Abstract
HOXB4 overexpression induces unique in vivo and in vitro expansion of hemopoietic stem cells (HSCs) without causing leukemia. Very little is known about the molecular basis underlying HOXB4-induced HSC self-renewal. We now report the in vitro proliferation and in vivo expansion capacity of primary bone marrow (BM) cells engineered to overexpress selected HOXB4 point mutants lacking either the capacity to directly bind DNA (HOXB4(A)), or to cooperate with members of the PBX family (HOXB4(W→G)) in DNA binding. The DNA binding–incompetent HOXB4 mutant failed to enhance the proliferation activity of transduced BM populations in vitro and HSC expansion in vivo. In contrast, the HOXB4(W→G) mutant conferred a pronounced in vitro proliferation advantage to the transduced BM populations, and dramatically enhanced their in vivo regenerative potential. We also demonstrate a correlation between HOXB4 protein levels and in vitro proliferative capacity of primary BM cells. Our observations thus suggest that the capacity of HOXB4 to induce HSC expansions is DNA-binding dependent and does not require direct HOX/PBX interaction, and sets the stage for identifying HOXB4-dependent targets involved in HSC expansion.
Introduction
Most mature blood cells have short life spans, and are continuously generated from totipotent hemopoietic stem cells (HSCs). The distinct feature of HSCs is their ability to undergo self-renewal divisions, and to generate progeny that differentiate into mature cells of myeloid and lymphoid lineages. Although the genetic and molecular mechanisms responsible for the control of self-renewal and differentiation outcome of HSC divisions remain to be elucidated, it is becoming evident that Hox genes are involved in regulation of normal and leukemic hemopoiesis.1,2
Specifically, there is growing body of data demonstrating that in the mouse bone marrow (BM) transplantation model, retroviral overexpression of HOXB4 enhances HSC self-renewal and induces up to 1000-fold in vivo expansion.3-5 Recent studies have also shown that HOXB4 induced rapid ex vivo expansion of the transduced HSCs6 and demonstrated that ex vivo HSC expansion is achievable using a soluble recombinant HOXB4 protein.7,8 Importantly, the HOXB4-expanded HSCs retained their normal differentiation and long-term repopulation potential, and no hematologic abnormalities have been detected in large groups of mice that received transplants with HOXB4-transduced HSCs. In one study however, differentiation of human CD34+ cells was perturbed by the high expression levels of a fusion HOXB4 protein.9 Except for this study, the accumulated results indicate that HOXB4 activates a distinct pathway or pathways that enhances self-renewal divisions of HSCs without overriding the regulatory mechanisms that maintain normal steady-state hemopoiesis.
Molecular mechanisms and target genes responsible for HOXB4-induced HSC expansion remain to be elucidated. Due to the high degree of conservation within the homeodomain, different Hox proteins recognize similar regulatory sequences in vitro,10 although their specificity of sequence recognition,11,12 affinity of DNA binding,13,14 and transactivating potential15-17 can be modified through heterodimerization with PBX and Meis/Prep members of the Three Amino acid Loop Extension (TALE) family of transcription factors. Hox proteins encoded by paralogs one to 10 cooperatively bind DNA with Pbx proteins through a conserved hexapeptide sequence (YPWM) preceding the homeodomain (HD) of Hox proteins.11 Hox 9 through 13 proteins directly interact with Meis/PREP proteins.18 Paralogs 9 and 10 encode distinct proteins that interact with both PBX and PREP, and functional trimeric interactions between Hox, Pbx, and Meis on native Hox enhancers have been reported.19,20 Genetic studies revealed the requirement for Hox/Pbx16,21,22 and Hox/Pbx/Meis interactions20,23,24 for execution of some developmental programs, whereas some functions of Drosophila Hox homologs Ubx and AbdA were shown to be Pbx independent.25,26 Requirement for Hox/Pbx collaboration in regulation of hemopoietic cell behavior appears to be Hox protein– and cellular context–dependent. Hoxa9/Pbx interactions were essential for some27 but not all aspects of Hoxa9-mediated immortalization of primary BM cells.28 Mutations in the PBX interaction motif of Hoxb8 abrogated its ability to prevent differentiation of myeloid cells,29 but not collaboration between Pbx1b and Hoxb3 or Hoxa9 in induction of leukemic transformation could be detected.30,31 An additional level of complexity regarding the homeodomain protein activity was uncovered with Ftz whose activity in tissue patterning can be accomplished by a mutant lacking its HD.32
To further characterize the molecular mechanisms underlying the HOXB4-induced HSC self-renewal, we analyzed the requirement of the HOXB4-DNA and HOXB4-PBX interaction in execution of this function, by examining the in vitro and in vivo proliferation and differentiation capacity of primary BM cells engineered to express HOXB4 proteins carrying inactivation amino acid substitutions in the DNA-binding domain, or in the surface required for HOX-PBX interactions. We demonstrate that DNA binding is essential for HOXB4-induced proliferation of BM cells in vitro, and expansion of the transduced HSC population in vivo. We also show that direct HOXB4-PBX interactions involving the YPWM motif of HOXB4 are not required for its HSC-expanding activity, and suggest that the total levels of HOXB4 protein determine the magnitude of HOXB4-induced proliferation of primitive BM cells.
Materials and methods
Animals
Bone marrow donors were (PepC3) (C57B1/6Ly-Pep3b × C3H/HeJ)F1 mice, and recipients were (C57B1/6J × C3H/HeJ)F1 (LY5.2+) mice and donor/recipients (C57B16/Pep3b) (Ly5.1+) and (C57B1/6J) (LY5.2+). All animals were housed and handled in accordance with the guidelines of the Clinical Research Institute of Montreal animal care and use committee, which also approved the experiments.
