Abstract
Oncogenic mutations in ras genes frequently occur in patients with myeloid disorders, and in these patients erythropoiesis is often affected. Previously, we showed that expression of oncogenic H-ras in purified mouse primary fetal liver erythroid progenitors blocks terminal erythroid differentiation and supports erythropoietin (Epo)-independent proliferation. As a first step in understanding the underlying molecular mechanisms we examined the signaling pathways downstream of Ras in primary erythroid cells. We found that 3 major pathways are abnormally activated by oncogenic H-ras: Raf/ERK (extracellular signal-regulated kinase), phosphatidyl inositol 3 (PI3)-kinase/Akt, and RalGEF/RalA. However, only constitutive activation of the MEK (MAPK [mitogen-activated protein kinase]/ERK kinase)/ERK pathway alone could recapitulate all of the effects of oncogenic H-ras expression in blocking erythroid differentiation and inducing Epo-independent proliferation. Although expression of a constitutively active Akt kinase (ca.Akt) in erythroid progenitors does not significantly affect erythroid differentiation in the presence of Epo, coexpression of ca.Akt together with a constitutively active MEK causes prolonged Epo-independent proliferation of erythroid progenitors in addition to a block in differentiation. Moreover, the effects of oncogenic H-ras expression on primary erythroid cells are blocked by the addition of U0126, a specific inhibitor of MEK1 and MEK2, allowing normal terminal erythroid proliferation and differentiation. Our data suggest that the interruption of constitutive MEK/ERK signaling is a potential therapeutic strategy to correct impaired erythroid differentiation in patients with myeloid disorders. (Blood. 2004;104: 1679-1687)
Introduction
Ras signaling plays an important role in erythropoiesis, and K-ras-/- and N-ras-/-/K-ras+/- mice die at early embryonic stages with anemia.1 Although apparent defects were identified in both hematopoietic cells and supporting stromal cells, the cellular mechanisms underlying this phenotype remain elusive.1,2 Additionally, mutations in the N- and K-ras genes that create constitutively active oncogenic Ras proteins are frequently identified in patients with myeloid disorders.3,4 In these diseases the erythroid lineage is often affected, suggesting a role for altered Ras signaling in the dysregulated erythropoiesis seen in these patients. We and others showed that transduction of human or murine erythroid progenitors with oncogenic Ras results in hyperproliferation and defective differentiation, leading to reduced production of mature erythrocytes.5-7 Consistent with these findings, a mouse line carrying a conditional oncogenic K-ras allele developed similar phenotypes when the oncogenic K-ras allele was activated somatically.8 These data support the notion that oncogenic Ras directly impairs erythropoiesis in patients with myeloid disorders, but the underlying mechanisms are largely unknown.
The effects of oncogenic Ras are mediated by its ability to activate multiple downstream effector proteins. The contribution of each individual effector to the effects of oncogenic Ras has been evaluated mainly in rodent fibroblast systems such as NIH 3T3 and mouse embryonic fibroblast cells. In these cells, activation of the Raf/MEK/ERK pathway is critical for Ras-mediated transformation. Like oncogenic Ras, expression of constitutively active Raf or MEK proteins leads to tumorigenic transformation of NIH 3T3 cells.9-12 In addition, inhibition of the Raf/MEK (MAPK [mitogen-activated protein kinase]/ERK [extracellular signal-regulated kinase] kinase)/ERK pathway by dominant-negative mutants or pharmacologic inhibitors blocks Ras-mediated transformation.13-20 Although constitutive activation of the phosphoinositide 3 (PI3)-kinase/Akt or RalGEF/RalA pathway alone fails to transform NIH 3T3 cells, their coexpression with activated Raf enhances the rate and extent of cell transformation.21-23
Because of the difficulty in purifying large numbers of primary erythroid progenitors, the signaling pathways abnormally activated by oncogenic Ras have not been extensively studied in these cells. For the same reason the contribution of each individual Ras downstream signaling pathway to the effects of oncogenic Ras has not been evaluated in these cells. Recently, we developed a method to isolate large numbers of erythroid progenitors from mouse fetal livers by an easy single-step purification involving removal of all TER119+ cells.5 Using these erythroid progenitors we developed an in vitro culture system that supports their normal terminal proliferation and erythroid differentiation.5 During the first day in culture these cells undergo several divisions and up-regulate the transferrin receptor. Additional divisions occur during the second day as the cells induce TER119 expression, down-regulate the transferrin receptor, induce hemoglobin synthesis, and enucleate. Overall the cell number increases 15- to 20-fold. We monitor erythroid differentiation step by step and quantitatively by using a flow cytometry analysis on living cells, following expression of the transferrin receptor (CD71) and the TER119 antigen. Because we can express any desired protein in these progenitors by using a bicistronic retrovirus, this is a valuable system to study the functions of any desired signaling molecule in erythroid differentiation. Using this system we found that oncogenic H-ras blocks terminal erythroid differentiation and supports Epo (erythropoietin)-independent proliferation in primary erythroid progenitors.5 As a first step in understanding the underlying mechanisms, we investigated signaling pathways downstream of H-ras in primary erythroid progenitors.
Here, we report that oncogenic H-ras expression constitutively activates the p44/42 ERK, Akt, and RalA pathways in primary erythroid progenitors. Expression of constitutively active MEK1 (ca.MEK) is sufficient to recapitulate the effects of oncogenic H-ras in these cells. In addition, expression of ca.Akt but not ca.Rlf acts synergistically with ca.MEK in supporting Epo-independent growth of primary erythroid progenitors. Moreover, the addition of U0126, a MEK1- and MEK2-specific inhibitor, to primary erythroid progenitors expressing oncogenic H-ras greatly reduces the activation level of the p44/42 ERK pathway. U0126 concomitantly inhibits Epo-independent growth and restores normal terminal erythroid differentiation.
Materials and methods
Cells and chemical inhibitors
The retrovirus packaging cell lines BOSC23 (a gift of Dr Xiaowu Zhang, Whitehead Institute for Biomedical Research, Cambridge, MA)24 and 293T were maintained in Dulbecco modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA).
Fetal liver cells were isolated from embryonic day (E)12.5 to E15.5 Balb/c (Jackson Laboratory, Bar Harbor, ME) embryos and mechanically dissociated by pipetting in phosphate-buffered saline (PBS) containing 2% FBS. Single cell suspensions were prepared by passing the dissociated cells twice through 70-μm cell strainers. TER119- cells were purified and cultured as previously described.5
U0126 (Calbiochem, San Diego, CA) was dissolved in dimethyl sulfoxide (DMSO) according to the manufacturer's instructions. The inhibitor was added into primary cell culture on day 1. For Western analyses, cells were harvested 6 hours after the addition of the inhibitor. For growth curve analysis, the inhibitor was replaced every 24 hours.
