR on cDNA from human marrow cells and CD34-based sorted primitive progenitors was performed. Notch4 transcript expression by RT-PCR was readily detectable in total RNA extracted from unfractionated marrow and in both the CD34+ and the CD34– subpopulations of 2 marrow samples (data not shown). To quantify Notch transcript expression in the most primitive progenitors (CD34+CD38–), more mature progenitors (CD34+CD38+), and nonprogenitor marrow (CD34–), real-time PCR was performed. Notch1 and Notch4 transcript expression was significantly higher in the CD34+CD38– populations as compared with unsorted cells (Figure 1), with the same trend observed for Notch2 and Notch3. Notch1 transcript expression levels varied approximately 4-fold as compared to a control. When expression of Notch2, Notch3, and Notch4 transcripts were compared with Notch1 transcript expression by means of the Ct analysis in 5 unsorted marrow samples, Notch2 transcript was expressed at 1.8 ± 1.0-fold higher than Notch1; Notch3 was at 0.5 ± 0.9-fold higher than Notch1; and Notch4 was at 0.3 ± 0.5-fold higher than Notch1. In the CD34+CD38– progenitor population, however, Notch4 transcript expression was 1.4 ± 1.7-fold higher as compared with Notch1, while Notch2 and Notch3 had a lower expression.
Relative Notch1, Notch2, Notch3, and Notch4 expression in human hematopoietic marrow progenitor subsets as compared with unfractionated marrow (uF) as measured by real-time PCR. Normal marrow cells were suspended in Hanks solution with 5% human serum at 5 to 10 × 106/mL and incubated for 30 minutes with CD34–fluorescein isothiocyanate (CD34-FITC) (gift from Dr P. Lansdorp) used at 4 μg/mL and CD38-PE (Becton Dickinson) used according to the manufacturer's instructions. Separate aliquots were stained with an irrelevant mouse immunoglobulin G1–FITC (IgG1-FITC) and IgG1-PE antibody (both from Becton Dickinson) as an isotype control. Viable CD34+CD38–, CD34+CD38+, and CD34– cells were sorted and used for RNA extraction. The cDNA of unsorted marrow and of marrow sorted for CD34 and CD38 was used for real-time PCR with Notch primers and SybrGreen. Notch expression in the sorted subpopulations was compared with its unfractionated population (uF) to allow comparisons and relative expression of Notch1 through Notch4 in the progenitors expressed. Error bars indicate the standard error of the mean. The expression of the sorted population was compared with the unsorted population in a paired t test. *P ≤ .05. Notch1 is indicated by ▪; Notch2, by □; Notch3, by ▧; and Notch4, by ▦.
Relative Notch1, Notch2, Notch3, and Notch4 expression in human hematopoietic marrow progenitor subsets as compared with unfractionated marrow (uF) as measured by real-time PCR. Normal marrow cells were suspended in Hanks solution with 5% human serum at 5 to 10 × 106/mL and incubated for 30 minutes with CD34–fluorescein isothiocyanate (CD34-FITC) (gift from Dr P. Lansdorp) used at 4 μg/mL and CD38-PE (Becton Dickinson) used according to the manufacturer's instructions. Separate aliquots were stained with an irrelevant mouse immunoglobulin G1–FITC (IgG1-FITC) and IgG1-PE antibody (both from Becton Dickinson) as an isotype control. Viable CD34+CD38–, CD34+CD38+, and CD34– cells were sorted and used for RNA extraction. The cDNA of unsorted marrow and of marrow sorted for CD34 and CD38 was used for real-time PCR with Notch primers and SybrGreen. Notch expression in the sorted subpopulations was compared with its unfractionated population (uF) to allow comparisons and relative expression of Notch1 through Notch4 in the progenitors expressed. Error bars indicate the standard error of the mean. The expression of the sorted population was compared with the unsorted population in a paired t test. *P ≤ .05. Notch1 is indicated by ▪; Notch2, by □; Notch3, by ▧; and Notch4, by ▦.
Constitutively active Notch1 or Notch4 decreases myeloid colony formation and short-term in vitro proliferation
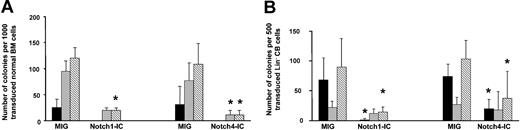
To examine the effect of constitutively active Notch1 and Notch4 on human myeloid differentiation, human marrow and lineage-depleted cord blood (cord) cells were transduced with Notch1-IC or Notch4-IC in the MIG vector or control MIG (Table 1). GFP+ marrow and cord cells were sorted and used to initiate primary colony assays. Total myeloid colony-forming cells were markedly (3- to 10-fold) and significantly reduced in the Notch1-IC– and Notch4-IC–transduced cultures as compared with control (Figure 2). Interestingly, although erythroid burst-forming units (BFU-Es) were observed with MIG control–transduced marrow, these colonies were not observed with Notch1-IC– or Notch4-IC–transduced marrow cells. Erythroid colonies were also significantly reduced in the Notch1-IC– and Notch4-IC–transduced cord cultures (P ≤ .001). With the use of marrow cells, a significantly lower number of granulocyte-macrophage colonies were seen with the Notch4-IC as compared with the MIG control; however, this effect was not significant when cord blood cells were assessed.
Decreased colony-forming ability of Notch1-IC– and Notch4-IC–transduced normal marrow (BM) and cord cells compared with MIG-transduced cord blood cells. Normal marrow cells (panel A) (Notch1-IC, n = 3; Notch4-IC, n = 4) and cord blood (panel B) (Notch1-IC, n = 4; Notch4-IC, n = 5) were retrovirally transduced with MIG, Notch1-IC, and Notch4-IC and sorted for GFP. Cultures were initiated with 1000 GFP+ normal marrow cells (panel A) or 500 cord blood cells (panel B) placed into methylcellulose in the presence of IL-3, IL-6, G-CSF, GM-CSF, and SF, and colonies were counted 10 days after initiation. A paired t test was performed to determine statistical differences between colony numbers in Notch1-IC or Notch4-IC and MIG. Error bars indicate the standard error of the mean. *P ≤ .05. Erythroid colonies are indicated by ▪; granulocyte and/or macrophage colonies, by ▦; and total colonies, by ▧.
Decreased colony-forming ability of Notch1-IC– and Notch4-IC–transduced normal marrow (BM) and cord cells compared with MIG-transduced cord blood cells. Normal marrow cells (panel A) (Notch1-IC, n = 3; Notch4-IC, n = 4) and cord blood (panel B) (Notch1-IC, n = 4; Notch4-IC, n = 5) were retrovirally transduced with MIG, Notch1-IC, and Notch4-IC and sorted for GFP. Cultures were initiated with 1000 GFP+ normal marrow cells (panel A) or 500 cord blood cells (panel B) placed into methylcellulose in the presence of IL-3, IL-6, G-CSF, GM-CSF, and SF, and colonies were counted 10 days after initiation. A paired t test was performed to determine statistical differences between colony numbers in Notch1-IC or Notch4-IC and MIG. Error bars indicate the standard error of the mean. *P ≤ .05. Erythroid colonies are indicated by ▪; granulocyte and/or macrophage colonies, by ▦; and total colonies, by ▧.
GFP+ cord cells were also used to initiate suspension cultures (SCs) in SFM with growth factors as described. GFP expression in GFP-sorted cultures after 1 and 3 weeks was 47% ± 33% and 19% ± 11%, respectively, from Notch1-IC cultures (n = 7); 42% ± 30% and 27% ± 18%, respectively, from Notch4-IC cultures (n = 10); and 75% ± 23% and 68% ± 12%, respectively, from MIG control cultures. GFP expression in Notch1-IC and Notch4-IC cultures was significantly reduced as compared with the MIG control (P ≤ .01 in all cases). Cell numbers in suspension cultures at day 3 were 2- to 4-fold lower in the Notch1-IC– and Notch4-IC–transduced cord cultures compared with control (P < .001 and P = .003, respectively) and remained reduced at day 7 (P = .1 and P = .05), day 14 (P = .03 and P = .02), and day 21 (P = .05 and P = .003). The phenotype of the cells was analyzed at weeks 1 and 3 (Table 2). Total GFP+ cells per 105 input cells from progenitor (CD34+), erythroid (glycophorin A+), myelomonocytic (CD33+, CD13+, and CD11b+), and megakaryocytic (CD41a+) lineages were all reduced in the Notch1-IC and Notch4-IC cultures as compared with MIG control, with the exception of megakaryocytic lineage cells, which were unchanged by Notch1-IC. Glycophorin A+ cells were reduced more profoundly than the others (18- to 37-fold). However, owing to heterogeneity of glycophorin A expression in the MIG control, this reached significance only in the Notch4-IC–transduced cultures at week 1. These results suggest that transduction with Notch1-IC and Notch4-IC inhibits myeloid differentiation when human cord progenitors are cultured with myeloid-promoting growth factors.
Notch1 or Notch4 activation results in increase in long-term culture initiating cells in vitro
To determine whether the long-term proliferative ability of cord cells was altered by Notch1-IC or Notch4-IC overexpression, a known number of GFP+ cord cells were maintained in long-term cultures for 5 weeks and then subcultured in semisolid media to determine colony numbers. A significant increase in colonies derived from long-term cultures initiated with Notch1-IC–transduced (P ≤ .05, n = 5) and Notch4-IC–transduced (P ≤ .05, n = 4) cord cells were observed as compared with the control (Table 3). No difference in the phenotype of the colonies of control and Notch-IC–transduced cells was observed, with all cultures producing similar proportions of mixed granulocytic-erythroid colonies, granulocyte-macrophage colonies, and erythroid colonies with similar morphology. This suggests that although Notch1 or Notch4 overexpression results in inhibition of short-term proliferation, it increases long-term proliferation of cord blood cells.
Cord cells overexpressing the intracellular domain of Notch4 give rise to a CD4+CD8+ population in bone marrow and spleen of β2-microglobulin–/– NOD/SCID mice
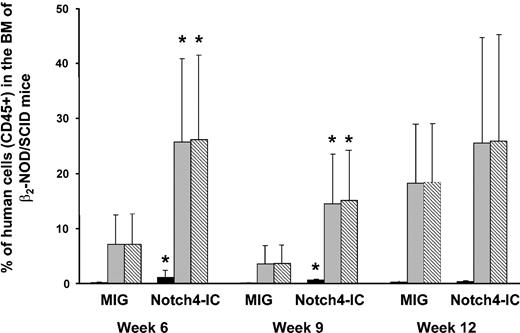
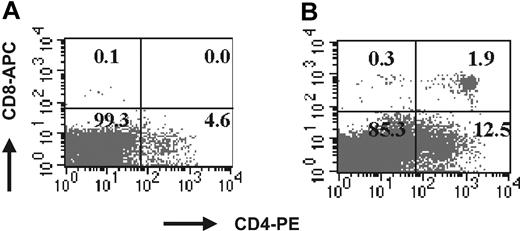
To assess the effect of Notch4 overexpression in lineage-depleted cord cells in vivo, human cord cells were retrovirally transduced with MIG or Notch4-IC for 36 hours. The average transduction efficiency of 4 cord samples used in these experiments was 22.6% ± 13.4% for MIG and 15.3% ± 10.7% for Notch4-IC. Unsorted transduced cord cells (1.5 × 105 to 1 × 106 cells per mouse, with 3 mice in each group) were injected into the tail vein of sublethally irradiated β2-microglobulin NOD/SCID mice. The bone marrow was evaluated for engraftment (CD45+ cells) at weeks 6, 9, and 12, and spleen and thymus were evaluated at week 12. The mean level of marrow engraftment in the 4 experiments was significantly higher in the mice injected with Notch4-IC cord cells as compared with the control at weeks 6 and 9 in both the GFP+ and the GFP– populations (Figure 3). Cells from week-9 and week-12 mouse marrow were costained with CD45 and the lineage markers CD71, CD34, CD33, glycophorin A, CD41a, CD11b, CD20, CD3, CD4, and CD8 (Table 4). CD34+ cells were more frequent in the GFP+CD45+ population of the Notch4-IC mice as compared with the control (14% ± 18% versus 1% ± 3%; P ≤ .05 at weeks 9 and 12). CD33 expression on GFP+CD45+ cells of Notch4-IC mice was lower compared with the control mice (5% ± 18% in Notch4-IC mice versus 44% ± 45% in MIG mice; P ≤ .05). CD11b expression, analyzed only at week 12, was significantly lower on GFP+CD45+ cells of Notch4-IC mice compared with the control (10% ± 9% versus 37% ± 24%, respectively; P ≤ .05). Notch4-IC mice had an increased GFP+CD45+CD3+ population, with 50% ± 20% of GFP+CD45+ cells expressing CD3 as compared with 8% ± 21% in MIG mice (P ≤ .01) at weeks 9 and 12 combined. Analysis of cells costained for CD4 and CD8 revealed a CD4+CD8+ population in the marrow of Notch4-IC mice (27% ± 21% of GFP+CD45+ cells), which was not seen in MIG mice (0% ± 0% of GFP+CD45+; P ≤ .01) (Figure 4). When cells were stained for CD20, a slightly lower percentage of GFP+CD45+ cells from the marrow of Notch4-IC mice (6% ± 11%) were positive for CD20 as compared with control (24% ± 19%, respectively, P = .13).
Increased engraftment of Notch4-IC–transduced cord cells compared with MIG-transduced cord cells in β2-microglobulin–/– NOD/SCID mice. Lineage-depleted cord cells were retrovirally transduced with MIG and Notch4-IC (n = 4) and injected into sublethally irradiated β2-microglobulin–/– NOD/SCID mice. Every 3 weeks, bone marrow aspirations were performed, and cells were stained with CD45 and GFP and analyzed by FACS to determine human engraftment. A paired t test was used to determine differences in engraftment. Error bars indicate the standard error of the mean. *P ≤ .05. CD45+GFP+ cells are indicated by ▪; CD45+GFP– cells, by ▦; total CD45+ cells, by ▧.
Increased engraftment of Notch4-IC–transduced cord cells compared with MIG-transduced cord cells in β2-microglobulin–/– NOD/SCID mice. Lineage-depleted cord cells were retrovirally transduced with MIG and Notch4-IC (n = 4) and injected into sublethally irradiated β2-microglobulin–/– NOD/SCID mice. Every 3 weeks, bone marrow aspirations were performed, and cells were stained with CD45 and GFP and analyzed by FACS to determine human engraftment. A paired t test was used to determine differences in engraftment. Error bars indicate the standard error of the mean. *P ≤ .05. CD45+GFP+ cells are indicated by ▪; CD45+GFP– cells, by ▦; total CD45+ cells, by ▧.
Representative FACS profile of Notch4-IC–transduced cord cells. FACS profile demonstrates that Notch4-IC–transduced cord cells give rise to a CD4+CD8+ population in the bone marrow of β2-microglobulin NOD/SCID mice. Marrow from week-9 and week-12 β2-microglobulin NOD/SCID mice receiving transplants of MIG- and Notch4-IC (n = 4)–transduced cord blood cells was analyzed for phenotype by FACS. Murine marrow cells were stained with CD4-PE and CD8–allophycocyanin (CD8-APC) along with propidium iodide (PI). (A) Marrow from MIG mouse within PI gates. (B) Marrow from Notch4-IC mouse within PI gates.
Representative FACS profile of Notch4-IC–transduced cord cells. FACS profile demonstrates that Notch4-IC–transduced cord cells give rise to a CD4+CD8+ population in the bone marrow of β2-microglobulin NOD/SCID mice. Marrow from week-9 and week-12 β2-microglobulin NOD/SCID mice receiving transplants of MIG- and Notch4-IC (n = 4)–transduced cord blood cells was analyzed for phenotype by FACS. Murine marrow cells were stained with CD4-PE and CD8–allophycocyanin (CD8-APC) along with propidium iodide (PI). (A) Marrow from MIG mouse within PI gates. (B) Marrow from Notch4-IC mouse within PI gates.
The average engraftment in the spleens of Notch4-IC mice was 10.2% ± 18.2% compared with 3.1% ± 5% in MIG mice. GFP+ cells were observed in the spleens of the mice in only 2 of the 4 experiments. In those 2 experiments, GFP+ cells in MIG mice contributed to the CD20+ B-cell population (63% ± 24% of GFP+CD45+ cells), while GFP+ cells in Notch4-IC mice did not (0.1% ± 0.1% cells of GFP+CD45+). Moreover, in 4 of 6 Notch4-IC mice, GFP+ cells gave rise to a GFP+CD3+ population (89% ± 16% of GFP+CD45+ cells), which was primarily CD4+CD8+. No GFP+CD3+ population in the spleens of MIG mice was observed. In these 4 experiments, no human engraftment was observed in the thymus of the killed animals. However, in an earlier individual experiment in which we cultured the transduced cord cells for 3 days before injecting them into the mice, engraftment was seen. In this single experiment, high levels of engraftment (CD45+ cells) were observed in the thymus of all 3 Notch4-IC mice (94% ± 3%), whereas in MIG mice engraftment in the thymus was lower (23% ± 37%). Interestingly, although a minority of CD45+ cells in the thymus of Notch4-IC mice were GFP+ (2% ± 1%), in MIG mice a substantial number of CD45+ cells were GFP+ (43% ± 32%). The mean percentage of total CD45+ cells expressing CD3 in the thymus was 69% ± 6% in Notch4-IC mice and 41% ± 36% in the MIG mice. The percentage of CD4+CD8+ cells in the thymus of the mice was 92% ± 4% of CD45+ cells in Notch4-IC and 52% ± 24% in MIG mice.
Discussion
Emerging evidence suggests an important role for Notch genes in influencing cell-fate decisions during early hematopoiesis. It is also known that Notch is important during development, and dysregulated Notch is associated with malignancy. However, with the availability in mammals of 4 Notch receptors, 5 ligands, and numerous coregulatory molecules, Notch signaling is complex, and the extent of redundancy that might exist in Notch functions is unknown. It is known that both Notch1-null mice and Jagged1-null mice exhibit embryonic lethality,34,35 suggesting nonoverlapping functions for Notch1 and Jagged1, at least during development. In contrast to Notch1–/– mice, Notch4–/– mice develop normally.36 However, embryos lacking both Notch1 and Notch4 have more severe vascular defects compared with Notch1–/– mice,36 suggesting that Notch4 is functionally redundant with Notch1 during murine embryogenesis. In hematopoiesis, expression of the Notch receptors appears to be cell-type specific,27 and cytokine specificity for Notch1 and Notch2 has been proposed.37 Therefore, specific roles for each Notch receptor in human hematopoiesis cannot be excluded. We have evaluated the expression and function of Notch4 in human hematopoiesis, a molecule previously poorly studied in this setting.
Here we demonstrate that Notch4 transcript, like Notch1, Notch2, and Notch3, is expressed in human marrow cells and like Notch1 has the highest expression in more primitive CD34+CD38– cells. Quantitative PCR revealed similar Notch1 and Notch4 transcript expression in primitive hematopoietic progenitors. To evaluate the role of Notch4 in the differentiation and maintenance of primitive progenitors, we overexpressed constitutively active forms of Notch4 in human lineage-negative (lin–) cord cells or marrow and functionally and phenotypically analyzed these cells.
Constitutively active Notch4 produced a marked reduction in primary colony formation and inhibited short-term in vitro proliferation affecting both the erythroid and the myelomonocytic lineages. This is consistent with previous reports, in which an inhibition of short-term proliferation and colony-forming ability was observed after Notch activation through its ligand Jagged1.38,39 Other groups reported an increase in colony-forming ability upon Jagged stimulation17-19 or overexpression of Notch1-IC.24 However, in most cases, cells were cultured for up to 14 days with Jagged1 before colony assays were performed. It is not clear whether these findings are a direct effect of Notch signaling or whether they can be attributed to soluble factors. A non–cell-autonomous effect of Notch1 has been proposed by Kawamata et al.40 We found no difference in short-term proliferative ability, colony-forming cell (CFC) frequency, or phenotype of CFCs in 3 experiments when nontransduced cord blood cells were cultured with the supernatant of Notch1-IC or Notch4-IC cultures and compared with cultures with the supernatant of MIG controls (data not shown). This suggests that the effects of Notch1-IC and Notch4-IC overexpression on short-term proliferative ability are due to a direct mechanism and not to a nonautonomous mechanism.
The transduction efficiency of the Notch vectors was lower compared with the control vectors. In addition, although GFP expression was greater than 99.9% at the start of cultures, GFP expression in Notch1-IC and Notch4-IC cultures decreased after 3 weeks in culture (19% ± 11% and 27% ± 18%, respectively) as compared with control cultures (68% ± 12%). It is possible that some of these effects are due to negative selection of Notch-IC–transduced cells, possibly as a result of the growth-inhibitory effect of the Notch-IC. However, it seems unlikely that these observations are all due to outgrowth of GFP– cells as they represent fewer than 0.1% of cells at initiation of the culture. More likely, gene silencing of the proviral integration site also occurs.
When we cultured Notch1-IC– or Notch4-IC–transfected cells in long-term cultures followed by a CFC assay, we observed an increase in CFC frequency, suggesting that Notch1 or Notch4 signaling results in an increase in primitive myeloid progenitors. This is consistent with higher expression levels of these genes in the CD34+CD38– populations. In this study, Notch1- and Notch4-induced inhibition of differentiation were most dramatic in the erythroid lineage cells. Hadland et al41 reported an increase in erythroid colony formation by Notch1–/– embryonic stem cells, suggesting the erythroid lineage may be differentially more influenced by Notch activity.
When Notch4-IC cord cells were evaluated for their engraftment potential in β2-microglobulin–/– NOD/SCID mice, engraftment levels were higher in marrow and spleen as compared with control cells. Increased repopulating ability has been observed upon overexpression of Notch1-IC in murine cells42,43 as well as upon stimulation of human cells with Delta1 and Jagged1 ligand,17,20,44 but increased engraftment of human hematopoietic cells upon overexpression of any Notch-IC has not been previously demonstrated. Engraftment was increased in both the GFP+ and the GFP– population, suggesting the possibility that Notch signaling might influence the expression levels of Notch receptor and/or ligands on both GFP+ and GFP– cells. However, it also may be that the expression of the Notch4-IC or Notch1-IC gene is differentially down-regulated after homing and/or engraftment, similarly to the proposed GFP down-regulation in the Notch-IC cultures. Consistent with higher levels of engraftment, we also observed a higher percentage of CD34+GFP+ cells and a decrease in the percentage of myeloid and monocytic cells in the marrow of the animals.
The function of Notch on the myeloid compartment in vivo is still controversial. Both promotion and inhibition of myeloid differentiation upon Notch signaling has been suggested.40,43,45,46 Human cord cells overexpressing the intracellular domain of Notch1 have limited potential to develop into monocytes in the marrow of NOD/SCID mice.45 This is consistent with a marked reduction in CD11b expression on Notch4-IC–transduced cord cells both in vivo and in vitro. This system is not suitable for analyzing the effect of Notch overexpression on erythroid differentiation because erythroid development does not occur in these mice upon transplantation of human cells.
Suppression of B-cell proliferation and abnormal maturation of T cells have been observed in mice receiving transplants of Notch1-IC–transduced murine marrow and human cord cells.40 Notch4-IC cord cells, like Notch1-IC–transduced cord cells, can become immature CD4+CD8+ T cells in marrow and spleen and are unable to differentiate into B cells. We found abnormal T cells and inhibited B-cell differentiation in the GFP+ population consistent with results from De Smedt et al,45 who reported abnormal B- and T-cell development in Notch1-IC–transduced human cord cells but not in untransduced cells. This suggests that the common lymphoid progenitor may adopt a T-cell fate instead of a B-cell fate when Notch4-IC is overexpressed. It is interesting that overexpression of the downstream effectors of Notch, HES1 and HES5, causes a partial block in B-cell development, but no T-cell abnormalities were seen, suggesting that other downstream effectors play a role.46 The thymic engraftment we observed in one experiment after culturing Notch4-IC cord cells for 3 days before injection into the mice could have been promoted by the culture. Ohishi et al44 showed that human cord cells cultured with soluble Delta1 resulted in high levels of thymic engraftment.
Despite structural differences, so far we have obtained similar results when Notch1-IC or Notch4-IC were overexpressed in human hematopoietic cells. The only domains homologous in Notch1-IC and Notch4-IC are (1) the RAM (RBP-Jκ–associated module) domain, which contains the high-affinity binding site for C-promoter binding factor 1 (CBF-1) (also called RBP-Jκ), a major effector of Notch-mediated regulation of gene expression, and (2) the ankyrin repeats, which are involved in protein-protein interaction. The ankyrin repeats and the C-terminal transactivating domain are essential for the transforming ability of Notch1 in T-cell leukemia, while the RAM domain is not.47 The inhibition of epithelial branching by Notch4 depends on both the RAM domain and the ankyrin repeats.48 This suggests that the 2 conserved domains in Notch1 and Notch4 play an important role in transformation and inhibition of differentiation and may explain the results obtained in this study.
All together, our data show that Notch4-IC, like Notch1-IC, plays a role in hematopoiesis in that it enhances engraftment, maintains stem cells, suppresses B-cell development while inducing abnormal double-positive T cells in both marrow and spleen, and suppresses myeloid differentiation. Therefore, Notch4 dysregulation could be involved in lymphohematopoietic malignancies. Further evaluation of this possibility is warranted.
Prepublished online as Blood First Edition Paper, July 1, 2004; DOI 10.1182/blood-2004-01-0204.
Supported by grants from the Leukemia Research Fund of Canada and from the National Cancer Institute of Canada with funds from the Canadian Cancer Society.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We wish to thank Keith Humphries and Donna Hogge for assistance with gene transfer and Richard Zapf, Cam Smith, and Gayle Thornbury for FACS operation. We also wish to thank Aly Karsan for Notch4 reagents and helpful discussions.