DNA constructs, expression vectors, and retroviral vectors
The HOXB4(W→G) mutation (Figure 1A row 3) was generated by replacing the 24-bp AccI–PmlI fragment of HOXB4 cDNA with the corresponding oligonucleotide containing a T472G substitution, and was verified by sequencing. Generation of HOXB4(N→A) mutant (Figure 1A row 2) was described previously.33
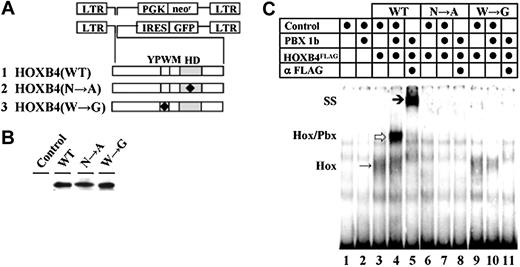
HOXB4, retroviral vectors, and DNA-binding properties of HOXB4 mutants examined. (A) Retroviral vectors and mutant HOXB4 cDNAs. (B) Western blot analyses of the in vitro–transcribed and translated WT and mutated HOXB4 proteins. (C) DNA-binding capacity of HOXB4, HOXB4(N→A), and HOXB4(W→G) proteins. Additions to each binding reaction are indicated above the lanes. Monomeric HOXB4 proteins bound to DNA are denoted by Hox to the left, and an arrow in lane 3. Heterodimers of HOX and PBX are denoted as Hox/Pbx, and by an arrow in lane 4. The position of anti-HOXB4 antibody supershifted complex is denoted by SS to the left, and an arrow in lane 5.
HOXB4, retroviral vectors, and DNA-binding properties of HOXB4 mutants examined. (A) Retroviral vectors and mutant HOXB4 cDNAs. (B) Western blot analyses of the in vitro–transcribed and translated WT and mutated HOXB4 proteins. (C) DNA-binding capacity of HOXB4, HOXB4(N→A), and HOXB4(W→G) proteins. Additions to each binding reaction are indicated above the lanes. Monomeric HOXB4 proteins bound to DNA are denoted by Hox to the left, and an arrow in lane 3. Heterodimers of HOX and PBX are denoted as Hox/Pbx, and by an arrow in lane 4. The position of anti-HOXB4 antibody supershifted complex is denoted by SS to the left, and an arrow in lane 5.
To generate expression vectors encoding C-terminal FLAG-tagged proteins, wild-type (WT) and mutated forms of HOXB4 were subcloned upstream and in frame of FLAG epitope in pCMV-Tag (Stratagene, La Jolla, CA). In vitro transcription vectors were created by subcloning FLAG-tagged WT and mutated HOXB4 cDNAs into pcDNA3 (Invitrogen, Carlsbad, CA). Retroviral vectors encoding the FLAG-tagged WT and HOXB4(W→G) mutant were generated by subcloning the corresponding cDNA into myeloproliferative sarcoma virus phosphoglycerate kinase and/or neomycin resistance gene (MSCV-PGKneor) upstream of the PGKneor cassette (MSCV-HOXB4-FLAG-PGK-neo no. 830, MSCV-HOXB4(W→G)-FLAG-PGK-neo no. 867). MSCV–internal ribosome entry site (IRES)–green fluorescent protein (GFP) retroviral vectors encoding WT and both mutated HOXB4 forms were created by subcloning the various HOXB4 cDNAs upstream of IRES (MSCV-HOXB4-IRES-GFP no. 812, MSCV-HOXB4(N→A)-IRES-GFP no. 815 and MSCV-HOXB4(W→G)-IRES-GFP no. 827).
Cell lines, viral production, and infection of primary murine bone marrow cells
Maintenance of ecotropic retrovirus packaging BOSC-23 and GP+E-86, and amphotropic vesicular stomatitis virus (VSV) pseudotyped retrovirus packaging cells 293 GPG, generation of high-titer helper-free GP+E-86 producer cells, and infection of primary BM cells were described.34 BaF/3 cells were maintained and infected as described.35
In vitro proliferation of primary bone marrow cells
To exclude potential pseudo-transduction effect, BM cells recovered after coculture with retroviral producers were cultured for 2 days in Iscove modified Dulbecco medium (IMDM) supplemented with 15% fetal calf serum (FCS) and 10 ng/mL interleukin 3 (IL-3), then the transduced GFP+(HOXB4+) cells were sorted. Competitor cells were prepared as described.36 Following a 1-day recovery period, liquid cultures were initiated by resuspending 5 × 104 GFP+ BM cells/mL in IMDM with 15% FCS and 10 ng/mL IL-3. After indicated periods of growth, the viable (trypan blue negative) cells were counted and diluted with fresh media so that cell density was maintained between 5 × 104 and 5 × 105 cells/mL. At the same points in time, suitable aliquots of cultures were plated in methylcellulose containing 10 ng/mL IL-3, 10 ng/mL IL-6, 50 ng/mL stem cell factor (SCF), and 5 U/mL of erythropoietin (Epo). Colonies were scored on day 10. To determine the in vitro competitive proliferation potential of the transduced cells, cultures comprising 10% GFP+ plus 90% nontransduced competitors were initiated at a density of 5 × 104 cells/mL, and the relative contents of GFP+ cells after 5- to 6- and 10- to 11-day incubations were determined by flow cytometry. To compare the in vitro competitive proliferation potential of HOXB4high and HOXB4low BM cells, liquid culture comprising 20% HOXB4high (Ly5.1+ GFPhigh) and 80% HOXB4low (Ly5.2+ GFPlow) was initiated at 1 × 105 cell/mL in IMDM supplemented with 15% FCS, 6 ng/mL IL-3, 10 ng/mL IL-6, and 100 ng/mL SCF, and proportions of HOXB4high (Ly5.1+) cells were determined by flow cytometry at indicated time points. IL-3–induced proliferation responses of the transduced BM populations were determined using a 3H-thymidine incorporation assay as described.37 Methylcellulose and CV-1 cell line transformed with an origin-defective SV40 virus (COS) cell supernatant-derived cytokines used for these experiments were prepared and quantitated at Institut de Recherches Cliniques de Montréal (IRCM). All other media components were purchased from GIBCO/Invitrogen (Burlington, ON, Canada).
Generation of bone marrow transplantation chimeras
Recipient mice were irradiated with 850 cGy (160 cGy/min, 137Cs γ; J.L., Shepherd and Associates, San Fernando, CA). Groups of control neor, HOXB4(N→A)neor and HOXB4(WT)neor BM transplantation chimeras were generated by transplanting 1.2-1.5 × 105 BM cells recovered from cocultures with retroviral producers as described.5 To generate groups of control GFP+, or HOXB4(W→G)GFP+ or HOXB4(WT)GFP+ recipients, BM cells recovered from cocultures with retroviral producers were cultured for an additional 3 days, then transplantation inocula (4 × 105 cells/recipient) comprising 10% GFP+ cells were prepared by diluting the infected BM populations with nontransduced competitors. The frequencies of myeloid and lymphoid progenitors in the hemopoietic tissues of mice undergoing transplantation were determined as described.38
CRU assays
HSC numbers in primary recipients were evaluated using a limiting dilution transplantation-based assay (competititive repopulation unit [CRU] assay) that detects cells with competitive, long-term lympho-myeloid repopulation capacity.39 Primary mice were killed 12 weeks after transplantation, and their BM cells were transplanted into a series of secondary recipients at varying dilutions (5 × 103 to 1 × 106 cells/recipient, 5-10 recipients/dilution) along with 1 × 105 freshly isolated helper bone marrow cells. The level of lymphoid and myeloid repopulation with Ly5.1+ or GFP+ (donor derived) cells in secondary recipients was determined 16 weeks after transplantation by flow cytometric analysis of peripheral blood.40 CRU frequencies were calculated by applying Poisson statistics to the proportion of negative recipients at different dilutions using Limit Dilution Analysis software (Stem Cell Technologies, Vancouver, BC, Canada).
Flow cytometry
GFP+ cells were sorted using FACSStarPlus equipped with a 488-nm argon laser or MoFlo (Cytomation, Fort Collins, CO). Proportions of Ly5.1+ cells were determined by flow cytometry at indicated days using phycoerythrin-conjugated Ly5.1 antibody (Pharmingen, Mississauga, ON, Canada). Discrimination by flow cytometry of myeloid and lymphoid cells was confirmed using allophycocyanin (APC)–conjugated antibodies recognizing cell surface lineage-specific markers (Mac-1, B220, CD4, and CD8; Pharmingen) as described.36
Southern and Western blot analyses
Southern blot analyses were performed as described previously.30 The probes used were 0.9-kb neor cDNA, 0.73-kb GFP cDNA, and 1.4-kb erythropoietin receptor cDNAs,35 labeled with 32P by random primer extension.
Total cell lysate and Western blot were performed as described.41 The primary antibodies used were mouse anti-FLAG antibody (Stratagene), rat anti-HOXB4 antibody (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City), mouse anti–β-tubulin (Sigma, St Louis, MO), and rabbit anti–c-Jun (Santa Cruz Biotechnology, Santa Cruz, CA) followed by horseradish peroxydase–conjugated antimouse, antirat, and antirabbit antibodies (Santa Cruz Biotechnology).
Pulse-chase analysis
Pulse-chase experiments were performed as described.42 The amount of radioactive and the total amount of FLAG-tagged proteins in each lane were measured using the STORM 860 and the ImageQuant 5.0 program (Molecular Dynamics, Sunnyvale, CA). The half-life was calculated from the proportions of radioactive proteins at the indicated time points using AllFit (Charles and André DeLéan, University of Montreal, QC, Canada).
Electrophoretic mobility shift assays (EMSAs)
EMSAs were performed as described previously13 using proteins produced by transcription and translation coupled reticulocyte lysate system (TNT) coupled as recommended in manufacturer's instructions (Promega, Madison, WI). The DNA probe was gel purified oligonucleotide 5′-CGA ATT GAT TGA TGC ACT AAT TGG AG-3′. For supershift analyses, DNA binding reactions were incubated for an additional 30 minutes with 1 μg anti-FLAG antibody (Stratagene).
Results
Generation of wild-type and mutant HOXB4 proteins
The requirement for HOXB4 DNA binding in regulation of HOXB4-induced expansion of HSCs was examined using point mutation N51→A within helix 3 of the homeodomain (Figure 1A row 2, HOXB4(N→A)),43,44 while the role of direct HOXB4-PBX interactions was assessed through W149G mutation in the conserved tetrapeptide (Figure 1A row 3, HOXB4(W→G)). 45 Wild-type (HOXB4(WT)) and mutant HOXB4 cDNAs produced proteins of the predicted sizes as detected by Western blot analyses of the in vitro–transcribed and translated protein carrying a C-terminal FLAG epitope (Figure 1B). EMSAs confirmed that the N51A mutation in the homeodomain of HOXB4(N→A) abolished the DNA binding capacity of protein (Figure 1C, compare lanes 3-5 with lanes 6-8). HOXB4(W→G) retained the ability to bind DNA as monomer (Figure 1C, lane 9 vs lane 3) but could not form heterodimers with PBX1 (Figure 1C, lanes 3-5 vs lanes 9-11).
DNA binding ability of HOXB4 is essential for enhancing the in vitro proliferation potential of BM cells
HOXB4 overexpression confers a pronounced in vitro proliferation advantage to transduced BM cells.5,6 To determine whether this HOXB4 function depends on its ability to bind DNA, mouse BM cells were infected with recombinant retroviruses encoding HOXB4(WT) and GFP, or HOXB4(N→A) and GFP, or GFP alone. In the in vitro competitive proliferation assays initiated with 10% of GFP+ cells (HOXB4(WT)+, HOXB4(N→A)+, or control GFP+), the proportions of HOXB4(WT) cells increased to approximately 80% within 10 days (Figure 2A). In cultures initiated with HOXB4(N→A), or with control GFP+ cells, no noticeable increase in the proportions of GFP+ cells above the starting 10% could be detected. The in vitro expansion of myeloid clonogenic progenitors (CFCs) was determined in cultures initiated with equal numbers of GFP+ cells sorted from the 3 populations examined. During a 10-day expansion, HOXB4(WT) CFCs increased more than 1000-fold over initial numbers, compared with a 5- to 8-fold increase determined for HOXB4(N→A) and control GFP CFCs (Figure 2B), suggesting that DNA-binding activity of HOXB4 is needed to enhance the in vitro proliferation capacity of the transduced BM cells.
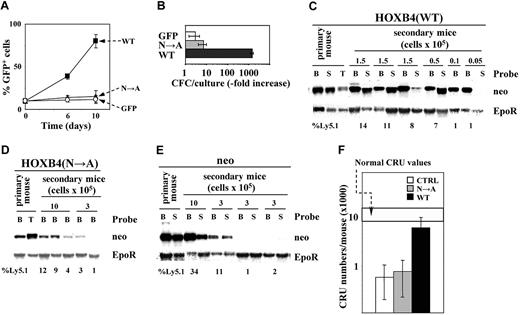
Intact HOXB4 homeodomain is essential for HOXB4-induced expansion of primitive BM cells. (A) Expansion of GFP+ cells in liquid cultures initiated with 10% GFP+ (control, or HOXB4(N→A)+, or HOXB4(WT)+) cells, and 90% of nontransduced competitors. ▪ indicates HOXB4(WT); ▴, HOXB4(N→A); and ○, control GFP. Results represent mean values ± the standard deviation (SD) of a representative experiment (n = 3) performed in duplicate. (B) In vitro expansion over a 10-day period of myeloid CFCs derived from indicated sorted populations of transduced BM cells. Results represent mean values ± SD of a representative experiment (n = 2) performed in quadruplicate cultures. (C) Southern blot analyses of proviral integrations in DNA isolated from bone marrow (B), spleen (S), and thymus (T) of primary recipients, and the secondary recipients of HOXB4(WT). Individual recipients and the numbers of transplanted cells are shown on top, and the proportions of transplant-derived Ly5.1 cells in these recipients are listed bellow each lane. DNA was digested with KpnI, which releases the integrated provirus. Probes are listed to the right. Erythropoietin receptor (EpoR)–derived signal is representative of DNA loading. Southern blot analyses of proviral integrations in DNA isolated from hemopoietic tissues of primary and secondary HOXB4(N→A) (D) and neor recipients (E) were performed as described for panel C. (F) Total CRU recovery following competitive reconstitution with neor-, HOXB4(N→A)-, or HOXB4(WT)-transduced BM cells determined for each group of recipients (mean ± 95% CI). □ indicates neor recipients; ▦, HOXB4(N→A) recipients; and ▪, HOXB4(WT) recipients.
Intact HOXB4 homeodomain is essential for HOXB4-induced expansion of primitive BM cells. (A) Expansion of GFP+ cells in liquid cultures initiated with 10% GFP+ (control, or HOXB4(N→A)+, or HOXB4(WT)+) cells, and 90% of nontransduced competitors. ▪ indicates HOXB4(WT); ▴, HOXB4(N→A); and ○, control GFP. Results represent mean values ± the standard deviation (SD) of a representative experiment (n = 3) performed in duplicate. (B) In vitro expansion over a 10-day period of myeloid CFCs derived from indicated sorted populations of transduced BM cells. Results represent mean values ± SD of a representative experiment (n = 2) performed in quadruplicate cultures. (C) Southern blot analyses of proviral integrations in DNA isolated from bone marrow (B), spleen (S), and thymus (T) of primary recipients, and the secondary recipients of HOXB4(WT). Individual recipients and the numbers of transplanted cells are shown on top, and the proportions of transplant-derived Ly5.1 cells in these recipients are listed bellow each lane. DNA was digested with KpnI, which releases the integrated provirus. Probes are listed to the right. Erythropoietin receptor (EpoR)–derived signal is representative of DNA loading. Southern blot analyses of proviral integrations in DNA isolated from hemopoietic tissues of primary and secondary HOXB4(N→A) (D) and neor recipients (E) were performed as described for panel C. (F) Total CRU recovery following competitive reconstitution with neor-, HOXB4(N→A)-, or HOXB4(WT)-transduced BM cells determined for each group of recipients (mean ± 95% CI). □ indicates neor recipients; ▦, HOXB4(N→A) recipients; and ▪, HOXB4(WT) recipients.
HOXB4-DNA interactions are essential for enhancing the repopulation potential of the transduced HSCs
The correlation between the in vivo HSC expansion of HOXB4 and its ability to bind DNA was assessed using the CRU assay.3,39 Transplantation chimeras were generated by injecting Ly5.1+ BM cells engineered to overexpress HOXB4(WT) or HOXB4(N→A) or control neor genes into lethally irradiated Ly5.2+ congenic recipients. At 12 weeks after transplantation, BM cells were harvested and transplanted in increasing dilutions into secondary recipients. The contribution of transplant-derived (Ly5.1+) transduced cells to lympho-myeloid reconstitution of secondary recipients was determined 16 weeks after transplantation by flow cytometry, and by Southern blot analyses of integrated proviruses in genomic DNA isolated from hemopoietic tissues of secondary recipients (Figure 2C-E). All integrated HOXB4(WT) (Figure 2C), HOXB4(N→A) (Figure 2D), and neor (Figure 2E) proviruses were free of rearrangements. There was a very good correlation observed between the percentage of donor-derived Ly5.1+ peripheral blood (PB) cell analysis, and the intensity of proviral signals revealed by a neor-specific probe in the autoradiograms (Figure 2D-E). Levels of HOXB4 expression also correlated with these parameters (not shown). These results suggested that low levels of transplant-derived reconstitution determined for HOXB4(N→A) recipients were due to the compromised function of this mutant.
The frequencies of transduced donor-derived CRUs were determined as described.3,39 At 16 weeks after transplantation, the frequency of HOXB4(WT)+ CRU was 1 in 30 000 (95% confidence interval [CI]: 1 in 10 000 to 1 in 85 000), compared with 1 in 260 000 (95% CI: 1 in 93 000 to 1 in 720 000) and 1 in 350 000 (95% CI: 1 in 120 000 to 1 in 1 000 000) as determined for HOXB4(N→A)+ and control neor CRUs, respectively. Assuming a total of 2 × 108 hemopoietic cells per adult mouse, the transplanted HOXB4(WT)+ HSCs generated a progeny of approximatively 6700 CRUs (Figure 2F). The transplanted HOXB4(N→A)+ and control neor HSCs, in contrast, generated progeny comprising approximatively 800 and 600 cells, respectively, suggesting that DNA binding is essential for HOXB4-induced in vivo expansion of HSCs, and implying a direct role for HOXB4 in transcriptional control of the gene or genes that regulate HSC self-renewal.
PBX binding–deficient HOXB4 mutant enhances the in vitro proliferation activity of the transduced BM cells
We previously reported that knockdown of endogenous PBX1 protein in HOXB4-overexpressing cells generated HSCs that were more than 20 times more competitive than those overexpressing HOXB4.36 To assess whether this increase in repopulation potential reflected direct de-repression of HOXB4 activity, or modulation of a parallel PBX-dependent pathway or pathways involved in regulation of self-renewal, we examined the in vitro and in vivo activity of BM cells engineered to express HOXB4 carrying amino acid substitution in the interface required for interactions with PBX (YPWM→YPGM).
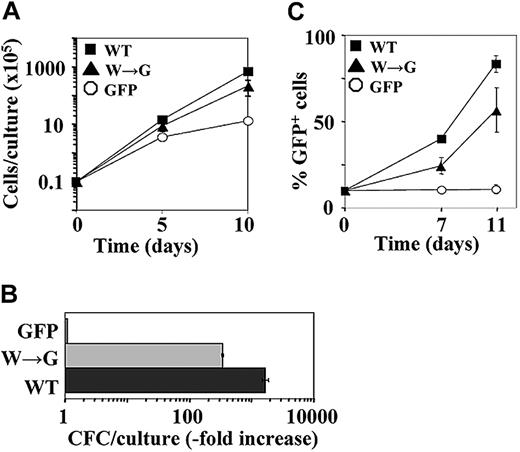
BM cells were infected with recombinant retroviruses encoding HOXB4(WT) and GFP, or HOXB4(W→G) and GFP, or GFP alone. In cultures initiated with sorted GFP+ cells, the numbers of HOXB4(W→G) and HOXB4(WT) cells increased 16- and 54-fold, respectively, above the input values compared with an approximately 10-fold increase determined for controls (Figure 3A). During a 10-day expansion period, the numbers of HOXB4(W→G) and HOXB4(WT) CFCs increased 380- and 1870-fold above the input, compared with a 7-fold increase determined for control CFCs (Figure 3B). Both HOXB4(W→G) and HOXB4(WT) also enabled the in vitro expansion of multipotent clonogenic progenitors (CFU-GEMM), which in control cultures could not be detected after day 4 (data not shown). In the in vitro competitive proliferation assays initiated with 10% GFP+ cells (control, HOXB4(W→G)+, or HOXB4(WT)+) and 90% nontransduced competitors, the proportions of HOXB4(W→G) and HOXB4(WT) cells increased within 11 days to 57% ± 13% and 83.5% ± 4.9, respectively, while in control cultures no increase of GFP+ cells above the starting 10% could be detected (Figure 3C). Together, these data indicate that HOXB4 mutant with dysfunctional PBX interacting surface retained the ability to confer the in vitro proliferation advantage to the transduced BM cells.
W→G substitution in the PBX-interacting motif abolishes the in vitro proliferation function of HOXB4. (A) Expansion of total nucleated cells over a 10-day period in cultures initiated with sorted GFP+ cells. ▪ indicates HOXB4(WT); ▴, HOXB4(W→G); and ○, control GFP. Results represent mean value ± SD of a representative experiment (n = 5) performed in duplicate. (B) In vitro expansion over a 10-day period of myeloid CFCs derived from the indicated populations of transduced BM cells. Results represent mean values ± SD of a representative experiment (n = 2) performed in quadruplicate cultures. ▪ indicates HOXB4 cells; ▦, HOXB4(W→G) cells; and □, GFP cells. (C) Expansion of GFP+ cells in liquid cultures initiated with 10% GFP+ cells (▪, HOXB4(WT)+; ▴, HOXB4(W→G)+; ○, control GFP+) and 90% nontransduced competitors. Results represent mean values ± SD of a representative experiment (n = 3) performed in duplicate.
W→G substitution in the PBX-interacting motif abolishes the in vitro proliferation function of HOXB4. (A) Expansion of total nucleated cells over a 10-day period in cultures initiated with sorted GFP+ cells. ▪ indicates HOXB4(WT); ▴, HOXB4(W→G); and ○, control GFP. Results represent mean value ± SD of a representative experiment (n = 5) performed in duplicate. (B) In vitro expansion over a 10-day period of myeloid CFCs derived from the indicated populations of transduced BM cells. Results represent mean values ± SD of a representative experiment (n = 2) performed in quadruplicate cultures. ▪ indicates HOXB4 cells; ▦, HOXB4(W→G) cells; and □, GFP cells. (C) Expansion of GFP+ cells in liquid cultures initiated with 10% GFP+ cells (▪, HOXB4(WT)+; ▴, HOXB4(W→G)+; ○, control GFP+) and 90% nontransduced competitors. Results represent mean values ± SD of a representative experiment (n = 3) performed in duplicate.
HOXB4(W→G) confers competitive repopulation advantage to the transduced HSCs
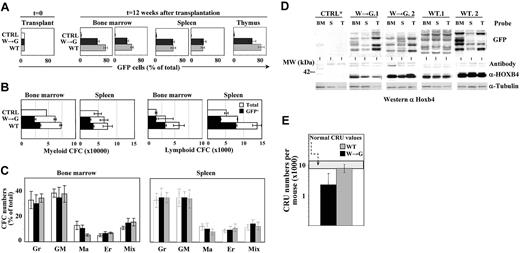
Three groups of BM transplantation chimeras were generated by transplanting a mixture of 10% GFP+ cells (HOXB4(W→G)+, HOXB4(WT)+, or control GFP+) and 90% nontransduced competitors in sublethally irradiated recipients. All recipients remained healthy for more than 12 months of observation. Contribution of the transplant-derived GFP+ cells to myeloid and lymphoid repopulation of recipients was determined 12 weeks after transplantation. In HOXB4(W→G) and HOXB4(WT) recipients (Figure 4A black and gray bars, respectively), GFP+ cells initially representing 10% of the graft accounted for 55% to 80% of bone marrow–derived myeloid (Mac-1+), 35% to 50% of splenic B-lymphoid (B-220+), and 60% to 80% of thymic CD4+CD8+ cell populations (Figure 4A). As expected, GFP+ control cells showed no proliferation advantage in this system (Figure 4A white bars). In both groups, GFP+ CFCs represented 45% to 55% of myeloid, and 30% to 45% of B-cell progenitor populations (Figure 4B), and no abnormalities in the relative proportions of myeloid CFCs could be detected (Figure 4C). Together, these observations suggest that HOXB4(W→G) retained the ability to confer competitive reconstitution advantage to the transduced cells without affecting their differentiation.
Hemopoietic reconstitution and expansion of HSCs induced by HOXB4 are independent of its collaboration with PBX. (A) Flow cytometric analysis of BM, splenic, and thymic cell populations of mice that received transplants of comparable numbers of GFP+ control, HOXB4(W→G), or HOXB4(WT)-transduced BM cells. Proportions of GFP+ cells in myeloid (Mac-1) and lymphoid (B-220 and CD4/CD8) populations were determined for recipients killed 12 weeks after transplantation. Results shown represent mean values ± SD determined for 4 mice analyzed in each group of recipients. (B-C) CFC content (B) and distribution (C) in BM and spleen of recipients described for panel A. Colonies were identified as containing predominantly granulocytes (Gr), granulocytes and macrophages (GM), macrophages (Ma), erythrocytes (Er), or a mixture of these cells together with megakaryocytes (Mix). (D) Top panel: representative Southern blot analysis of proviral integration patterns in hemopoietic tissues isolated from primary recipients of control GFP, HOXB4(W→G), or HOXB4(WT)-transduced BM cells presented in panel A. DNA was digested with EcoRI, which cuts once within the integrated provirus, such that each band represents a unique integration event on blots probed with GFP. Bottom panel: Western blot of HOXB4 levels in bone marrow, spleen, and thymus of recipients identified in panel D. Tubulin levels are shown as control for protein loading and transfer. (E) HSC regeneration in recipients of HOXB4(W→G) or HOXB4(WT)-transduced BM cells. For each group of recipients, results represent mean values ± 95% CI determined for 2 primary recipients.
Hemopoietic reconstitution and expansion of HSCs induced by HOXB4 are independent of its collaboration with PBX. (A) Flow cytometric analysis of BM, splenic, and thymic cell populations of mice that received transplants of comparable numbers of GFP+ control, HOXB4(W→G), or HOXB4(WT)-transduced BM cells. Proportions of GFP+ cells in myeloid (Mac-1) and lymphoid (B-220 and CD4/CD8) populations were determined for recipients killed 12 weeks after transplantation. Results shown represent mean values ± SD determined for 4 mice analyzed in each group of recipients. (B-C) CFC content (B) and distribution (C) in BM and spleen of recipients described for panel A. Colonies were identified as containing predominantly granulocytes (Gr), granulocytes and macrophages (GM), macrophages (Ma), erythrocytes (Er), or a mixture of these cells together with megakaryocytes (Mix). (D) Top panel: representative Southern blot analysis of proviral integration patterns in hemopoietic tissues isolated from primary recipients of control GFP, HOXB4(W→G), or HOXB4(WT)-transduced BM cells presented in panel A. DNA was digested with EcoRI, which cuts once within the integrated provirus, such that each band represents a unique integration event on blots probed with GFP. Bottom panel: Western blot of HOXB4 levels in bone marrow, spleen, and thymus of recipients identified in panel D. Tubulin levels are shown as control for protein loading and transfer. (E) HSC regeneration in recipients of HOXB4(W→G) or HOXB4(WT)-transduced BM cells. For each group of recipients, results represent mean values ± 95% CI determined for 2 primary recipients.
To verify that hemopoietic regeneration of HOXB4(W→G) and HOXB4(WT) recipients was mediated by pluripotent transduced HSCs, and to determine the numbers of cellular clones that participated in reconstitution, Southern blot analyses were performed to analyze proviral integrations in genomic DNA isolated from BM, spleen, and thymus of selected recipients (Figure 4D top panels). In individual HOXB4(W→G) and HOXB4(WT) recipients, the same proviral integration pattern could be detected in BM, spleen, and thymus, illustrating the pluripotent nature of several of the reconstituting HSC clones. In both groups, multiple proviral integrations of varying signal intensities indicated the ongoing activity of several independent clones in the indicated mice. Importantly HOXB4 overexpression was demonstrated in the hemopoietic tissues of recipients in both groups (Figure 4D bottom panel).
The numbers of transduced HSCs that reconstituted HOXB4(W→G) and HOXB4(WT) transplantation chimeras were determined using CRU assay. At 16 weeks after transplantation, the CRU frequency in recipients of HOXB4(W→G)-transduced cells was 1 in 92 000 (95% CI: 1 in 51 000 to 1 in 164 000) for a total of approximately 2200 CRU per recipient, compared with a frequency of 1 in 26 000 (95% CI: 1 in 20 000 to 1 in 36 000) and 7700 total CRUs in mice reconstituted with HOXB4(WT)-transduced cells (Figure 4E). Given that all groups of primary recipients received an estimated 15 transduced HSCs, CRU expanded by approximately 150-fold and approximately 500-fold in the HOXB4(W→G) and HOXB4(WT) chimeras, respectively. This expansion occurred in conditions that resulted in complete loss of control GFP+ HSCs (not shown).
Levels of HOXB4 protein determine the magnitude of HOXB4-induced BM expansion
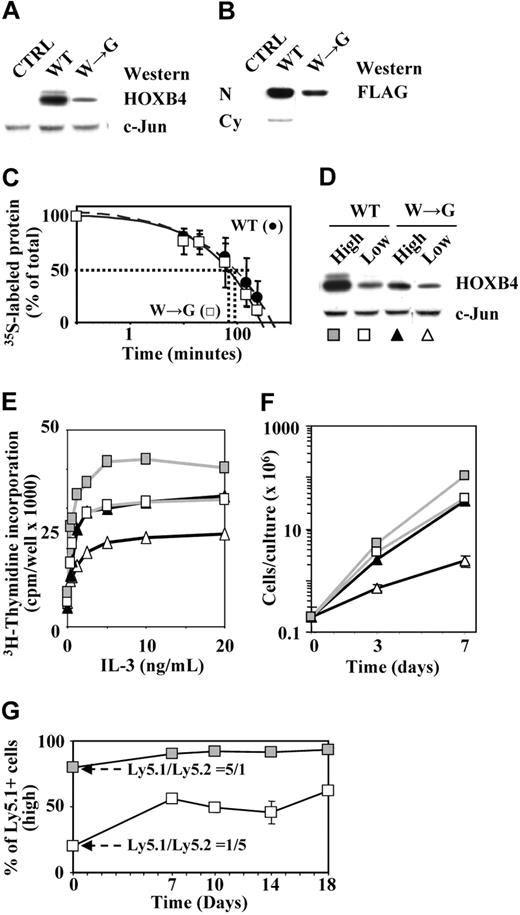
Results presented in the previous section indicated that HOXB4(W→G) was less efficient in inducing HSC expansion than HOXB4(WT), suggesting a possible functional impairment of HOXB4(W→G) protein. In freshly infected cells, HOXB4(W→G) consistently appeared to be expressed at lower levels than HOXB4(WT) (Figure 5A). Importantly, HOXB4(W→G) was capable of translocating into nuclear compartments (Figure 5B), and had an intracellular half-life (70 minutes) comparable to that determined for HOXB4(WT) (Figure 5C), indicating that neither intracellular compartmentalization nor protein stability was affected by this mutation. The apparently diminished biologic activity of HOXB4(W→G) could thus simply reflect lower levels of available HOXB4(W→G) protein. To examine this possibility, we sorted HOXB4(WT)- and HOXB4(W→G)-transduced BM cells in populations expressing low or high levels of HOX proteins (GFPlowHOXB4low or GFPhighHOXB4high; Figure 5D) and determined their in vitro proliferation activity. Cells expressing comparable levels of HOXB4(W→G) (Figure 5D, W→Ghigh, filled triangles) and HOXB4(WT) (Figure 5D, WTlow, squares) proteins were indistinguishable in their proliferation responses to cytokine stimulation as determined by 3H-thymidine incorporation (Figure 5E), and generated comparable numbers of progeny during a 7-day expansion (Figure 5F). The apparently diminished potential of HOXB4(W→G) thus likely reflected lower levels of available HOXB4(W→G) protein, and not functional impairment resulting from the loss of ability to interact with PBX in DNA binding. To complement these experiments and further validate this point, in vitro competitive assays were initiated with mixturecomprising20%ofHOXB4HighGFPHighLy5.1+and80%HOXB4Low-GFPLowLy5.2+. The proportion of Ly5.1+ (HOXB4high) cells increased over time in culture, and represented by day 18 approximately 60% of the total cell population (Figure 5G), indicating that an increase in HOXB4 levels enhanced the competitive proliferation advantage of the transduced cells.
Levels of available HOXB4 protein determine the magnitude of HOXB4-induced proliferation responses. (A) Western blot analyses of HOXB4 levels in HOXB4(WT)- or HOXB4(W→G)-transduced primary BM cells. (B) Accumulation of HOXB4(WT) and HOXB4(W→G) proteins in nuclear compartments of the transduced BaF3 cells. N indicates nuclear extracts; and Cy, cytoplasmic fraction. (C) Pulse-chase analysis of HOXB4(WT) and HOXB4(W→G) proteins. (D) HOX protein levels in HOXB4(WT) and HOXB4(W→G)-transduced BM cells sorted in subpopulations expressing high (filled symbols) or low (open symbols) levels of GFP. c-Jun levels are shown as a loading control. (E) IL-3–induced proliferation responses of populations shown in panel D. (F) Expansion of total nucleated cells over a 7-day period in cultures initiated with populations of cells sorted as described for panel D. Results represent mean values ± SD of a representative experiment (n = 2) performed in duplicate. (G) Representative assay showing preferential expansion of HOXB4highLy5.1+ cells in liquid cultures initiated with a 1:5 mixture of HOXB4highLy5.1+ and HOXB4lowLy5.2+ BM cells.
Levels of available HOXB4 protein determine the magnitude of HOXB4-induced proliferation responses. (A) Western blot analyses of HOXB4 levels in HOXB4(WT)- or HOXB4(W→G)-transduced primary BM cells. (B) Accumulation of HOXB4(WT) and HOXB4(W→G) proteins in nuclear compartments of the transduced BaF3 cells. N indicates nuclear extracts; and Cy, cytoplasmic fraction. (C) Pulse-chase analysis of HOXB4(WT) and HOXB4(W→G) proteins. (D) HOX protein levels in HOXB4(WT) and HOXB4(W→G)-transduced BM cells sorted in subpopulations expressing high (filled symbols) or low (open symbols) levels of GFP. c-Jun levels are shown as a loading control. (E) IL-3–induced proliferation responses of populations shown in panel D. (F) Expansion of total nucleated cells over a 7-day period in cultures initiated with populations of cells sorted as described for panel D. Results represent mean values ± SD of a representative experiment (n = 2) performed in duplicate. (G) Representative assay showing preferential expansion of HOXB4highLy5.1+ cells in liquid cultures initiated with a 1:5 mixture of HOXB4highLy5.1+ and HOXB4lowLy5.2+ BM cells.
Together, the results presented in this report show that the capacity of HOXB4 to enhance HSC proliferation depends on its ability to bind DNA. Our observations also indicate that HOXB4-induced HSC expansion represents a PBX-independent function of this transcription factor, and establish a strong correlation between protein levels of HOXB4 and in vitro biologic activity. These studies also validate future efforts to identify HOXB4-dependent target genes involved in HSC activity.
Discussion
Results presented in this paper indicate that the DNA-binding ability of HOXB4 is essential for its HSC-expanding function and suggest that HOXB4 acts as a transcriptional regulator of genes that govern self-renewal. These observations are consistent with results of studies showing that the transforming potentials of NUP98-Hoxa946 and NUP98-Hoxd1347 are absolutely dependent on the integrity of their respective homeodomains. Moreover, these homeodomains fused to NUP98 retained their leukogenic potential,48 indicating that upon acquisition of transactivating function provided by NUP98, homeodomains were sufficient for activation of Hox-responsive genes.
In transactivation assays, only a weak suppressive effect of HOXB4 on expression of reporter genes driven by synthetic promoters comprising HOX target sites could be detected (M. Featherstone, unpublished observations, June 2001). In agreement with the repressive role proposed for HOX4 paralogs,17 HOXB4 suppressed transcription of Ras antagonist X-Rap1 during Xenopus embryonic development,49 and repressed expression of c-MYC in hemopoietic cell line HL-60.50,51 However, HOXB4 also acted as a positive regulator of Drosophila Iroquois 552 and Flash transcription,53 indicating that cellular context plays a major role in defining the nature of HOXB4 regulatory activity.
HOXB4 could thus promote HSC self-renewal by suppressing transcription of genes implicated in commitment and differentiation, or by enhancing expression of a distinct element or elements that enables self-renewal divisions and maintenance of “stemness.” To discriminate between these 2 possibilities we generated chimeras comprising HOXB4 and engrailed repression domain,54 or HOXB4 and VP16 activation domain,54,55 and examined their activity in the mouse BM model system (N.B. and M.L., unpublished observations, February 2004). Preliminary results suggest that addition of strong repressor domain abolished the ability of HOXB4 to confer the in vitro and in vivo competitive proliferation advantage to the transduced BM cells. In contrast, addition of VP-16 transactivating domain failed to enhance activity of HOXB4 above the levels determined for HOXB4(WT), implying that HOXB4 acts as a positive regulator of a stem cell–specific gene or genes. Moreover, in the transduced BM populations VP-16/HOXB4 induced a major up-regulation of endogenous HOXB4 protein, suggesting an evolutionally conserved HOXB4 auto-regulatory loop.56 An increase in HOXB4 levels has also been reported in conjunction with Tpo-promoted57 and Wnt3a-promoted58 HSC self-renewal, implying that up-regulation of HOXB4 could represent an initiating event in this process.
We reported previously that HOXB4 and PBX cooperated in enhancing proliferation in a fibroblast model system.33 PBX knockdown, in contrast, significantly enhanced the competitive repopulation capacity of HOXB4-transduced HSCs,36 suggesting that requirement for HOX-PBX interactions depends on the cellular context. The latter study relied on examination of genetic interactions between HOXB4 and PBX1, and could not exclude the possibility that other members of the TALE family compensated for the absence of PBX1 in HOXB4-promoted HSC expansion. However, the HOXB4(W→G) mutant examined in this study retained the HOXB4-specific stem cell–expanding potential despite the W→G substitution that prevented the cooperative HOX/PBX DNA binding. The Hoxa9-induced HSC expansion during the preleukemic phase38 is comparable to expansions determined for HOXB4. Interestingly, the Hoxa9 mutant lacking the ability to interact with PBX was capable of immortalizing myeloid CFCs,28 and NUP98-HOXA9 carrying a W→G substitution enhanced proliferation of the transduced BM cells34 and induced leukemic transformation (E. Kroon and G.S., manuscript in preparation). Direct HOX/PBX interactions thus appear to be dispensable in the context of HOX-promoted stem cell proliferation.
Differences in protein expression levels in cells transduced with wild-type versus W→G mutant constructs remain uncertain. Our observations indicate that there was no difference in titers between the 2 retroviruses and the half-life of both wild-type and mutant W→G proteins are comparable (Figure 3C). This, combined with the results indicating that mRNA levels are comparable between bone marrow cells transduced with wild-type versus mutant Hoxb4 cDNA, suggest that the W→G point mutation may interfere with Hoxb4 translation.
Based on our recent studies demonstrating that down-regulation of PBX1 significantly enhanced the competitive activity of HOXB4-transduced BM cells,36 the expectation was that our HOXB4(W→G) mutant would behave as a “super HOXB4” protein. Although we cannot formally exclude the possibility of some in vivo HOXB4-PBX interactions involving nonidentified HOX domains as suggested with HOXA9,28 it appears that the likely explanation for these observations is that HOXB4 and PBX genes act on distinct pathways in these cells.
In summary, studies presented in this report show that the homeodomain-mediated HOXB4-DNA interactions are essential for a HOXB4-induced increase in the self-renewal activity of HSCs and indicate that direct HOXB4-PBX interactions involving the YPWM motif are not required for this function. In future studies, these observations will form the basis for the identification of HOX-activated genes contributing to HSC self-renewal.
Prepublished online as Blood First Edition Paper, June 29, 2004; DOI 10.1182/blood-2004-04-1653.
Supported by National Institutes of Health (NIH) grant RO1 HL65430-01A1 and by the Stem Cell Network of Canada. N.B. and M.L. are fellows of the American Society of Hematology and the Fond de Recherches en Sante du Quebec, respectively, and G.S. is a scholar of the Leukemia Lymphoma Society of America.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Mrs Melanie Frechette for her expertise and help regarding maintenance of animals. The help of Mr Eric Massicotte and Mrs Martine Dupuis with flow cytometry is also acknowledged.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal