Abstract
Infusion of parental lymph node (LN) cells into sublethally irradiated hybrid F1 recipients created a murine model for bone marrow (BM) failure. Affected animals developed fatal pancytopenia within 2 to 3 weeks, accompanied by BM oligoclonal T-cell infiltration and severe marrow hypoplasia indicated by approximately 10-fold declines in total BM cellularity, 15-fold declines in BM Lin-Sca1+c-Kit+ cells, 100-fold declines in spleen colony-forming units, and 100-fold declines in hematopoietic progenitor and stem cells as estimated by irradiation protection in vivo. LN cells of both H2b/b and H2d/d haplotypes were effectors. Serum interferon-γ (IFN-γ) concentration increased 2- to 3-fold. Marrow cells were severely apoptotic, with high proportions of Fas+ and annexin V+ cells. Cotransplantation of 5 × 105 BM cells from clinically affected donors and 106 BM cells from H2 identical healthy mice could not rescue lethally irradiated recipients. Recipients had significantly lower cellularity in peripheral blood and BM, and cell mixtures failed to produce a stromal feeder layer to support marrow cell growth in vitro. Pathogenic T cells from donors after BM failure appeared capable of destroying hematopoietic progenitor, stem, and stromal cells from fully compatible healthy donors as “innocent bystanders.” This effect can be partially abrogated by anti-IFN-γ antibody. (Blood. 2004;104:1671-1678)
Introduction
Bone marrow (BM) failure occurs in several closely related human diseases, including aplastic anemia (AA), myelodysplastic syndromes (MDS), and paroxysmal nocturnal hemoglobinuria (PNH). A decrease in the number and function of hematopoietic stem and progenitor cells leads to anemia, neutropenia, and thrombocytopenia, which, when severe and untreated, are fatal.1,2 In all these syndromes, clinical observations and laboratory studies have implicated activated cytotoxic T cells, producing type 1 cytokines, as the effectors of an active process of stem cell destruction, mainly through Fas-mediated apoptosis.2 BM failure can be cured by stem cell replacement from an allogeneic donor, and it can be alleviated by immunosuppressive drugs that allow the recovery of hematopoietic function.2 An immune mechanism is dramatically illustrated by transfusion-associated graft-versus-host disease (GVHD), in which foreign donor lymphocytes contained in a blood product and infused into a susceptible host provoke uniformly lethal marrow aplasia.3
Animal models have been developed to better understand the pathophysiology of BM failure and to test new treatments.4-15 In mice, the alkylating drug busulfan produces latent AA. After a course of therapy, animals maintain normal blood counts and bone marrow cellularity for a year before demonstrating pancytopenia and frank aplasia. Spleen colony-forming units (CFU-Ss), representative of early hematopoietic progenitor cells, decline during this intervening period to very low numbers.6 This busulfan-induced model was useful for assessing myelotoxic agents, and it illustrated the enormous compensatory capacity of the marrow, which allows limited numbers of stem cells to provide normal levels of blood cells, but it did not address the likely immune mechanism of most acquired hematopoietic failure in humans. Marrow failure has also been produced in some mouse strains through inoculation of allogeneic lymphocytes.5,7,12 Barnes and Mole4 transplanted 1-10 × 106 C3H/H lymph node (LN) cells into sublethally irradiated CBA/H mice and found that variable numbers of recipients with marrow aplasia eventually died within days or weeks. This approach was extended to C3H/He mice by injecting LN cells from B10.BR mice; donor and recipient shared the same H2k haplotype but differed in a mixed lymphocyte reaction locus (Mls).15 Total body irradiation (TBI) at 6 Gy plus injection of 10 million B10.BR LN cells produced fatal pancytopenia in C3H/He mice within 2 to 3 weeks, accompanied by dramatic declines in CFU-S and colony-forming cells (CFU-Cs) in vitro. However, this model could not be extended to other strains12 ; mice were H2k identical but Mls mismatched, but certain Mls combinations induced marrow aplasia in one strain combination but not in others. Despite speculation that a macrophage-like cell might be a mediator of immunologic injury,12,13 T lymphocytes, not B cells, were later shown to be responsible for marrow aplasia.7
In the current study, we induced BM failure using the combination of sublethal TBI and parent into hybrid F1 (major histocompatibility complex [MHC]-mismatch) LN cell infusion and specifically studied the effects of infusion-induced BM failure on the phenotype and function of BM hematopoietic progenitor and stem cells. In this model, massive lymphocyte infiltration in the BM led to rapid destruction of hematopoietic progenitor and stem cells. Using a cotransplantation approach, we showed that hematopoietic progenitor and stem cells could be eliminated as “innocent bystanders,” not necessarily as specific targets. We also showed that the indiscriminate destruction of BM cells can be partially abrogated by anti-interferon-γ (anti-IFN-γ) antibody.
Materials and methods
Mice and induction of BM failure
Inbred C57BL/6 (B6, H2b/b) and BALB/cBy (BALB, H2d/d) mice, hybrid B6D2F1 (H2b/d) and CByB6F1 (H2b/d) mice, and B6-CD45.1 congenic mice were purchased from the Jackson Laboratory (Bar Harbor, ME). They were maintained at animal facilities of the National Institutes of Health and received standard care and nutrition.16 Males and females were used at 6 to 16 weeks of age. Donor and recipient animals were matched for sex in each specific experiment.
Inguinal, axillary, and lateral axillary LNs were removed from B6 or BALB donor mice, homogenized in phosphate-buffered saline (PBS) using a mini-tissue grinder (A. Daigger & Company, Vernon Hills, IL), filtered through 100-μm nylon mesh (Small Parts, Miami Lakes, FL), washed in PBS, and counted using a Z2 Coulter Counter (Coulter, Hialeah, FL). In some experiments, each B6D2F1 or CByB6F1 mouse received 5 to 6 Gy TBI from a Gammacell 40 (K2K 1 × 8; MDS Nordion, Ontario, Canada) source at approximately 0.9 Gy/min and was injected with a single dose of 5 to 10 × 106 B6 or BALB LN cells through the lateral tail vein 4 to 6 hours after irradiation. Treated mice received normal care and nutrition and were monitored 2 to 3 times a week until they died or were humanely killed for tissue/cell collection. In 1 experiment, LN cell-infused and untreated mice were killed at day 14 after LN cell infusion and were examined for gross GVHD-related lesions in the skin, liver, stomach, intestines, and spleen. Complete blood cell (CBC) counts were performed on these same mice to ensure that pancytopenia had developed.
Cellular composition analyses
Mice received no treatment (untreated) or they received 5 to 6 Gy TBI (TBI only), 5 to 6 Gy TBI plus 5 to 10 × 106 B6 LN cells (B6 LN cells), or 5 Gy TBI plus 10 × 106 BALB LN cells (BA-LN cells) and were bled from the retro-orbital sinus 14 to 21 days after LN cell injection. Red blood cells (RBCs) and white blood cells (WBCs) were analyzed using a Z2 Coulter Counter in experiments 1 and 3, whereas CBCs were performed in experiment 4 using a Hemavet 1500 analyzer (Drew Scientific, Oxford, CT). Some experimental mice were humanely killed at 14 to 21 days after treatment, and BM cells were extracted from tibiae and femurs of both legs, counted with a Coulter Counter, and processed for further analysis or transplantation.
Procedures for flow cytometry were as previously described.17 To lyse RBCs, peripheral blood and BM cells were incubated twice in Gey solution (130.68 mM NH4Cl, 4.96 mM KCl, 0.82 mM Na2HPO4, 0.16 mM KH2PO4, 5.55 mM dextrose, 1.03 mM MgCl2, 0.28 mM MgSO4, 1.53 mM CaCl2, and 13.39 mM NaHCO3) for 10 minutes each incubation. After that, they were stained and washed in flow buffer (2.68 mM KCl, 1.62 mM Na2HPO4, 1.47 mM KH2PO4, 137 mM NaCl, 7.69 mM NaN3, and 1% bovine serum albumin [BSA]), first with a premixed antibody cocktail and then with diluted streptavidin-conjugated quantum red (SAQR). Monoclonal antibodies directed against murine CD3 (clone 145-2C11), CD4 (clone GK 1.5), CD8 (clone 53-6.72), CD11b (clone M1/70), CD19 (clone ID3), CD34 (clone RAM34), CD45R (B220; clone RA3-6B2), CD95 (Fas; clone Jo2), CD117 (c-Kit; clone 2B8), erythroid cells (clone Ter119), granulocytes (Gr1/Ly6-G; clone RB6-8C5), stem cell antigen 1 (Sca1; clone E13-161), and a panel of 15 T-cell receptor-β variable region (TCR-Vβ) were all from BD Biosciences (San Diego, CA). Each antibody was conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE), biotin, or allophycocyanin (APC). FITC-conjugated annexin V was also from BD Biosciences. SAQR was from Sigma (St Louis, MO). Cells were recovered by centrifugation at 500g for 5 minutes in 4°C after each staining/washing step. All antibody cocktails were premixed, and their performances were pretested. Analyses were carried out using a BD-LSR flow cytometer (Becton Dickinson, San Jose, CA). The acquisition threshold was predetermined to exclude debris and residual erythrocytes. Each acquisition was stopped when 2000, 10 000, 20 000, 100 000, or 500 000 cells were collected, depending on the type of analysis and the availability of cells.
ELISA
Orbital sinus blood was kept at room temperature (RT) for 30 minutes and then underwent centrifugation at 1020g for 10 minutes. Serum was removed and stored at -20°C. Serum IFN-γ concentration was analyzed using enzyme-linked immunosorbent assay (ELISA) with the BD OptEIA Set Mouse IFN-γ kit (BD Biosciences). Briefly, 96-microwell MaxiSorp plates (Nalge Nunc International, Rochester, NY) were coated with capture antibody at 4°C overnight and were blocked with PBS containing 10% fetal bovine serum (FBS) for 60 minutes at RT. Serum samples and standards were added to plates at 100 μL/well. Plates were sealed and incubated for 2 hours at RT. Diluted (1:250) avidin-horseradish peroxidase-conjugated anti-mouse IFN-γ detection antibody was added at 100 μL/well and was incubated for 60 minutes at RT. Five to 10 washing steps with washing buffer (PBS containing 0.05% Tween-20) were performed between each incubation. Finally, each well received 100 μL tetramethylbenzidine and hydrogen peroxide (1-Step Ultra TMB-ELISA; Pierce, Rockford, IL) and was incubated in the dark (unsealed) for 30 minutes at RT. Reaction was stopped by adding 50 μL/well of 1 M sulfuric acid. Light absorbance was analyzed using the Wallac1420 Victor 3 reader (Perkin Elmer, Wellesley, MA) at 450 nm.
CFU-S and irradiation protection assays
Ten CByB6F1 mice each received 5 Gy TBI, and 5 × 106 LN cells from B6-CD45.1 donors were killed on day 14. BM cells were extracted from 2 tibiae and 2 femurs of each mouse and were combined to form 3 pools (P1, mice 1-4; P2, mice 5-7; P3, mice 8-10). Cells from P2 and P3 were injected into 4 lethally irradiated (10 Gy; Gammacell 40; MDS Nordion, Ontario, Canada) CByB6F1 recipients at 105 cells per recipient. Eight more recipients each receiving 105 BM cells from 2 healthy CByB6F1 donors were used as controls. After 12 days, all 16 recipients were killed, and spleens were removed for examination of colonies. Numbers of colonies per 105 BM cells were multiplied by total numbers of BM cells per mouse to calculate total CFU-S per mouse.
BM cells in P1 (from treated mice 1-4) were washed once in PBS and were resuspended in 4 mL PBS. Half these cells were injected into 4 lethally irradiated (10 Gy) CByB6F1 recipients at 0.5 mL cells per recipient, equivalent to 50% BM cells from the 2 tibiae and 2 femurs of each donor mouse (1:2 dilution), and the remaining cells (2 mL) were transferred to a new tube and were combined with 2 mL PBS. This process was carried out for 6 dilutions from 1:2 to 1:64. We injected cells from each dilution into 4 lethally irradiated recipients. In parallel, BM cells from 2 untreated CByB6F1 control donors were diluted from 1:32 to 1:128 and were injected into lethally irradiated CByB6F1 recipients. Recipient survival was monitored for 2 months.
Cotransplantation
B6D2F1 or CByB6F1 mice treated with 5 Gy TBI (TBI only) plus 5 × 106 B6 LN cells (B6 LN cells) were humanely killed 14 to 15 days after initial treatment. BM cells extracted from each donor mouse were mixed 1:2 with BM cells from a B6-CD45.1 congenic standard mouse, and the cell mixtures were injected into new sets of lethally irradiated (11 Gy; Gammacell 40; MDS Nordion), young B6D2F1 or CByB6F1 recipients. Each recipient was injected with 5 × 105 BM cells from a designated donor and 106 BM cells from the B6-CD45.1 congenic standard. Recipient survival was monitored 2 to 3 times per week for the first 4 weeks. In both experiments, recipients alive at 10 weeks after transplantation were bled and analyzed for donor contribution by flow cytometry.
In other experiments, CByB6F1 recipients were divided into 4 treatment groups: (1) BM failure plus B6-CD45.1 donor cells with 500 μg anti-IFN-γ (Research Diagnostics, Flanders, NJ); (2) BM failure plus B6-CD45.1 donor cells without anti-IFN-γ; (3) normal CByB6F1 plus B6-CD45.1 donor cells; and (4) CByB6F1 mice without any treatment. Recipients were analyzed 2 to 3 weeks after transplantation for peripheral blood pancytopenia and BM cellularity.
Stromal feeder layer development and cell culture
BM cells from CByB6F1 mice treated with 5 Gy TBI and 5 × 106 B6 LN cells 14 days earlier were mixed 1:2 with BM cells from a fresh B6 donor, and the cell mixtures were transplanted into a new set of lethally irradiated CByB6F1 recipients at 1.5 × 106 cells per recipient. Two weeks later, BM cells were extracted from 3 recipients and from 2 CByB6F1 mice to establish stromal feeder layers in 96-well, flat-bottomed cell culture plates by culturing in α-modified Eagle medium (α-MEM) (80% α-MEM, 10% fetal bovine serum, 10% horse serum, 2 mM l-glutamine, 50 μg(U)/mL penicillin-streptomycin, 50 μM β-mercaptoethanol, and 10 μM hydrocortisone) in an incubator (Forma Scientific, Marietta, OH) at 33°C with 5% CO2. Cells from each of the 3 treated donors and the 2 controls were plated into 24 wells, with each well initially containing 2 × 105 BM cells in 200 μL α-MEM. After 4 weeks, each well was overlaid with 2 × 104 fresh BM cells from a new CByB6F1 donor. Half the medium (100 μL) in each well was replaced by fresh medium each week. Cell growth was observed weekly under a phase-contrast microscope. Digital images were captured to assess feeder layer morphology, using a Zeiss Axiovert 200 inverted phase contrast microscope (Carl Zeiss, Germany) under ph1 10 × magnification with a Zeiss AxioCam HR camera equipped with the Axiovision 3.0.6 image-capture software (Zeiss).
Data analysis
Data from blood, BM cell composition analyses, and flow cytometry analyses were analyzed using JMP statistical discovery software (SAS Institute, Cary, NC).18 Data were presented as mean ± SEM, and statistical significance was declared at the P less than .05 and P less than .01 levels.
Results
Induction of BM failure
We induced BM failure by infusing preirradiated hybrid B6D2F1 and CByB6F1 mice with parental B6 or BALB LN cells. In experiment 1, 6 Gy TBI plus 10 × 106 B6 LN cell injection caused recipients to die at approximately 12 days, whereas mice that received 6 Gy TBI without LN cell infusion survived without marrow replacement (Table 1). Injecting 10 × 106 B6 LN cells into B6D2F1 mice preirradiated at 5 Gy TBI produced BM failure, as follows: 9-fold reductions in WBC counts (P < .0001), 2.5-fold reductions in RBC counts (P < .0001), and more than 10-fold reductions in total BM cell counts (P < .0001) (Table 2). This success led us to test BM failure induction in a different hybrid strain, CByB6F1. In experiment 2, injecting 5 × 106 or 10 × 106 B6 LN cells into CByB6F1 recipients preirradiated at 5 Gy TBI caused all recipients to die by day 14 (Table 1). In experiment 3, we administered 5 Gy TBI and injected 5 × 106 B6 LN cells into 15 CByB6F1 mice. Seven mice died between days 14 and 21 (Table 1), and the remaining 8 mice showed more than 12-fold reductions in WBC counts (P < .0001) and 3-fold reductions in RBC counts (P < .0001) (Table 2). Thus, sublethal irradiation plus B6 LN cell infusion caused BM failure in B6D2F1 and CByB6F1 hybrid mice.
Because B6 LN cells were effective at inducing BM failure in preirradiated F1 mice, we next questioned whether the marrow-destructive effect was restricted to lymphocytes carrying the H2b/b allele. Toward this end, we conducted experiment 4 in which newly preirradiated CByB6F1 mice were infused with 5 × 106 LN cells from B6 (H2b/b) donors or with 10 × 106 LN cells from BALB (H2d/d) donors. By day 14, 8 of 10 B6 LN cell-injected recipients died (Table 1), and the 2 remaining recipients had severe pancytopenia (Table 2). The 5 recipients that received BALB LN cells were alive but had severe lymphocytopenia, thrombocytopenia, and mild anemia, with 15-fold reductions in WBC counts (P < .0001), 31% reductions in RBC counts (P < .05), 7-fold reductions in platelet counts (P < .0001), and 10-fold reductions in total BM cells (P < .0001) (Table 2). Thus, LN cells from BALB donors of the H2d/d haplotype also induced BM failure in CByB6F1 mice, though LN cells from B6 donors of the H2b/b haplotype appeared more effective.
Infusing parental LN cells resulted in BM failure in the hybrid F1 recipients. No gross GVHD-related lesions were found in skin, stomach, intestines, liver, or spleen of 4 mice that were killed on day 14 after LN infusion and had pancytopenia and marrow hypoplasia at the time of analysis. Mice receiving sublethal TBI without injection of parental LN cells had slight decreases in RBC counts, 60% decreases in WBC counts, and 40% decreases in platelet and total BM cell counts compared with untreated control mice, as measured 2 to 3 weeks after irradiation. The degree of pancytopenia in these control animals was more modest than in the experimental group; in addition, all animals showed spontaneous recovery, and no deaths were associated with sublethal TBI (Table 2).
Marrow aplasia and lymphocyte infiltration
In addition to peripheral pancytopenia, affected mice also showed severe marrow aplasia with more than 10-fold declines in marrow cellularity on average (Table 2). Affected animals had 2- to 3-fold increases in serum IFN-γ concentration (P < .01) (Figure 1, top left) with a 35-fold increase in BM CD4+ cell percentage (P < .01) (Figure 1, top center) and a 400-fold increase in BM CD8+ cell percentage (P < .01) (Figure 1, top right). The proportion of BM cells expressing Fas (CD95), an antigen associated with immune-mediated apoptosis, was significantly increased in residual BM CD8+ and CD8- cells from BM failure mice compared with healthy mice (P < .01) (Figure 1, middle panels). Similarly, the number of annexin V+ cells was also significantly increased in T and non-T cells in BM failure mice compared with controls (P < .01) (Figure 1, bottom panels).
BM lymphocyte infiltration and Fas-mediated apoptosis in BM failure mice. Five CByB6F1 mice each received 5 Gy TBI and injections of 5 × 106 B6 LN cells to induce BM failure. Two CByB6F1 mice that received 5 Gy TBI without LN cell injection were used as controls. At 14 days, all mice were bled, and serum IFN-γ concentrations were analyzed using ELISA (top left). BM cells were analyzed by flow cytometry for the proportions of CD4 (top center) and CD8 (top right) lymphocytes, for the expression of CD95 (Fas, middle row), and for the binding to annexin V (bottom row). Data in the top row are presented as means with standard error bars.
BM lymphocyte infiltration and Fas-mediated apoptosis in BM failure mice. Five CByB6F1 mice each received 5 Gy TBI and injections of 5 × 106 B6 LN cells to induce BM failure. Two CByB6F1 mice that received 5 Gy TBI without LN cell injection were used as controls. At 14 days, all mice were bled, and serum IFN-γ concentrations were analyzed using ELISA (top left). BM cells were analyzed by flow cytometry for the proportions of CD4 (top center) and CD8 (top right) lymphocytes, for the expression of CD95 (Fas, middle row), and for the binding to annexin V (bottom row). Data in the top row are presented as means with standard error bars.
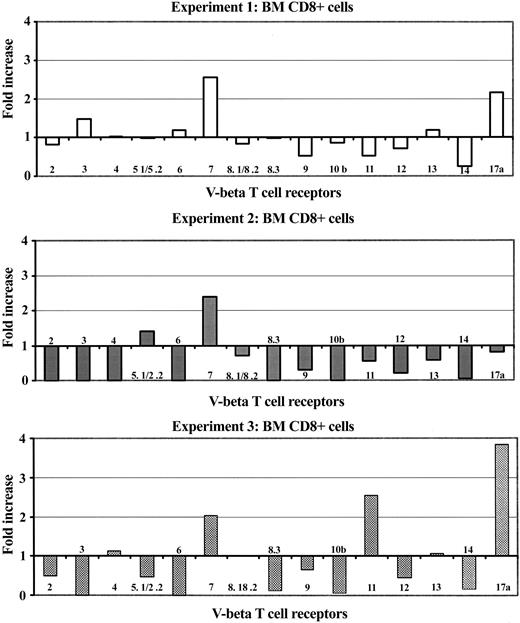
To analyze the composition of infiltrated T lymphocytes in the marrow of BM failure mice, we examined selected Vβ T-cell receptor (TCR) repertoires (Vβ 2, 3, 4, 5.1/5.2, 6, 7, 8.1/8.2, 8.3, 9, 10, 11, 12, 13, 14, and 17a) by flow cytometry. In 3 separate experiments, BM failure mice showed larger proportions of T cells expressing a limited number of specific Vβ TCRs compared with controls (Figure 2). The classes of up-regulated Vβ-TCR were Vβ 7 and Vβ 17a in experiment 1 (Figure 2, upper panel), Vβ 7 in experiment 2 (Figure 2, middle panel), and Vβ 7, Vβ 11, and Vβ 17a in experiment 3 (Figure 2, bottom panel). Thus, the infiltrated and expanded T cells in BM failure mice (mainly CD8+ T cells) were oligoclonal, as defined by TCR subfamily analysis.
BM oligoclonal T-cell expansion in mice with BM failure. BM cells from control and BM failure B6D2F1 mice were analyzed for the expression of Vβ TCR repertoire. Proportions of each specific Vβ TCR repertoire relative to the sum of all 15 Vβ TCR repertoires (Vβ 2, 3, 4, 5.1/5.2, 6, 7, 8.1/8.2, 8.3, 9, 10, 11, 12, 13, 14, 17a) on BM CD8+ T cells were compared between BM failure mice and control mice. Data shown are fold increases in each Vβ TCR repertoire in BM failure mice from 3 independent experiments.
BM oligoclonal T-cell expansion in mice with BM failure. BM cells from control and BM failure B6D2F1 mice were analyzed for the expression of Vβ TCR repertoire. Proportions of each specific Vβ TCR repertoire relative to the sum of all 15 Vβ TCR repertoires (Vβ 2, 3, 4, 5.1/5.2, 6, 7, 8.1/8.2, 8.3, 9, 10, 11, 12, 13, 14, 17a) on BM CD8+ T cells were compared between BM failure mice and control mice. Data shown are fold increases in each Vβ TCR repertoire in BM failure mice from 3 independent experiments.
Elimination of functional hematopoietic progenitor and stem cells
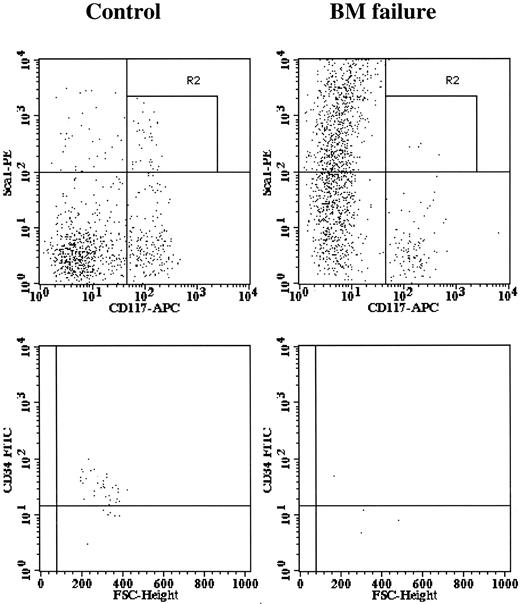
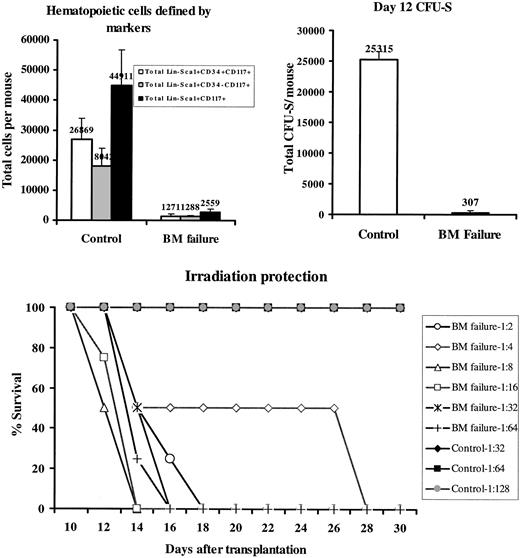
A characteristic feature of human BM failure syndrome is a deficiency of hematopoietic progenitor and stem cells. We studied the stem cell compartment using 3 different systems. First, we analyzed BM cells for the Lin-Sca1+c-Kit+ marker phenotype and further divided cells into CD34+ and CD34- fractions. It was shown previously that mouse BM Lin-Sca1+c-Kit+CD34- cells contain long-term hematopoietic stem cells, whereas BM Lin-Sca1+c-Kit+CD34+ cells were less primitive.19,20 Compared with control mice, BM failure mice had normal proportions of Lin- cells, drastically reduced (P < .01) proportions of Lin-Sca1+c-Kit+ cells (Figure 3, top panels), and significantly reduced (P < .01) proportions of Lin-Sca1+c-Kit+CD34- and Lin-Sca1+c-Kit+CD34+ cells (Figure 3, bottom panels). Calculated total Lin-Sca1+c-Kit+, Lin-Sca1+c-Kit+CD34+, and Lin-Sca1+c-Kit+CD34- cells per mouse were reduced 17.5-fold, 21-fold, and 14-fold, respectively, in BM failure mice (all at P < .01) (Figure 4, upper left).
Elimination of BM Lin-Sca1+CD117+ hematopoietic progenitor and stem cells. BM cells from 15 control and 15 BM failure mice were stained with an antibody cocktail containing CD34-FITC, Sca1-PE, Lin (CD3, CD4, CD8, CD11b, CD19, Gr1, Ter119)-biotin, and CD117 (c-Kit)-APC and were analyzed using a BD-LSR flow cytometer. Data shown are expressions of Sca1 and CD117 on BM Lin- cells (top panels) and expressions of CD34 on Lin-Sca1+CD117+ BM cells (bottom panels) of control and BM failure mice.
Elimination of BM Lin-Sca1+CD117+ hematopoietic progenitor and stem cells. BM cells from 15 control and 15 BM failure mice were stained with an antibody cocktail containing CD34-FITC, Sca1-PE, Lin (CD3, CD4, CD8, CD11b, CD19, Gr1, Ter119)-biotin, and CD117 (c-Kit)-APC and were analyzed using a BD-LSR flow cytometer. Data shown are expressions of Sca1 and CD117 on BM Lin- cells (top panels) and expressions of CD34 on Lin-Sca1+CD117+ BM cells (bottom panels) of control and BM failure mice.
Elimination of BM hematopoietic progenitor and stem cells. BM Lin-Sca1+c-Kit+CD34+, Lin-Sca1+c-Kit+CD34-, and Lin-Sca1+c-Kit+ cell proportions from 15 control and 15 BM failure mice were each multiplied by the total number of BM cells of each mouse, assuming that 2 tibiae and 2 femurs contained 25% of total BM cells, to calculate total cell numbers (top left). Two BM cell pools, each from 3 BM failure mice, and BM cells from 2 untreated control mice were tested for day 12 CFU-S, shown as total CFU-S per mouse (top right). BM cells pooled from 4 BM failure CByB6F1 mice were serial diluted from 1:2 to 1: 64, whereas BM cells from 2 untreated CByB6F1 control donors were diluted from 1:32 to 1:128, and each cell dilution was used to rescue 4 or 2 lethally irradiated (10 Gy) recipients. Recipient survival was monitored for 2 months (for those that received control BM) but only shown for 30 days here (bottom). Data presented in the top row are means with standard error bars.
Elimination of BM hematopoietic progenitor and stem cells. BM Lin-Sca1+c-Kit+CD34+, Lin-Sca1+c-Kit+CD34-, and Lin-Sca1+c-Kit+ cell proportions from 15 control and 15 BM failure mice were each multiplied by the total number of BM cells of each mouse, assuming that 2 tibiae and 2 femurs contained 25% of total BM cells, to calculate total cell numbers (top left). Two BM cell pools, each from 3 BM failure mice, and BM cells from 2 untreated control mice were tested for day 12 CFU-S, shown as total CFU-S per mouse (top right). BM cells pooled from 4 BM failure CByB6F1 mice were serial diluted from 1:2 to 1: 64, whereas BM cells from 2 untreated CByB6F1 control donors were diluted from 1:32 to 1:128, and each cell dilution was used to rescue 4 or 2 lethally irradiated (10 Gy) recipients. Recipient survival was monitored for 2 months (for those that received control BM) but only shown for 30 days here (bottom). Data presented in the top row are means with standard error bars.
Second, we tested hematopoietic progenitor cell function using the day-12 CFU-S assay. The concentration of day 12 CFU-S was reduced 8-fold (P < .01) in BM failure mice (data not shown), equivalent to an overall 82-fold decline (P < .01) in total day-12 CFU-S in BM failure mice compared with untreated controls (Figure 4, upper right), considering the 10- to 11-fold reduction in total BM cells per mouse.
Third, we tested the ability of donor BM cells to rescue lethally irradiated recipients. When serially diluted BM cells from BM failure donors and untreated healthy donors were injected into lethally irradiated young animals, cells from BM failure donors could not rescue recipients even at the lowest dilution (1:2); all recipients died within 3 weeks. Cells from healthy control donors prevented recipient death for 2 months at the highest (1:128) dilution tested (Figure 4, bottom). From these data, we estimated a 64- to 128-fold decrease in functional hematopoietic progenitor and stem cells in BM failure mice.
Hematopoietic stem cells are destroyed as innocent bystanders
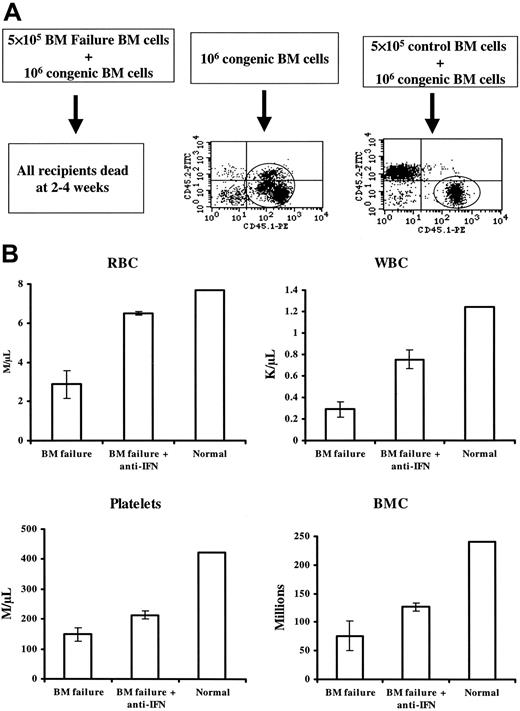
Although marrow cell destruction was severe and extensive in LN cell-infused mice, a key question was whether hematopoietic progenitor and stem cells were specifically targeted or were destroyed as innocent bystanders. To address this issue, we examined cell function in vivo in cotransplantation experiments in which BM cells from BM failure B6D2F1 or CByB6F1 donors (previously treated with sublethal TBI and B6 LN cell injection) and control B6D2F1 and CByB6F1 donors (sublethal TBI only) were mixed with BM cells from healthy B6-CD45.1 congenic codonors, and the cell mixtures were then injected into lethally irradiated B6D2F1 recipients. In the first experiment, 3 recipients that received 10 × 105 BM cells from the B6-CD45.1 congenic codonor survived long-term and showed 100% contribution of the transplanted B6-CD45.1 cell type, indicating that 10 × 105 B6-CD45.1 BM cells were sufficient to rescue recipients from lethal irradiation. Five recipients that received 5 × 105 BM cells from a control donor and 10 × 105 cells from the B6-CD45.1 congenic codonor all survived long term and showed engraftment from both the donor and the B6-CD45.1 codonor, whereas 10 recipients that received 5 × 105 BM cells from 1 of the 2 BM failure donors and 10 × 105 BM cells from the B6-CD45.1 congenic codonor all died within 2 to 4 weeks of marrow transplantation (Figure 5A). In a second experiment, the same design was used to test bystander effects in CByB6F1 recipient mice; similar results were obtained: death of recipients of BM failure donor plus codonor cells and normal engraftment in recipients of BM cells from control donor and codonor (data not shown). Thus, marrow cells from BM failure donors not only failed to contribute to recipient reconstitution but also indiscriminately destroyed BM cells from the B6-CD45.1 congenic codonors as bystanders, preventing engraftment and causing recipient death.
Destruction of BM hematopoietic progenitor and stem cells as bystanders. Sublethally irradiated B6D2F1 mice (5 Gy TBI) were infused with 5 × 106 normal B6 (CD45.2) LN cells to induce BM failure. BM cells from 2 affected mice and 1 control mouse that did not receive LN cell infusion were mixed 1:2 with BM from a B6-CD45.1 congenic codonor, and the cell mixtures were then transplanted into new lethally irradiated, B6D2F1 recipients at 5 recipients per donor. Three recipients each received 10 × 105 B6-CD45.1 congenic codonor BM cells and were used as controls. Recipient survival and donor engraftment were monitored for 10 weeks (A). Sublethally irradiated CByB6F1 mice (5 Gy TBI) were infused with 5 × 106 normal B6 (CD45.2) LN cells to induce BM failure. BM from 1 affected mouse (moderate for BM failure to ensure recipient survival) was mixed 1:2 with BM from a B6-CD45.1 congenic codonor and was injected into 6 lethally irradiated CByB6F1 recipients. Of these, 3 recipients were intraperitoneally injected with 500 μg/d anti-IFN-γ on days 0 and 9, and the other 3 recipients were not. BM cells from 1 control CByB6F1 mouse that did not receive LN cell infusion were mixed 1:2 with BM from a B6-CD45.1 congenic codonor and were injected into 3 lethally irradiated CByB6F1 recipients. Recipients were analyzed 3 weeks after transplantation for CBC and total BM cellularity (B). In both parts of study, each recipient received 5 × 105 donor and 10 × 105 codonor BM cells. Data in panel B are means ± standard error.
Destruction of BM hematopoietic progenitor and stem cells as bystanders. Sublethally irradiated B6D2F1 mice (5 Gy TBI) were infused with 5 × 106 normal B6 (CD45.2) LN cells to induce BM failure. BM cells from 2 affected mice and 1 control mouse that did not receive LN cell infusion were mixed 1:2 with BM from a B6-CD45.1 congenic codonor, and the cell mixtures were then transplanted into new lethally irradiated, B6D2F1 recipients at 5 recipients per donor. Three recipients each received 10 × 105 B6-CD45.1 congenic codonor BM cells and were used as controls. Recipient survival and donor engraftment were monitored for 10 weeks (A). Sublethally irradiated CByB6F1 mice (5 Gy TBI) were infused with 5 × 106 normal B6 (CD45.2) LN cells to induce BM failure. BM from 1 affected mouse (moderate for BM failure to ensure recipient survival) was mixed 1:2 with BM from a B6-CD45.1 congenic codonor and was injected into 6 lethally irradiated CByB6F1 recipients. Of these, 3 recipients were intraperitoneally injected with 500 μg/d anti-IFN-γ on days 0 and 9, and the other 3 recipients were not. BM cells from 1 control CByB6F1 mouse that did not receive LN cell infusion were mixed 1:2 with BM from a B6-CD45.1 congenic codonor and were injected into 3 lethally irradiated CByB6F1 recipients. Recipients were analyzed 3 weeks after transplantation for CBC and total BM cellularity (B). In both parts of study, each recipient received 5 × 105 donor and 10 × 105 codonor BM cells. Data in panel B are means ± standard error.
Because recipients of BM cell mixtures from BM failure donors and congenic codonors had all died within 2 to 4 weeks in both experiments, we conducted another experiment in which some of the recipients were analyzed at an earlier time point. We also tested the potential role of anti-IFN-γ monoclonal antibodies in preventing bystander marrow destruction. At 14 days after transplantation of BM cell mixtures, living recipients were bled for blood count determination and then humanely killed for BM cellularity analyses. Recipients of BM failure plus codonor BM cell mixtures had lower peripheral blood RBC (P < .01), WBC (P < .05), platelet (P < .01) and total BM cell counts (P < .05) than did recipients of normal CByB6F1 plus codonor BM cell mixtures (Figure 5B). Injecting 500 μg/mouse anti-IFN-γ to recipients of BM failure plus codonor BM cell mixtures significantly improved all peripheral blood cell counts and preserved total BM cellularity (all at P < .05) (Figure 5 B), though cell counts were still lower than those in the recipients of normal CByB6F1 plus codonor BM cell mixture. These data were consistent with earlier results indicating that infiltrated LN cells were capable of destroying BM cells from healthy codonors and suggested that this bystander marrow destructive effect was at least partially mediated by IFN-γ.
Destruction of BM stromal cells as bystanders
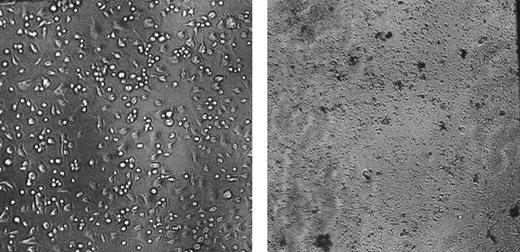
To investigate whether bystander cell destruction damaged not only hematopoietic cells but also stromal cells, we cotransplanted BM cells from BM failure CByB6F1 mice with BM cells from a healthy B6 codonor into lethally irradiated CByB6F1 recipients. Two weeks later, BM cells from humanely killed recipients were cultured in 96-well plates to develop feeder layers; at 4 weeks, they were overlaid with fresh BM cells from an untreated healthy CByB6F1 donor. For comparison, feeder layers were also established from BM cells of untreated healthy CByB6F1 mice, which were then overlaid with fresh BM cells from the same untreated healthy CByB6F1 donor. Stromal feeder layers that developed from normal CByB6F1 BM cells supported marrow cell growth (Figure 6, upper panel), but BM cells from cotransplantation recipients did not form a stromal feeder layer and thus could not support the growth of overlaid normal BM cells (Figure 6, low panel). These data indicate that BM cells from BM failure donors indiscriminately destroyed stromal cells from BM of healthy codonors, causing functional failure in the establishment of a feeder layer to support marrow cell growth (Figure 6, low panel).
Stromal cells as bystanders. BM cells from CByB6F1 mice treated with 5 Gy TBI and 5 × 106 B6 LN cell infusion 14 days earlier were mixed 1:2 with BM cells from a healthy B6 mouse, and the cell mixtures were then transplanted into new lethally irradiated CByB6F1 recipients. After 2 weeks, BM cells extracted from the recipients were cultured in α-MEM at 33°C with 5% CO2 along with BM cells from 2 fresh CByB6F1 mice to establish stromal feeder layers After 4 weeks in cell culture, fresh BM cells were overlaid on the established feeder layers. Stromal feeder developed from normal CByB6F1 marrow (left) supported marrow cell growth, but stromal cells from cotransplantation recipients could not establish a feeder layer to support marrow growth (right).
Stromal cells as bystanders. BM cells from CByB6F1 mice treated with 5 Gy TBI and 5 × 106 B6 LN cell infusion 14 days earlier were mixed 1:2 with BM cells from a healthy B6 mouse, and the cell mixtures were then transplanted into new lethally irradiated CByB6F1 recipients. After 2 weeks, BM cells extracted from the recipients were cultured in α-MEM at 33°C with 5% CO2 along with BM cells from 2 fresh CByB6F1 mice to establish stromal feeder layers After 4 weeks in cell culture, fresh BM cells were overlaid on the established feeder layers. Stromal feeder developed from normal CByB6F1 marrow (left) supported marrow cell growth, but stromal cells from cotransplantation recipients could not establish a feeder layer to support marrow growth (right).
Discussion
In our mouse model, injecting 5-10 × 106 LN cells of a parent animal into sublethally irradiated F1 recipients produced a disease that closely resembled human BM failure. RBC, WBC, and platelet counts dramatically decreased within 2 to 3 weeks to levels approximately 10% to 20% of normal, and severe pancytopenia was accompanied by profound marrow aplasia. These are clinical characteristics of human AA.2 Infused donor LN cells infiltrated and expanded in recipient BM; these cells are likely the effectors of the massive destruction of more than 90% of all cells in the BM.
Lack of typical GVHD responses in tissues other than BM, such as skin, stomach, intestines, liver, and spleen, is a useful if not fully explained feature of this mouse model. In a previous study, Ellison et al21 reported that infusing 100 × 106 B6 LN-spleen cell mixture into healthy B6D2F1 mice induced acute GVHD responses, with apoptotic lesions in recipient intestines 2 weeks after cell infusion. BM was not examined. It may be that we did not observe gross intestinal damage because of our lower dose of LN cells in the infusion and because BM is more vulnerable than intestine in acute GVHD.
The destruction of functional hematopoietic progenitor and stem cells in our BM failure mouse model was severe and extensive, as evidenced by an approximate 100-fold decline in CFU-S and in cells capable of protecting and repopulating a lethally irradiated recipient (Figure 4). These numbers differ from the approximate 10-fold lower numbers of total BM cells (Table 2) and Lin-Sca1+c-Kit+CD34- cells (Figures 3, 4). The dissociation between the Lin-Sca1+c-Kit+CD34- marker phenotype (Figure 3) and functional hematopoietic stem cells (Figure 4) in BM failure mice is similar to that reported previously for in vivo expanded BM cells22 and for BM cells from Il2-deficient mice.23 The total number of BM cells and Lin-Sca1+c-Kit+CD34- cells likely underestimates the severity of marrow destruction because most residual cells in the BM infiltrated expanded populations of donor T cells (Figure 1), and only a small fraction represent residual host marrow cells.
Activated T lymphocytes, producing type 1 cytokines, cause extensive stem cell destruction and the development of pancytopenia and marrow aplasia in patients with BM failure.24 We compared a 2- to 3-fold increase in serum IFN-γ concentration in mice that developed marrow failure with that in healthy mice. An important question in AA and in other autoimmune diseases is whether destroyed cells are specifically targeted or are victims of a toxic immune environment. In studies of AA, 30% of patients showed autoreactivity and 50% showed alloreactivity in lymphocyte toxicity assays using autologous or HLA-identical target cells.25,26 More recent studies have found increased numbers of cytotoxic T cells that react to cells from histocompatible siblings and have found that T lymphocytes from AA patients can lyse autologous target cells as well.27,28 In our mouse model, GVHD-induced BM failure originated from an allogenic LN cell attack and was not generated by an autoimmune response, as seen in most AA patients. However, our finding that cotransplantation of marrow cells from BM failure mice and large numbers of cells from B6-CD45.1 congenic mice, containing more than sufficient repopulating stem cells, did not rescue lethally irradiated recipients provides direct evidence that pathogenic T-cell clones from affected marrow may destroy normal BM cells from B6-CD45.1 congenic animals as bystanders (Figure 5A). Further studies confirmed that peripheral blood and BM cellularity were significantly reduced in recipients that underwent cotransplantation with BM failure and normal congenic BM cells (Figure 5B). The bystander marrow destruction model provides an explanation for the efficiency of immune-mediated destruction in AA and other human clinical marrow failure syndromes. Regardless of inciting antigen or specificity of T-cell recognition, extensive hematopoietic cell destruction may follow more generally from lymphocyte attack and from the release of cytokines deleterious to stem cell survival. That normal B6 stromal cells were also destroyed by mixture with marrow cells from BM failure mice (Figure 6) further supports the conclusion that BM cell destruction was not specifically targeted in BM failure mice. This result is consistent with previous findings of stromal injury in mouse models of AA.14
Our results in general are concordant with findings of others in mouse models of autoimmunity in which indiscriminate cell destruction has been assigned 2 distinct mechanisms: cytokine effect and epitope spreading. For viral-induced pancytopenia, perforin-deficient mice infected with lymphocytic choriomeningitis virus (LCMV) showed progressive pancytopenia within 14 days of infection.5 Disease was prevented by depleting CD8+ T lymphocytes and was accelerated by increasing the frequency of LCMV-specific CD8+ T cells. There was no cognate interaction between the TCR and hematopoietic progenitors presenting either the LCMV-derived antigens or self-antigens on the major histocompatibility complex, and damage to hematopoiesis was interpreted as the result of excessive secretion and activity of TNF-α, IFN-γ, and lymphotoxin-α. LCMV infection of mice deficient in tumor necrosis factor (TNF) receptor rescued animals and partially prevented hematopoietic malfunction, further suggesting the involvement of cytokines in cell destruction that caused pancytopenia. In the nonobese diabetic mouse, a favored model of autoimmune T-cell-mediated type 1 diabetes in humans,29,30 animals initially develop nondestructive peri-insular infiltration of dendritic cells, accessory macrophages, T cells, and B cells that persists for several weeks and then progresses to destructive insulitis. The autoimmune response is specific to islet cells, but in some cases thyroid cells30 and Schwann cells31 are also casualties of the autoimmune response. In experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis, epitope spreading is highly associated with,32 if not necessarily required for,33 disease progression. The development of epitope spreading or the lack of response by disease-initiating T cells may indicate the activity of regulatory cells. The recent finding that CD4+CD25+ regulatory T cells suppress an innate immune abnormality and inhibit a variety of autoimmune and inflammatory diseases34 may identify, at least partially, such regulatory cells.
We report here that infusing parent LN cells into hybrid F1 recipients induced BM failure in mice that can serve as a model for human immune-mediated BM failure. LN from B6 and BALB donors can provide effector cells. We demonstrate that residual marrow cells from infusion-induced BM failure mice are infiltrated with expanded donor T lymphocytes, which, on mixing with fresh BM cells from healthy donors, were able to destroy H2 identical hematopoietic progenitor and stem cells from healthy BM as innocent bystanders.
Prepublished online as Blood First Edition Paper, May 27, 2004; DOI 10.1182/blood-2004-03-1115.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Keyvan Keyvanfar from the Hematology Branch and David Caden from the Laboratory of Animal Medicine of the National Heart, Lung, and Blood Institute for technical assistance in flow cytometry and complete blood counts.