Retroviral constructs
cDNAs encoding constitutively active MEK (ca.MEK) (kindly provided by Dr Randolph S. Watnick, Children's Hospital, Boston, MA),15 ca.Akt (kindly provided by Dr Janine Zieg, Children's Hospital, Boston),25 and ca.Rlf (kindly provided by Dr Anapoorni Rangarajan, Whitehead Institute for Biomedical Research),26 were subcloned 5′ to the internal ribosome entry site (IRES) in the XZ201 and MICD4 retroviral vectors.5
Generation of retroviral supernatants and infection of primary cells
BOSC23 or 293T cells were seeded on 100-mm dishes 1 day before transfection. Retroviral plasmid (4-9 μg) together with 1 to 3 μg pCL-Eco vector27 were used to cotransfect BOSC23 or 293T cells by the FuGENE 6 Transfection Reagent (Roche, Indianapolis, IN) according to the manufacturer's protocol. The retroviral supernatants were collected 40 to 48 hours after transfection and stored in aliquots at -80°C. Purified TER119- fetal liver cells were infected with retroviral constructs, plated on fibronectin-coated plates, and cultured for 2 days in medium containing Epo exactly as described previously.5
Immunostaining and flow cytometric analysis of erythroid differentiation
Cultured cells were harvested and stained for CD71, TER119, and hCD4 (when infected with MICD4 constructs only) or TER119 and hCD4 (when infected with both XZ201 and MICD4 constructs) as previously described.5 hCD4- and hCD4+ cells were distinguished by staining mock-infected cells with the same protocol. Flow cytometry was carried out on a Becton Dickinson FACSCalibur (BD Biosciences, Franklin Lakes, NJ).
Cytospin preparations and histologic staining
Between 20 000 and 40 000 in vitro cultured TER119- cells were centrifuged onto slides for 3 minutes at 800 rpm (Cytospin 3; Thermo Shandon, Pittsburgh, PA) and air dried. Cells were fixed in methanol for 2 minutes at -20°C and stained with 3,3′-diaminobenzidine Giemsa stains according to the manufacturer's recommendations (Sigma, St Louis, MO). The slides were mounted in DPX Mountant for histology (Sigma) and observed under a 100× oil objective lens on an Olympus BH-2 microscope (Olympus, Melville, NY). The color pictures were taken with an Olympus C-35AD-2 camera with Kodak Ektachrome 64T films (Eastman Kodak, Rochester, NY). The developed color slides were scanned by a Nikon Super cool scan 4000 (Nikon, Melville, NY) and processed with Photoshop software (Adobe, San Jose, CA).
Cell cycle analysis
Retrovirally transduced TER119- fetal liver cells were cultured in vitro for 1 day, and infected cells were stained and sorted by a fluorescence activated cell sorting (FACS) Moflo machine (Cytomation, Fort Collins, CO) according to their surface expression of the hCD4 marker inserted downstream of the IRES in the retroviral vector. The sorted cells were cultured for another day on fibronectin-coated wells. Cultured cells were then harvested and stained in hypotonic propidium iodide (PI) solution (0.1% sodium citrate and 50 mg/mL PI). Flow cytometry was carried out on a BD FACSCalibur machine, and collected data were analyzed by ModFit software (Verity Software House, Topsham, ME).
In vitro growth assay
Retrovirally transduced TER119- fetal liver cells were cultured for 1 day, and infected cells were sorted. Sorted cells were further maintained and counted as previously described.5
Immunoprecipitation and Western analysis
Retrovirally transduced cells were cultured overnight and starved in Iscove modified Dulbecco medium (IMDM) containing 1% bovine serum albumin (BSA) for 2 hours at 37°C. Cells were pelleted and resuspended in RIPA Buffer (1 × PBS, 1% Igepal CA-630 [Sigma], 0.5% sodium deoxycholate, 0.1% SDS [sodium dodecyl sulfate]) containing complete proteinase inhibitors (Roche), 1 mM sodium orthovanadate, 5 mM sodium fluoride, and 50 nM Calyculin A (Cell Signaling Technology, Beverly, MA).
Immunoprecipitation of RalA-GTP (guanosine triphosphate) was performed with the Ral Activation Assay Kit (Upstate, Lake Placid, NY) according to the manufacturer's protocol. All of the primary antibodies were from Cell Signaling Technology unless specified; primary antibodies used for immunoprecipitations and Western blotting were as follows: Ras (BD Transduction Laboratories), Phospho-Stat5 (signal transducer and activator of transcription 5) (Tyr694), Stat5b (C-17; Santa Cruz Biotechnology, Santa Cruz, CA), Phospho-Akt (Ser473) (587F11), Akt, Phosphop44/42 MAP kinase (Thr202/Tyr204), p44/42 MAP Kinase, Phospho-p38 MAP Kinase (Thr180/Tyr182), p38 MAP Kinase, Phospho-SAPK/JNK (stress-activated protein kinase/c-Jun N-terminal kinase) (Thr183/Tyr185), and SAPK/JNK.
Results
Oncogenic H-ras constitutively activates the p44/42, Akt, and RalA pathways in primary erythroid progenitors
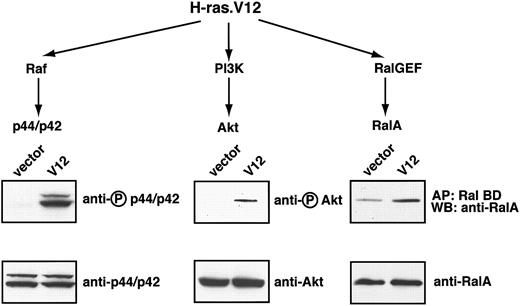
We first determined the signaling pathways constitutively activated by oncogenic H-ras (H-ras.V12 mutant) expression in mouse erythroid progenitors. In other cell lines transformed with the H-ras.V12 mutant 3 major pathways become constitutively activated: the Raf, PI3-kinase, and RalGEF pathways (reviewed in Campbell et al28 ). Thus. we first focused on these pathways in H-ras.V12-transduced erythroid progenitors.
To this end we used freshly isolated TER119- primary fetal liver cells; previously we showed that at least 85% of this population is erythroid progenitors and early erythroblasts.5 These cells were infected either with a control retrovirus or a retrovirus encoding H-ras.V12. Because an infection efficiency of 60% to 80% was routinely achieved, total cell lysates were prepared without further enrichment or purification of infected cells. After the cells were cultured for approximately 16 hours in the presence of serum and Epo, the extent of phosphorylation of p44/42 ERK, an indicator of its activation state, was the same in control vector-transduced cells and H-ras.V12-transduced cells (data not shown). However, following depletion of both serum and Epo for 2 hours, p44/42 ERK remained active in H-ras.V12-transduced cells but not in the control cells. No difference was seen in the expression of total p44/42 ERK in the 2 populations of cells (Figure 1). Similarly, H-ras.V12 expression induced constitutive activation of Akt in primary erythroid cells; the basal activation level of Akt was extremely low in control vector-transduced cells (Figure 1). In control cells deprived of serum and Epo, the GTP-bound form of RalA was maintained at a significant basal level (Figure 1). However, expression of H-ras.V12 caused a 2- to 3-fold elevation in the level of RalA-GTP (Figure 1).
Oncogenic H-ras activates 3 major pathways in erythroid progenitors. Freshly isolated fetal liver TER119- cells were infected with bicistronic retroviruses encoding hCD4 alone (control vector) or oncogenic H-ras (H-ras.V12 mutant) and cultured overnight. The cells were starved for 2 hours and harvested. Levels of RalA-GTP, the active form of RalA, were analyzed by affinity purification of lysates with use of a GST fusion with RalA-binding domain of Ral BP immobilized on agarose beads. Phosphorylated p44/p42 ERK and Akt were measured by Western blotting (described in “Materials and methods”). For each tested signaling protein, the activated or phosphorylated form is shown in the top blot (indicated by a circled P) and the total protein on the bottom blot.
Oncogenic H-ras activates 3 major pathways in erythroid progenitors. Freshly isolated fetal liver TER119- cells were infected with bicistronic retroviruses encoding hCD4 alone (control vector) or oncogenic H-ras (H-ras.V12 mutant) and cultured overnight. The cells were starved for 2 hours and harvested. Levels of RalA-GTP, the active form of RalA, were analyzed by affinity purification of lysates with use of a GST fusion with RalA-binding domain of Ral BP immobilized on agarose beads. Phosphorylated p44/p42 ERK and Akt were measured by Western blotting (described in “Materials and methods”). For each tested signaling protein, the activated or phosphorylated form is shown in the top blot (indicated by a circled P) and the total protein on the bottom blot.
We also measured activation of other signaling pathways in these primary erythroid progenitors expressing H-ras.V12, including Stat5, p38, and JNK MAPKs. We failed to detect any abnormal activation of these pathways (data not shown). In summary, expression of oncogenic H-ras in primary fetal liver erythroid progenitors leads to constitutive activation of 3 major signaling pathways: p44/42 ERK, Akt, and RalA.
Constitutively active MEK1 recapitulates the effects of oncogenic H-ras in mouse erythroid progenitors
Previously, we showed that expression of oncogenic H-ras (H-ras.V12 mutant) blocks terminal erythroid differentiation and triggers Epo-independent growth.5 To determine how individual Ras pathways contribute to the effects of oncogenic H-ras.V12 in erythroid progenitors, we expressed constitutively active mutants of key signaling proteins in primary erythroid progenitors and examined their differentiation profiles, cell cycle profiles, and Epo-independent growth.
We first analyzed the well-characterized H-ras double mutants that carry the V12 mutation and an additional mutation in the effector loop domain.21,23 Each of these double mutants constitutively activates only 1 of the 3 Ras downstream signaling pathways. We introduced the double mutants individually (constitutive activation of 1 pathway) or 2 mutants simultaneously (constitutive activation of 2 pathways) into primary erythroid progenitors. To our surprise, none of these could recapitulate the effects of oncogenic H-ras on terminal erythroid differentiation and Epo-independent growth (data not shown). We then checked the extent that individual pathways were activated by the double mutants, for example p44/42 ERK activation by the H-ras.V12;E38 mutant. Compared with H-ras.V12, expression of the H-ras.V12;E38 mutant resulted in very poor constitutive activation of p44/42 ERK (data not shown). We were not sure whether the failure to see any effects of expression of the H-ras.V12 double mutants alone or in any combination was caused by insufficient pathway activation in primary erythroid progenitors. Therefore, we switched to another group of constitutively active mutants. As shown in Figure 2, constitutively active (ca) Raf activated the p44/42 ERK pathway to a level comparable to that of the oncogenic H-ras.V12 mutant, whereas ca.MEK activated the p44/42 ERK pathway to a lesser extent. Moreover, ca.MEK expression did not cause abnormal activation of either Akt or RalA in primary erythroid progenitors (data not shown). In contrast, expression of ca.Akt and ca.Rlf (a RalGEF) activated the Akt and RalA proteins, respectively, to an even higher level than did H-ras.V12 (Figure 2).
Constitutively active mutant signaling proteins activate specific signaling pathways downstream of H-ras in erythroid progenitors. Freshly isolated fetal liver TER119- cells were infected with bicistronic retroviruses encoding hCD4 alone (control vector), H-ras.V12, constitutively active (ca.) Raf, ca.MEK, ca.Akt, or ca.Rlf. The cells were cultured and harvested, and lysates were analyzed exactly as described in the legend to Figure 1. The extent of activation of each individual pathway by each ca. mutant was compared with that induced H-ras.V12. ca.Akt was created by the deletion of its PH domain and the addition of an src myristoylation signal at its N-terminus.25 Therefore, the size of ca.Akt protein is smaller than that of wild-type Akt.
Constitutively active mutant signaling proteins activate specific signaling pathways downstream of H-ras in erythroid progenitors. Freshly isolated fetal liver TER119- cells were infected with bicistronic retroviruses encoding hCD4 alone (control vector), H-ras.V12, constitutively active (ca.) Raf, ca.MEK, ca.Akt, or ca.Rlf. The cells were cultured and harvested, and lysates were analyzed exactly as described in the legend to Figure 1. The extent of activation of each individual pathway by each ca. mutant was compared with that induced H-ras.V12. ca.Akt was created by the deletion of its PH domain and the addition of an src myristoylation signal at its N-terminus.25 Therefore, the size of ca.Akt protein is smaller than that of wild-type Akt.
We then expressed each of these constitutively active mutant proteins individually in purified primary fetal liver TER119- erythroid progenitors. As before we used a bicistronic retroviral vector (MICD4) that also encodes a truncated hCD4 protein.5 Thus, cells expressing these signal-transducing proteins simultaneously expressed hCD4 on their surface (hCD4+ cells). After 2 days in culture, the infected cells were first analyzed for erythroid differentiation profiles by the flow cytometry analysis described previously.5 Essentially, cells were double-labeled for erythroid-specific TER119 and nonerythroid-specific transferrin receptor (CD71). Five distinct populations of cells can be defined by their characteristic staining patterns: CD71medTER119low (R1, progenitor cells and proerythroblasts), CD71highTER119low (R2, proerythroblasts and early basophilic erythroblasts), CD71highTER119high (R3, early and late basophilic erythroblasts), CD71medTER119high (R4, chromatophilic and orthochromatophilic erythroblasts), and CD71lowTER119high (R5, late orthochromatophilic erythroblasts and reticulocytes).
In erythroid cells infected with control vector, approximately 87% of cells induced TER119 expression and differentiated into R3 to R5 cells (Figure 3). Erythroid cells transduced with ca.Rlf maintained a roughly normal differentiation profile (“Discussion”). Expression of ca.Akt exerted a mild inhibitory effect on erythroid differentiation; 29.4% of infected cells remained TER119-. Expression of ca.Raf resulted in massive cell death in erythroid cells (“Discussion”). In contrast, expression of ca.MEK in erythroid progenitors blocked terminal erythroid differentiation to the same level as seen in H-Ras.V12-transduced cells; approximately 49% of ca.MEK-infected cells and approximately 53% of H-Ras.V12 expressing cells remained TER119-.
Expression of constitutively active MEK1 inhibits erythroid differentiation. Freshly isolated fetal liver TER119- cells were infected with bicistronic retroviruses encoding hCD4 alone, H-ras.V12, ca.MEK, ca.Akt, or ca.Rlf and cultured for 2 days on fibronectin-coated plates; Epo was added during the first day.5 The cells were then simultaneously stained with a fluorescein isothiocyanate (FITC)-conjugated anti-CD71 monoclonal antibody (mAb), a phycoerythrin (PE)-conjugated anti-TER119 mAb, and an anti-hCD4 mAb followed by cyanine 5 (Cy5)-conjugated donkey anti-mouse immunoglobulin G (IgG) antibody. Their differentiation profiles were analyzed by flow cytometry. Dead cells (7-aminoactinomycin D [7-AAD]-positive), debris, and most terminally differentiated reticulocytes (low forward scatter) were excluded from analysis. The panels display the density plots of infected cells (hCD4+ cells). The percentages of TER119- cells (presented as total of R1 and R2 cells) and TER119+ cells (presented as total of R3-R5 cells) are labeled at the bottom of each density plot.
Expression of constitutively active MEK1 inhibits erythroid differentiation. Freshly isolated fetal liver TER119- cells were infected with bicistronic retroviruses encoding hCD4 alone, H-ras.V12, ca.MEK, ca.Akt, or ca.Rlf and cultured for 2 days on fibronectin-coated plates; Epo was added during the first day.5 The cells were then simultaneously stained with a fluorescein isothiocyanate (FITC)-conjugated anti-CD71 monoclonal antibody (mAb), a phycoerythrin (PE)-conjugated anti-TER119 mAb, and an anti-hCD4 mAb followed by cyanine 5 (Cy5)-conjugated donkey anti-mouse immunoglobulin G (IgG) antibody. Their differentiation profiles were analyzed by flow cytometry. Dead cells (7-aminoactinomycin D [7-AAD]-positive), debris, and most terminally differentiated reticulocytes (low forward scatter) were excluded from analysis. The panels display the density plots of infected cells (hCD4+ cells). The percentages of TER119- cells (presented as total of R1 and R2 cells) and TER119+ cells (presented as total of R3-R5 cells) are labeled at the bottom of each density plot.
Next, we examined the cell cycle progression in cells expressing individual constitutively active proteins, after selecting the cells that expressed the GFP marker and, thus, the protein coexpressed by the retroviral vector. As expected for terminally differentiated cells, by the end of the two-day culture period approximately 82% of erythroid cells expressing GFP only (control vector) had permanently exited the cell cycle and accumulated in the G1 phase (Table 1). Consistent with our previous report, cells expressing H-ras.V12 were undergoing abnormal proliferation, because approximately 46% were in S phase. Although expression of ca.Akt and ca.Rlf showed mild effects on promoting cell cycle progression, only ca.MEK-transduced cells displayed a similar cell cycle profile as those expressing the oncogenic H-ras.V12 (Table 1); approximately 40% of the cells expressing ca.MEK were in S phase and, thus, actively proliferating.
Cell cycle analysis of erythroblasts expressing different signaling proteins
Bicistronic retrovirus construct . | G1 phase, % . | S phase, % . | G2/M phase, % . |
|---|---|---|---|
| IRES-GFP | 81.8 ± 4.1 | 7.8 ± 2.2 | 10.4 ± 2.5 |
| H-ras. V12-IRES-GFP | 53.9 ± 0.6 | 40.0 ± 0.6 | 6.1 ± 0 |
| ca.MEK-IRES-GFP | 53.9 ± 6.0 | 39.8 ± 5.6 | 6.2 ± 1.8 |
| ca.Akt-IRES-GFP | 65.3 ± 7.3 | 20.1 ± 7.6 | 14.5 ± 1.0 |
| ca.Rlf-IRES-GFP | 70.2 ± 5.0 | 24.5 ± 6.4 | 5.3 ± 2.2 |
Bicistronic retrovirus construct . | G1 phase, % . | S phase, % . | G2/M phase, % . |
|---|---|---|---|
| IRES-GFP | 81.8 ± 4.1 | 7.8 ± 2.2 | 10.4 ± 2.5 |
| H-ras. V12-IRES-GFP | 53.9 ± 0.6 | 40.0 ± 0.6 | 6.1 ± 0 |
| ca.MEK-IRES-GFP | 53.9 ± 6.0 | 39.8 ± 5.6 | 6.2 ± 1.8 |
| ca.Akt-IRES-GFP | 65.3 ± 7.3 | 20.1 ± 7.6 | 14.5 ± 1.0 |
| ca.Rlf-IRES-GFP | 70.2 ± 5.0 | 24.5 ± 6.4 | 5.3 ± 2.2 |
Total fetal liver cells were freshly isolated from E13.5 to E15.5 embryos, and TER-119− erythroblasts were purified. Purified erythroblasts were infected with different bicistronic retrovirus constructs and cultured for 1 day in vitro. Infected cells were sorted by FACS and cultured in erythroid differentiation medium (EDM) for another day. Cultured cells were then incubated in hypotonic PI solution and analyzed by flow cytometry; the data were analyzed by ModFit software for cell cycle distribution. Results of infected cells are presented here as percentage of total cells analyzed.
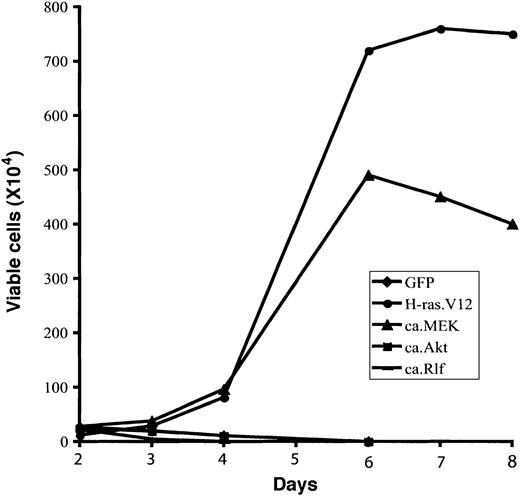
Finally, we examined whether the expression of any constitutively active signal-transducing protein could support Epo-independent growth in vitro. To this end, 2 days after infection retrovirally transduced erythroid cells were continuously cultured in fibronectin-coated wells in medium without Epo (described in “Materials and methods”). Total viable cells were counted, and the percentage of infected cells (GFP+) was determined by flow cytometry analysis. As before, erythroid cells expressing GFP only (control vector) differentiated normally, and the number of total viable cells started to decrease after 2 days in culture (Figure 4). In contrast, expression of oncogenic H-ras.V12 resulted in abnormal Epo-independent proliferation of erythroid progenitors; the viable cell number peaked around 6 to 7 days. Expression of ca.MEK, but not of ca.Akt or ca.Rlf, triggered Epo-independent growth of erythroid progenitors in vitro similar to that of cells expressing H-ras. V12.
Expression of constitutively active MEK1 induces Epo-independent growth of erythroid progenitors. Freshly isolated fetal liver TER119- cells were infected with bicistronic retroviruses encoding GFP alone, H-ras.V12, ca.MEK, ca.Akt, or ca.Rlf and cultured for 1 day. GFP+ cells were isolated by FACS sorting and continuously cultured in fibronectin-coated wells in the absence of Epo. Cell numbers are presented as total viable cells. Data are averages from triplicate cultures of a representative experiment and standard deviations were always less than 5% of the averages.
Expression of constitutively active MEK1 induces Epo-independent growth of erythroid progenitors. Freshly isolated fetal liver TER119- cells were infected with bicistronic retroviruses encoding GFP alone, H-ras.V12, ca.MEK, ca.Akt, or ca.Rlf and cultured for 1 day. GFP+ cells were isolated by FACS sorting and continuously cultured in fibronectin-coated wells in the absence of Epo. Cell numbers are presented as total viable cells. Data are averages from triplicate cultures of a representative experiment and standard deviations were always less than 5% of the averages.
In summary, expression of ca.MEK in primary erythroid progenitors is sufficient to recapitulate all of the effects of oncogenic H-ras.V12; it blocked terminal erythroid differentiation and induced Epo-independent cell cycling and proliferation.
Constitutive activation of both MEK and Akt in primary erythroid progenitors supports more extensive Epo-independent growth than does oncogenic H-ras
We further assessed the contribution of H-ras downstream signaling pathways by simultaneously expressing 2 constitutively active mutant signaling proteins in primary erythroid progenitors. In NIH 3T3 and other cell lines, constitutive activation of any 2 Ras downstream signaling pathways recapitulates the effects of oncogenic H-ras (H-ras.V12 mutant) expression at least to some extent (eg, stimulation of foci formation, but less efficiently than H-ras.V12).21,23 We were interested to know whether this is the case in primary erythroid progenitors.
To this end we expressed all 3 possible combinations of 2 constitutively active mutant signaling proteins, ca.MEK, ca.Akt, and ca.Rlf, in TER119- fetal liver erythroid progenitor-enriched cells. To select for double-infected cells we used 2 bicistronic retroviral constructs encoding different markers, GFP and hCD4. Doubly infected cells (GFP+hCD4+) were sorted 1 day after infection, then cultured for an additional day on fibronectin-coated plates before analysis of erythroid differentiation and cell cycle progression.
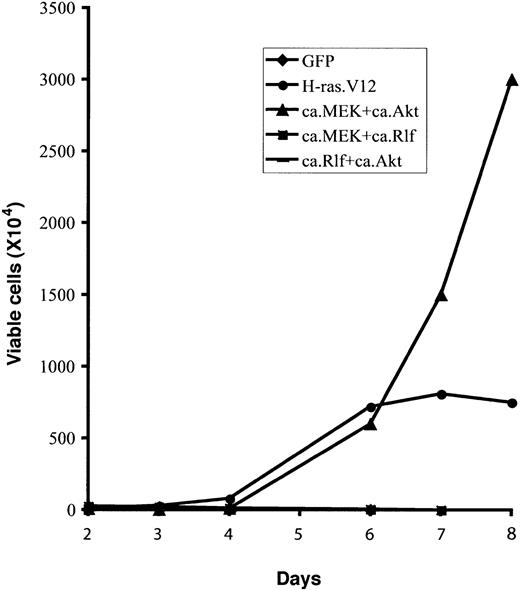
Only constitutive activation of MEK and Akt together resulted in Epo-independent growth of erythroid progenitors (Figure 5). Moreover, these cells underwent abnormal proliferation in the absence of Epo for 4 to 6 weeks, much longer than those expressing H-ras.V12 (Figure 5 and data not shown). These data suggest that the PI3-kinase/Akt pathway acts synergistically with the MEK/ERK pathway to support Epo-independent growth in primary erythroid progenitors. Alternatively, constitutive activation of the Akt pathway could prevent apoptosis of cells expressing a constitutively active MEK, allowing a continuous increase in cell numbers (“Discussion”).
Constitutive activation of MEK1 and Akt together supports longer Epo-independent growth of erythroid progenitors than does H-ras.V12. Freshly isolated fetal liver TER119- cells were infected with bicistronic retroviruses encoding GFP alone, H-ras.V12, ca.MEK and ca.Akt, ca.MEK and ca.Rlf, or ca.Rlf and ca.Akt, and cultured for 1 day. For double infection, one retroviral construct encodes hCD4 and the other encodes GFP. Doubly infected cells were stained for hCD4. Then, GFP+ cells (single infection) or GFP+hCD4+ cells (double infection) were sorted by FACS. Cells were then replated in fibronectin-coated wells in medium lacking Epo. Cell numbers are presented as total viable cells. Data are averages from triplicate cultures of a representative experiment and standard deviations were always less than 5% of the averages.
Constitutive activation of MEK1 and Akt together supports longer Epo-independent growth of erythroid progenitors than does H-ras.V12. Freshly isolated fetal liver TER119- cells were infected with bicistronic retroviruses encoding GFP alone, H-ras.V12, ca.MEK and ca.Akt, ca.MEK and ca.Rlf, or ca.Rlf and ca.Akt, and cultured for 1 day. For double infection, one retroviral construct encodes hCD4 and the other encodes GFP. Doubly infected cells were stained for hCD4. Then, GFP+ cells (single infection) or GFP+hCD4+ cells (double infection) were sorted by FACS. Cells were then replated in fibronectin-coated wells in medium lacking Epo. Cell numbers are presented as total viable cells. Data are averages from triplicate cultures of a representative experiment and standard deviations were always less than 5% of the averages.
Coexpression of constitutively active Rlf with constitutively active Akt did not induce Epo-independent proliferation (Figure 5). Nor did it have any significant effect on cell cycle progression or erythroid differentiation (data not shown). Interestingly, coexpression of constitutively active Rlf with constitutively active MEK blocked the Epo-independent proliferation of erythroid progenitors observed after expression of constitutively active MEK alone (Figures 4 and 5). These cells underwent normal erythroid differentiation, indicating that signaling downstream of Rlf inhibits cell proliferation induced by constitutively active MEK (“Discussion”).
Inhibition of the MEK/ERK pathway restores normal differentiation in erythroid progenitors expressing oncogenic H-ras
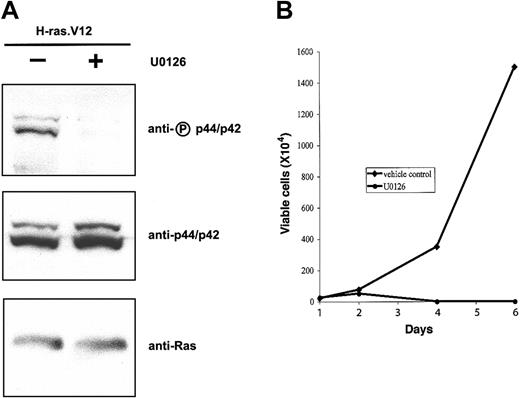
Constitutive activation of the MEK/ERK pathway in primary erythroid progenitors is sufficient to mimic the effects of oncogenic H-ras.V12 expression. Next, we wanted to know whether constitutive activation of this pathway is essential for the proliferative and antidifferentiation effects of oncogenic Ras. To this end TER119- fetal liver cells were infected with the bicistronic retrovirus encoding H-ras.V12. After 1 day in culture, we added a MEK1- and MEK2-specific inhibitor, U0126, and placed the cells in medium lacking Epo. As expected, U0126 greatly reduced the phosphorylation level of p44/42 ERK in erythroid progenitors expressing H-ras.V12 (Figure 6A) but exhibited no effects on Akt and RalA activation in these cells (data not shown). When these cells were cultured further in the presence of U0126 (with replacement of U0126 every 24 hours), the number of total viable cells peaked around 2 to 3 days (Figure 6B). As before, cells expressing H-ras.V12 but cultured in the presence of DMSO (vehicle control) proliferated actively for 6 to 7 days. Thus, U0126 inhibited Epo-independent growth of erythroid progenitors expressing H-ras.V12.
U0126 inhibits H-ras.V12-induced Epo-independent growth of erythroid progenitors. Freshly isolated fetal liver TER119- cells were infected with a bicistronic retrovirus encoding H-ras.V12 and hCD4 and cultured in fibronectin-coated wells in medium containing serum and Epo. U0126 (20 μM) was added into cells on day 1 and replaced every 24 hours. (A) The cells were harvested 6 hours after the addition of DMSO (control) or U0126. Expression of H-ras.V12 protein and activation of p44/42 were examined by Western blotting as in Figure 1. (B) The cells were cultured in fibronectin-coated wells in medium lacking Epo and in the absence or presence of U0126. Cell numbers are presented as total viable cells. Data are averages from triplicate cultures of a representative experiment and standard deviations were always less than 5% of the averages.
U0126 inhibits H-ras.V12-induced Epo-independent growth of erythroid progenitors. Freshly isolated fetal liver TER119- cells were infected with a bicistronic retrovirus encoding H-ras.V12 and hCD4 and cultured in fibronectin-coated wells in medium containing serum and Epo. U0126 (20 μM) was added into cells on day 1 and replaced every 24 hours. (A) The cells were harvested 6 hours after the addition of DMSO (control) or U0126. Expression of H-ras.V12 protein and activation of p44/42 were examined by Western blotting as in Figure 1. (B) The cells were cultured in fibronectin-coated wells in medium lacking Epo and in the absence or presence of U0126. Cell numbers are presented as total viable cells. Data are averages from triplicate cultures of a representative experiment and standard deviations were always less than 5% of the averages.
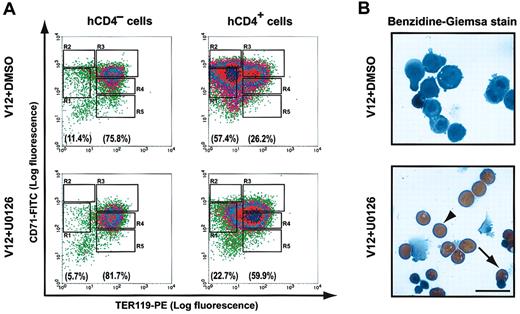
We further investigated the cellular mechanisms underlying the U0126-mediated inhibition of Epo-independent growth in erythroid progenitors expressing H-ras.V12. We examined the erythroid differentiation profile, cell cycle profile, and extent of apoptosis rate every 24 hours. On day 2, we found that U0126 exerted a mild inhibition of cell cycle progression on cells expressing H-ras.V12 (data not shown and see “Discussion”). The apoptotic rate in cells cultured in the presence of U0126 was similar to that in vehicle control cells (data not shown). Importantly, in the presence of U0126 approximately 60% of H-ras.V12-expressing cells underwent apparently normal erythroid differentiation and became TER119+ (R3-R5) cells (Figure 7A). In contrast, in the absence of U0126 approximately 60% of H-ras.V12-transduced cells remained undifferentiated as TER119- (R1 + R2) cells.
U0126 restores erythroid differentiation in H-ras.V12-transduced erythroid progenitors. Freshly isolated fetal liver TER119- cells were infected with a bicistronic retrovirus encoding H-ra.V12 and hCD4 and cultured as described in Figure 6. (A) After 2 days in culture in the absence or presence of U0126, erythroid differentiation profiles were analyzed by FACS as described in Figure 3. hCD4+ cells express H-ras.V12, whereas hCD4- cells are uninfected and provide an internal control to the experiment. The percentages of TER119- cells (presented as total of R1 and R2 cells) and TER119+ cells (presented as total of R3-R5 cells) are indicated at the bottom of each density plot. (B) After 3 days in culture in the absence or presence of U0126, the cells were processed with Benzidine-Giemsa stain. Cells from one representative field are shown here. The arrow indicates a late orthochromatophilic erythroblast, and the arrowhead indicates an enucleated reticulocyte. Scale bar: 20 μm.
U0126 restores erythroid differentiation in H-ras.V12-transduced erythroid progenitors. Freshly isolated fetal liver TER119- cells were infected with a bicistronic retrovirus encoding H-ra.V12 and hCD4 and cultured as described in Figure 6. (A) After 2 days in culture in the absence or presence of U0126, erythroid differentiation profiles were analyzed by FACS as described in Figure 3. hCD4+ cells express H-ras.V12, whereas hCD4- cells are uninfected and provide an internal control to the experiment. The percentages of TER119- cells (presented as total of R1 and R2 cells) and TER119+ cells (presented as total of R3-R5 cells) are indicated at the bottom of each density plot. (B) After 3 days in culture in the absence or presence of U0126, the cells were processed with Benzidine-Giemsa stain. Cells from one representative field are shown here. The arrow indicates a late orthochromatophilic erythroblast, and the arrowhead indicates an enucleated reticulocyte. Scale bar: 20 μm.
On day 3, we examined the H-ras.V12-transduced cells by Benzidine-Giemsa staining. Benzidine stains for hemoglobin, whose elevated expression is a hallmark of terminal erythroid differentiation. When cultured in the absence of U0126, H-ras.V12-transduced cells displayed a morphology characteristic of undifferentiated erythroid cells and were mainly benzidine negative (Figure 7B). In contrast, when cultured in the presence of U0126, H-ras.V12-transduced cells displayed morphologies characteristic of differentiated erythroid cells (Figure 7B). Most of them had differentiated into late orthochromatophilic erythroblasts and enucleated reticulocytes, all of which were benzidine positive. The sizes of the differentiated reticulocytes were comparable to those of reticulocytes freshly isolated from fetal livers (data not shown). Consistent with their morphologies, more than 80% of the remaining nucleated cells had exited the cell cycle and accumulated in the G1 phase (data not shown). These data suggest that the MEK/ERK pathway is essential for H-ras.V12-mediated effects in primary erythroid progenitors. Blocking constitutive activation of this pathway by U0126 inhibited Epo-independent growth of erythroid cells expressing H-ras.V12 and restored normal erythroid differentiation.
Discussion
Here, we showed that expression of oncogenic H-ras (H-ras.V12) abnormally activates 3 major downstream signaling pathways in primary erythroid cells. Among these constitutive activation of the MEK/ERK pathway is the key mediator of the effects of H-ras.V12 expression in erythroid progenitors: Expression of ca.MEK blocked terminal erythroid differentiation and triggered Epo-independent growth, closely resembling the effects of H-ras.V12 (Figures 3 and 4). Our findings are consistent with previous studies performed in human acute myelogenous leukemia (AML) cell lines and primary leukemic blasts, in which the ERK pathway is constitutively activated in most cases (reviewed in Platanias29 and Lee and McCubrey30 ). These data suggest that constitutive activation of the MEK/ERK pathway is sufficient to cause impaired erythropoiesis in patients with myeloid disorders. However, unlike the case in NIH 3T3 cells, ca.Raf, which constitutively activates the p44/42 ERK pathway to the same extent as does H-ras.V12 in primary erythroid cells (Figure 2), failed to recapitulate the cell proliferation and antidifferentiation effects of H-ras.V12 expression. Rather, ca.Raf expression induced massive cell death (data not shown). It is likely that primary erythroid progenitors are highly sensitive to the extent of ERK activation and that very high activation of this pathway alone may be toxic. Alternatively, as previously reported that this construct confers a weak Ras-independent transforming ability,12 ca.Raf may activate additional pathways that antagonize the constitutive activation of the p44/42 ERK.
Consistent with previous findings in NIH 3T3 cells, expression of ca.Akt or ca.Rlf alone did not mimic the effects of H-ras.V12 expression (Figures 3 and 4). Erythroid cells expressing ca.Rlf showed a roughly normal differentiation profile, although the R5 cells, mainly enucleated reticulocytes, appeared underrepresented (Figure 3). We examined ca.Rlf transduced cells after Benzidine-Giemsa staining. The morphology of these cells was indistinguishable from that of control cells and reticulocytes were present in the normal ratio (data not shown). One possibility is that expression of ca.Rlf blocks the normal down-regulation of cell surface transferrin receptor CD71 and caused an apparent reduction of the R5 population on the flow cytometry plot. In any case, the effects of ca.Rlf expression on erythropoiesis appear minor. Expression of ca.Akt in primary erythroid cells caused a slight inhibition of their differentiation (Figure 3). Consistent with this differentiation profile, Benzidine-Giemsa staining of these cells showed that a significantly higher percentage of them were not terminally differentiated (data not shown). Overall, however, the effects of ca.Akt expression on erythroid terminal proliferation and differentiation are minor.
When coexpressed with ca.MEK, ca.Rlf opposed many of the pro-proliferation and antidifferentiation effects induced by ca.MEK. Expression of ca.Rlf inhibited the abnormal cell cycle progression induced by ca.MEK (data not shown) and coexpression of ca.Rlf with ca.MEK abolished the Epo-independent growth induced by ca.MEK (Figure 5). This opposing effect of ca.Rlf expression was likely not due to the higher activation level of the Rlf/RalA pathway than that seen in H-ras.V12-expressing cells (Figure 3), because similar results were obtained when we used another constitutively active mutant (RalA.L71) that activates the RalA pathway less than does ca.Rlf22,31 (data not shown). Our observation is consistent with a previous report that Rlf opposes the action of Raf and PI3-kinase in PC12 cells.32
Similarly to ca.Rlf, ca.Akt exerted certain antiproliferation and prodifferentiation effects in cells coexpressing ca. MEK. Primary erythroid progenitors coexpressing ca.Akt and ca.MEK showed a much lower percentage of cells in the S and G2/M phases compared with cells expressing ca.MEK alone (data not shown). Consistent with this effect on the cell cycle, more cells coexpressing ca.Akt and ca.MEK became TER119+ and underwent terminal erythroid differentiation than did cells expressing only ca.MEK (data not shown). However, and unlike the cells coexpressing ca.MEK and ca.Rlf, most TER119- cells coexpressing ca.Akt and ca.MEK underwent a very prolonged period of Epo-independent proliferation, much longer than did cells expressing either ca.MEK or H-ras.V12 (Figures 4 and 5). We examined the apoptotic rate in double-infected cells and did observe lower apoptotic rate between day 1 and day 3 than in cells expressing oncogenic Ras or ca.MEK alone. However, beyond day 3, apoptosis in cells expressing oncogenic Ras or ca.MEK alone was negligible and was similar to the cells expressing ca.MEK and ca.Akt together. Therefore, it is likely that enhancement of cell viability by Akt activation is a contributing factor to the prolonged Epo-independent growth, but other as yet unknown synergistic mechanisms are also involved. Similar conflicting effects of ca.Akt and ca.MEK have been well documented before (reviewed in Lee and McCubrey30 ). Some studies suggest that the PI3-kinase/Akt pathway enhances and/or synergizes with the MEK/ERK signaling to provide a more robust growth-promoting signal, whereas other reports state that Akt is able to efficiently abrogate Raf activation of downstream signaling proteins. Different states of cell differentiation might account for the apparent discrepancies in these effects.33 Alternatively, erythroid cells expressing a constitutively active MEK/ERK pathway might undergo significant apoptosis, which could be prevented by constitutive activation of the Akt pathway, thus allowing a continuous increase in cell numbers in cells coexpressing ca.Akt and ca.MEK.
We used U0126, a specific MEK1 and MEK2 inhibitor, and a dominant-negative MEK mutant (dn.MEK)17 to inhibit the ERK activation in erythroid cells expressing H-ras.V12 (Figure 6 and data not shown). In both cases, these agents greatly reduced ERK activation but showed no inhibition of Akt or p70 S6 kinase activation (Figure 6 and data not shown). These agents efficiently blocked Epo-independent proliferation of H-Ras.V12-transduced cells and restored normal terminal erythroid differentiation (Figures 6 and 7). The effects on erythroid differentiation induced by U0126 were similar to those induced by a farnesylation inhibitor, FTI-276, on H-Ras.V12-transduced cells (data not shown). These observations are consistent with a previous report that a novel Ras farnesylation inhibitor (HR12) reverses the Ras-induced transformed phenotype through inhibition of the MEK/ERK pathway.34
Our results suggest the possibility of targeting the MEK/ERK signal transduction cascade to restore normal erythroid differentiation in myeloid leukemia patients. Pharmacologic inhibitors of MEK have been used in human AML cell lines to inhibit the activation of ERK pathway.35-37 These inhibitors inhibited cell growth and induced apoptosis and also sensitized the cells to other chemotherapeutic drugs. However, our results are different from those obtained from AML cell lines. When examined 6 to 10 hours after addition, U0126 did not inhibit cell cycle progression in primary erythroid cells expressing oncogenic H-ras, although it showed a mild inhibition 24 hours after addition (data not shown). We hypothesize that U0126 does not inhibit the cell cycle progression per se. Rather, the cell cycle inhibition observed 24 hours after U0126 addition might be an indirect consequence of initiation of terminal erythroid differentiation in these cells. We did not observe any significant increase of apoptosis in H-ras.V12-transduced erythroid cells in the presence of U0126 (data not shown). Such differences on the effects of MEK/ERK inhibition between our data on primary erythroid progenitors with that obtained with AML cell lines is not surprising given the very different properties of these cells.
In addition to the 3 signaling pathways constitutively activated by oncogenic H-ras in primary erythroid progenitors, H-Ras.V12 appeared to inactivate certain protein phosphatases. We found (J.Z. and H.F.L., unpublished data, March 2004) that in primary erythroid cells expressing H-ras.V12, p70 S6 kinase was phosphorylated (and thus activated) to the same level as in control cells. However, the phosphorylation level of its downstream target, S6 ribosomal protein, was much higher in cells transduced by H-ras.V12 than that in cells transduced by the control vector. An elevated phosphorylation level of S6 ribosomal protein correlates well with an increase in translation of proteins involved in cell cycle progression and the translational machinery.38,39 Because the level of phosphorylation of any protein is regulated simultaneously by its kinase(s) and phosphatase(s), we reasoned that the phosphatase(s) that dephosphorylate S6 ribosomal protein must be inactivated by H-ras.V12 expression in primary erythroid progenitors. To test this, we treated primary erythroid progenitors transduced by control vector with okadeic acid, an inhibitor of protein phosphatases 1 and 2A.40 The phosphorylation level of S6 ribosomal protein in these cells increased to a level comparable to that in cells transduced by H-ras.V12 (and untreated with okadeic acid; data not shown). This observation is consistent with a previous report that phosphatase PAC1 is down-regulated in human acute leukemia cells.41 Taken together, these data suggest that inactivation of some downstream signaling proteins, protein phosphatases 1 and 2A as examples, by oncogenic Ras in primary erythroid progenitors may be an important aspect of oncogenic transformation.
Although in patients with myeloid disorders Ras mutations occur predominantly in the N-ras and K-ras genes,3,4 we found that overexpression of oncogenic N-ras in erythroid progenitors has similar effects to overexpression of oncogenic H-ras, blocking terminal erythroid differentiation and promoting abnormal proliferation of erythroid colony-forming units (CFU-Es) and early erythroblasts.5 Consistent with our findings, a mouse line carrying a conditional oncogenic K-ras allele developed similar phenotypes when the oncogenic K-ras allele was activated somatically; erythroid progenitors show hyperproliferation and diminished terminal differentiation.8 Moreover, we found that overexpression of oncogenic N-ras abnormally activates p44/42 MAPK, Akt, and RalA pathways, indistinguishably from overexpression of oncogenic H-ras (data not shown). Taken together, our data suggest that ectopic expression of oncogenic H-ras in primary erythroid progenitors recapitulates the effects of oncogenic N-ras expression.
In summary, we found that in purified primary erythroid progenitor cells expressing oncogenic H-ras, the Raf/ERK, PI3-kinase/Akt, and RalGEF/RalA pathways become constitutively activated. However, constitutive activation only of the MEK/ERK pathway alone is sufficient and necessary for the proliferative and antidifferentiation effects of oncogenic H-ras expression. Expression of ca.MEK blocks terminal erythroid differentiation and triggers Epo-independent growth, whereas inhibition of MEK/ERK activation reverses H-ras.V12-mediated effects by restoring terminal erythroid differentiation in H-ras.V12-transduced cells.
Prepublished online as Blood First Edition Paper, May 27, 2004; DOI 10.1182/blood-2004-04-1362.
Supported by a grant from the National Institutes of Health (grant PO1 HL 32262 to H.F.L.) and a Postdoctoral Fellowship from the Leukemia and Lymphoma Society (J.Z.).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Qiang Chang, Alec W. Gross, and Anapoorni Rangarajan for helpful discussion and critical comments on the manuscript, and Glen Pardis for help with flow cytometry. We also thank Dr Julian Downward (Imperial Cancer Research Fund, London, United Kingdom) for permission to use the H-ras double mutant constructs, Dr Chris J. Marshall (Cancer Research United Kingdom Centre for Cell and Molecular Biology, Institute of Cancer Research, London, United Kingdom) for permission to use the ca.MEK construct, Dr Larry A. Feig (Tufts University, Boston, MA) for permission to use the Rlf and RalA mutant constructs, and Dr Richard A. Roth (Stanford University School of Medicine, Stanford, CA) for permission to use the ca.Akt construct.


![Figure 3. Expression of constitutively active MEK1 inhibits erythroid differentiation. Freshly isolated fetal liver TER119- cells were infected with bicistronic retroviruses encoding hCD4 alone, H-ras.V12, ca.MEK, ca.Akt, or ca.Rlf and cultured for 2 days on fibronectin-coated plates; Epo was added during the first day.5 The cells were then simultaneously stained with a fluorescein isothiocyanate (FITC)-conjugated anti-CD71 monoclonal antibody (mAb), a phycoerythrin (PE)-conjugated anti-TER119 mAb, and an anti-hCD4 mAb followed by cyanine 5 (Cy5)-conjugated donkey anti-mouse immunoglobulin G (IgG) antibody. Their differentiation profiles were analyzed by flow cytometry. Dead cells (7-aminoactinomycin D [7-AAD]-positive), debris, and most terminally differentiated reticulocytes (low forward scatter) were excluded from analysis. The panels display the density plots of infected cells (hCD4+ cells). The percentages of TER119- cells (presented as total of R1 and R2 cells) and TER119+ cells (presented as total of R3-R5 cells) are labeled at the bottom of each density plot.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/6/10.1182_blood-2004-04-1362/6/m_zh80180466700003.jpeg?Expires=1769156848&Signature=q0BYy2OzialjZIY9b6s0qvc18XsAuV7gbbpedZdbnrogX4H9BdpIvPOeAXRqXZ-5TjdYh1g6NGlZe6K0kO7a7Ms-jnQpjOGjrtYVx~Ndxkx9s91Kaxe-yyNBiMxFqBd4IjylLgVTJCcdmoqE6E5YEaNbfjGksaluV-aLotrUH8rkWtlTEKYcPE-jCTYdpkwkrOpBWsjAv47OBYCBk9ZYLk3dQRLjGsBLqyJzzws~-D8UJ90dIJloGILMG1CSjMdCHoEebYUT6DmDuQ3xUS14GQbuUS0HrwUHtpI0RUDS8Y1DlYlWL5EL4x5o~EejIC3LfysGqAW3wzSNr6sA3RD29A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal