Abstract
Homozygous natural white-spotted (W) mutations in the gene encoding the receptor tyrosine kinase c-Kit are associated with hypoplastic bone marrow, severe macrocytic anemia, and lethality during early postnatal life. c-KitW/W mice can be rescued by wild-type hematopoietic stem cells (HSCs), but it is not known whether the lethality of c-KitW/W mice is the result of HSC failure or defects specific for erythropoiesis. Here we show that transgenic expression of erythropoietin (EPO) can overcome the lethality caused by the c-KitW/W mutation. In W mutant mice rescued by EPO, termed WEPO, erythrocyte colony-forming units (CFU-Es) are rescued to normal frequencies. Hence, Epo receptor signals can partially bypass the strict requirement for c-Kit signaling in erythropoiesis in the absence of c-Kit in vivo. Using a series of W and rescue mouse strains, we define here the erythropoietic threshold permitting survival in vivo. The lethality of c-KitW/W mice has precluded analysis of this crucial receptor-ligand pair in adult stem/progenitor cells. Our strategy to generate viable c-KitW/W mice will be useful to analyze the role of this important receptor tyrosine kinase in adult life in vivo. (Blood. 2004; 104:1688-1695)
Introduction
Mutations in the receptor tyrosine kinase c-Kit lead to sterility, white coat color, intestinal disorders, and anemia reflecting various contributions of c-Kit-mediated signals to differentiation and maintenance of germ cells, melanocytes, intestinal pacemaker cells, and hematopoietic cells (for reviews, see Bernstein et al,1 Besmer,2 and Broudy3 ). The c-KitW allele encodes a shortened protein lacking the transmembrane portion, which, therefore, fails to be expressed on the cell surface.4 Heterozygous c-KitW/+ mice have a white spotted belly, hence the designation “W mutation.” c-KitW/+ mice are hematologically normal compared with wild-type mice.5 Mice homozygous for the W allele (c-KitW/W) are entirely white and die before postnatal day 10.6 To obtain live-born white mice with a robust frequency of 25% from c-KitW/+ × c-KitW/+ intercrosses, Russell and Lawson6 tested inbred lines of c-KitW/+ mice by continuous brother-sister matings for large litter size and longevity of c-KitW/W mutants. In this breeding program, the W-spotted line B (WB) c-KitW/+ inbred mouse strain was established.6 Thus, the W mutation was fixed on the WB background. In the original WB mouse strain, a low percentage (6 of 133 c-KitW/W mice born) of weak white mice survived into adulthood.6 The basis for this survival of c-KitW/W mice is unknown, and this survival trait has been lost. In 2002, 75 years after the first description of the c-KitW mutation,7 an adult c-KitW/W mouse strain was reported from outcrossing WB mice to mice carrying a mixed genetic background.8 The molecular nature of the survival trait in these viable c-Kit-deficient (Vickid) mice remains unknown; therefore, carriers of the survival trait cannot be identified by molecular means. Vickid mice are sterile and have been maintained solely by intensive brother-sister matings of c-KitW/+ Vickid littermates. In addition, backcrosses onto the WB background showed that the unknown modifier is dominant. Because viable c-Kit null mice are valuable tools for studying the biology of this receptor tyrosine kinase during adult life, we searched for a new way to generate viable c-KitW/W mice, hoping to obtain more predictable numbers of surviving mice compared with Vickid mice.
In hematopoiesis, c-Kit-mediated signals play a role in mast cell development (for example, see Kitamura et al9 ), lymphopoiesis (for example, see Waskow et al8 and Rodewald et al10 ), and erythropoiesis (for a review, see Broudy3 and Russell5 ). Viable compound heterozygous mice (c-KitW/Wv) that have, in addition to c-KitW, a kinase-weak hypomorphic allele (c-KitWv) develop macrocytic anemia. This anemia is even more severe in nonviable c-KitW/W mice, suggesting that c-KitW/W mice die of the anemia (for a review, see Russell5 ). c-Kit-mediated signals, however, play important functional roles before erythrocyte lineage commitment in hematopoietic stem cells (HSCs) and in multipotent progenitors. Lack of c-Kit in HSCs or in early progenitors causes functional defects, including failure of c-Kit-deficient bone marrow (BM) cells to provide radioprotection from lethal irradiation and failure to produce macroscopic spleen colony-forming units (CFU-Ss) after transfer into irradiated recipients.8,11-13 Anemia, radioprotection, and CFU-S formation can be permanently corrected by the transfer of histocompatible BM cells to c-KitW/Wv mice.14 Moreover, in utero transfer of wild-type BM cells into c-KitW/W embryos rescues c-KitW/W mice.15 These findings clearly show that defects intrinsic to c-KitW/W BM cells cause hematopoietic failure. However, it was unknown whether, in the absence of c-Kit, HSCs give rise to too few erythroid progenitors or whether c-Kit signals are crucial at the stage of committed erythroid progenitors. When we noticed that Vickid mice had improved red blood cell (RBC) parameters compared with lethal-type c-KitW/W mice, we speculated that additional support of erythropoiesis might be sufficient to overcome the lethality of c-KitW/W mice. To test this idea, we generated EPO-overexpressing c-KitW/W mice by crossing WB mice to an EPO transgenic mouse line, tg6,16 which is termed EPO-tg. Interestingly, this strategy led to viable c-KitW/WEPO-tg mice, termed WEPO. Erythrocyte colony-forming units (CFU-Es), a late stage in erythropoiesis, are strongly reduced in lethal c-KitW/W mice. We show here that erythropoiesis can be partially rescued at the CFU-E stage by EPO overexpression.
Materials and methods
Mice
WB c-KitW/+ mice6 (obtained from Japan-SLC, Shizuoka, Japan), EPO overexpressing transgenic mice (line tg6),16 and WEPO mice were kept under specific pathogen-free conditions. To generate WEPO mice, EPO-tg mice, maintained on a C57BL/6 genetic background, were crossed twice to WB c-KitW/+ mice. All WEPO mice analyzed are WBB6F2c-KitW/WEPO-tg animals. Heterozygosity and homozygosity for the W mutation was determined by polymerase chain reaction (PCR) on genomic DNA, as described previously.8 Peripheral blood analysis was performed using an automated hemocytometer according to the manufacturer's instructions (ADVIA120; Bayer, Leverkusen, Germany).
In vitro colony assays
To assay for erythroid colony formation, cells were plated into medium containing 1% methylcellulose (M3334; Stem Cell Technologies, Lyon, France) supplemented with 3 U/mL recombinant human EPO (Erypo, FS2000; Janssen-Cilag, Neuss, Germany), or with EPO and 1% interleukin-3 (IL-3)-containing supernatant from an IL-3 gene-transfected cell line17 for growth of CFU-E and erythroid burst-forming unit (BFU-E), respectively. After 48 hours (CFU-E) or 7 days (BFU-E), hemoglobin was stained by overlaying the culture with an equal volume of benzidine staining solution (0.4% benzidine [Sigma B3530, Taufkirchen, Germany], 12% acetic acid, 0.3% H2O2). For BFU-E colony numbers, the sum of purely blue and mixed colonies was calculated as the total yield of hemoglobinized colonies.
Flow cytometry
BM and spleen cell suspensions were prepared and stained as described.8 Briefly, nonspecific staining was blocked by incubation with murine immunoglobulin G (IgG; Dianova, Hamburg, Germany; used at 500 μg/mL for 30 minutes on ice). All stainings were performed using 106 cells in 50 μL phosphate-buffered saline (PBS)/5% fetal calf serum (FCS) for 30 minutes on ice. Antibodies used were anti-CD3 (145-2C11), anti-CD4 (129.19), anti-CD8 (53-6.7), anti-CD34 (RAM34), anti-CD45R (RA3-6B2), anti-c-Kit (CD117) (2B8), DX5, Ter119, anti-Gr-1 (RB6-8C5), and anti-Mac-1 (M1/70).
EPO measurements
Serum EPO levels were quantified using sandwich enzyme-linked immunosorbent assay (ELISA) (EPO Quantikine; R&D Systems, Wiesbaden, Germany) according to the manufacturer's guidelines. This assay detects human and murine Epo but does not discriminate between them. Increased EPO levels indicated murine Epo in the serum of mice after phenylhydrazine-induced hemolytic anemia (data not shown). To determine EPO levels in EPO-tg, WEPO, and Vickid mice, serial 2-fold serum dilutions were titrated in 0.9% NaCl. Sera obtained from day 2 mice (20-30 μL) were diluted 3- to 5-fold in 0.9% NaCl. A typical dilution row of recombinant EPO gave the following optical density (OD) readings: 0 IU/L ≈ OD < minimum; 2.5 IU/L ≈ OD < minimum; 5 IU/L ≈ OD 0.062; 20 IU/L ≈ OD 0.199; 50 IU/L ≈ OD 0.46; 100 IU/L ≈ OD 0.814; 200 IU/L ≈ OD > maximum. EPO levels from mice were calculated by comparison with the linear part of the standard titration curve.
Results
Survival of EPO transgenic c-KitW/W mice
Mice lacking c-Kit cell surface expression (c-KitW/W) die before they are 10 days old (for a review, see Russell5 ). The cause of death is within the hematopoietic system because c-KitW/W mice can be rescued by transplantation of c-Kit+/+ HSCs.15 It is, however, unknown whether lethality in c-KitW/W mice results from HSC failure or from defects specific to erythropoiesis. We previously obtained a viable adult c-KitW/W mouse line (Vickid) by outcrossing c-KitW/+ mice of the WB strain to animals of mixed genetic background.8 Vickid mice show elevated numbers of reticulocytes and mature erythrocytes compared with lethal c-KitW/W mice. This finding suggested that improving erythropoiesis in the absence of c-Kit expression might overcome lethality of c-KitW/W mice. To test this hypothesis directly, we introduced an EPO-encoding transgene into c-KitW/W mice. In the EPO transgenic mouse (tg6), the transgene is expressed under the control of the platelet-derived growth factor-B (PDGF-B) chain promoter, which drives expression of the transgene preferentially, but not exclusively, to neuronal cells.18 In addition to the brain,19 the transgene is expressed in the retina20 and in the lung (M. Gassmann, unpublished observation, May 2004). The transgene-encoded EPO is secreted in a hypoxia-independent manner21 as a soluble, systemically acting growth factor, and it leads to an approximately 250-fold higher EPO level compared with that achieved in normal mice. The hematocrit, 40% to 50% in wild-type mice, can reach very high levels (up to 85%) in this EPO-tg line with a c-Kit+ background.16,21,22
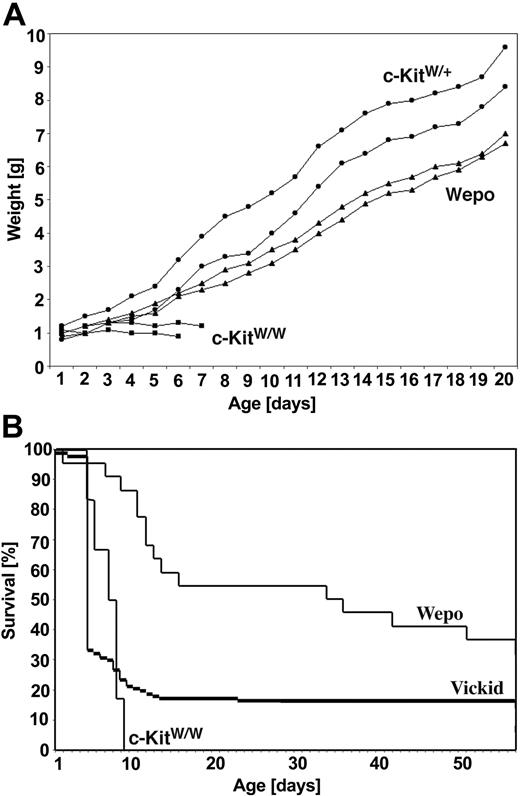
To introduce the EPO transgene into c-KitW/W mice, we crossed c-KitW/+ mice (WB strain) to hemizygous EPO-tg mice (C57Bl/6 background). F1 c-KitW/+EPO-tg male mice were subsequently backcrossed to c-KitW/+ mice to generate c-KitW/WEPO-tg mice. Interestingly, the latter cross-yielded c-KitW/W mice that survived into adulthood (Figure 1A). We term this viable c-KitW/W mouse strain “W rescued by EPO” (WEPO).
Survival of WEPO mice. (A) Growth of a single litter of 6 mice, including wild-type (c-KitW/+) (•), lethal c-KitW/W (▪), and WEPO (c-KitW/WEPO-tg) (▴) mice. The weight of each mouse was determined daily for 3 weeks. (B) Kaplan-Meier plot for the survival of WEPO mice (n = 24) and viable c-Kit-deficient mice (n = 238) (Vickid; a viable c-KitW/W line distinct from WEPO8). Of all WEPO mice, approximately 40% survived long term. In the Vickid mouse colony, the overall survival of all white (c-KitW/W) pups is approximately 15%.
Survival of WEPO mice. (A) Growth of a single litter of 6 mice, including wild-type (c-KitW/+) (•), lethal c-KitW/W (▪), and WEPO (c-KitW/WEPO-tg) (▴) mice. The weight of each mouse was determined daily for 3 weeks. (B) Kaplan-Meier plot for the survival of WEPO mice (n = 24) and viable c-Kit-deficient mice (n = 238) (Vickid; a viable c-KitW/W line distinct from WEPO8). Of all WEPO mice, approximately 40% survived long term. In the Vickid mouse colony, the overall survival of all white (c-KitW/W) pups is approximately 15%.
Crosses of c-KitW/+EPO-tg to c-KitW/+ mice yield, in addition to c-KitW/WEPO-tg mice (WEPO), c-KitW/+ (wild type for c-Kit and nontransgenic for EPO) and c-KitW/+EPO-tg (wild type for c-Kit and transgenic for EPO) mice. In comparing postnatal growth, measured by increases in body weight, of mice of the various genotypes, we found that the weight gain of WEPO mice was slightly less than that of c-KitW/+ mice but that the weight gain was comparable between WEPO and wild-type mice (Figure 1A). In contrast, the body weight of c-KitW/W mice nontransgenic for EPO increased only until postnatal day 3 and then remained constant until death at approximately 1 week after birth.
Littermates from crosses of c-KitW/+EPO-tg to c-KitW/+ mice were genotyped as c-KitW/+, c-KitW/+EPO-tg, c-KitW/W, and c-KitW/W EPO-tg and were analyzed for survival beyond postnatal day 20 (Table 1). As expected, all c-KitW/+ and c-KitW/+EPO-tg mice survived longer than 20 days, whereas none of the c-KitW/W mice survived. In contrast, 8 of 10 c-KitW/WEPO-tg mice survived in this experiment. In a separate experiment, we analyzed 50 adult c-KitW/W mice from crosses of c-KitW/+EPO-tg to c-KitW/+ mice for the presence of the EPO transgene. Without exception, all surviving c-KitW/W mice were positive for EPO-tg. Thus, survival of c-KitW/W mice is strictly dependent on the EPO transgene. Of all c-KitW/WEPO-tg mice born, approximately 40% survive long term (Figure 1B). The fact that not all c-KitW/WEPO-tg mice survive long term might be attributed to a variable onset of EPO transgene expression23 or to epigenetic factors responsible for EPO transgene expression in individual mice. In our Vickid colony, approximately 15% of all white mice survive. Of note, the molecular nature of the rescue mechanism in Vickid mice is unknown; hence, c-KitW/W mice without the so-called survival factor are included in this graph. Therefore, for Vickid mice, the Kaplan-Meier plot does not represent penetrance of the survival factor.
Survival as a result of EPO-tg
c-Kit . | EPO-tg . | No. mice . | No. dead before day 20 . | No. surviving past day 20 . |
|---|---|---|---|---|
| W/W | − | 4 | 4 | 0 |
| W/W | + | 10 | 2 | 8 |
| W/+ | − | 14 | 0 | 14 |
| W/+ | + | 22 | 0 | 22 |
c-Kit . | EPO-tg . | No. mice . | No. dead before day 20 . | No. surviving past day 20 . |
|---|---|---|---|---|
| W/W | − | 4 | 4 | 0 |
| W/W | + | 10 | 2 | 8 |
| W/+ | − | 14 | 0 | 14 |
| W/+ | + | 22 | 0 | 22 |
Fifty mice from mixed litters were monitored for survival beyond postnatal day 20 as a function of the presence of the EPO transgene. All mice were typed for c-Kit deficiency based on extreme pallor. The presence of the EPO transgene was determined using PCR analysis. Only c-KitW/W mice carrying the EPO-tg survived longer than 20 days. Also, in an independent experiment, 50 white (c-KitW/W) adult mice were tested for the presence of EPO-tg. Without exception, all 50 mice were positive for EPO-tg, indicating that survival strictly depends on its presence.
WEPO mice have a c-KitW/W genotype and lack c-Kit on the cell surface
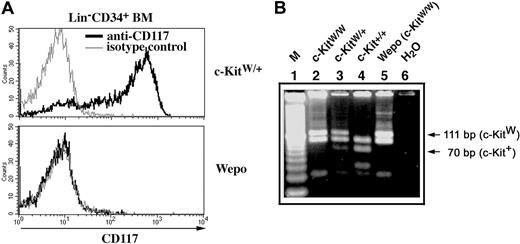
Using flow cytometry, we next analyzed c-Kit expression in hematopoietic stem/progenitor cells to exclude a somatic reversion of the c-KitW loci. Such reversion has been previously reported for melanocytes in mice carrying a different c-Kit mutant allele (c-KitWrio).24 Early progenitor cells from WEPO and c-Kit+ BM, defined by lack of mature hematopoietic surface markers (CD3, CD8, CD4, Mac-1, Gr-1, Ter119, DX5, CD45R) and by expression of CD34, were analyzed for surface expression of c-Kit (Figure 2A). Progenitor cells in WEPO mice completely lacked cell surface expression of c-Kit, whereas c-Kit was strongly expressed on most progenitors of wild-type mice. PCR and subsequent digestion analysis of genomic DNA for the detection of c-Kit+ (70-bp band) or c-KitW (111-bp band) alleles8 distinguished heterozygosity from homozygosity for the W mutation in the various genotypes (Figure 2B). Wild-type mice yielded a 70-bp band and lacked the 111-bp band (lane 4). Heterozygous mice showed bands of 70 bp and 111 bp (lane 3). From WEPO and lethal c-KitW/W mice we detected only the 111-bp band (lanes 2 and 5), demonstrating the c-KitW/W genotype of WEPO mice. Hence, WEPO animals are adult c-KitW/W mice that lack surface expression of c-Kit.
WEPO mice have a c-KitW/W genotype and lack c-Kit cell surface expression. (A) Lin- CD34+ BM cells from WEPO mice lack expression of c-Kit (CD117) on the cell surface. (B) WEPO mice are homozygous for the W mutation. The DNA product amplified by PCR from genomic DNA from c-Kit+, but not from c-KitW templates, has an HphI restriction site. After HphI digestion of the PCR product, a 70-bp band indicates a c-Kit+ allele (lanes 3, 4), and a 111-bp band indicates a c-KitW allele (lanes 2, 3, 5).
WEPO mice have a c-KitW/W genotype and lack c-Kit cell surface expression. (A) Lin- CD34+ BM cells from WEPO mice lack expression of c-Kit (CD117) on the cell surface. (B) WEPO mice are homozygous for the W mutation. The DNA product amplified by PCR from genomic DNA from c-Kit+, but not from c-KitW templates, has an HphI restriction site. After HphI digestion of the PCR product, a 70-bp band indicates a c-Kit+ allele (lanes 3, 4), and a 111-bp band indicates a c-KitW allele (lanes 2, 3, 5).
WEPO hematopoietic stem/progenitor cells are functionally c-Kit null
Hematopoietic stem/progenitor cells from Vickid mice lack CFU-S acitivity.8 We applied this assay to verify whether WEPO BM is functionally c-Kit deficient. To this end, we injected WEPO BM cells into lethally irradiated wild-type recipients. In contrast to c-Kit+ BM, WEPO BM lacked CFU-S activity (data not shown). Because WEPO mice are on a mixed MHC background and because CFU-S formation is sensitive to allogeneic MHC differences between donor and host, we also transferred WEPO BM cells into recipients lacking T, B, and NK cells (recombination activation genes (Rag)-2-/- and common cytokine receptor gamma chain (γc)-/-). Moreover, CFU-S formation appears to depend on hierarchical differences in the strength of the c-Kit alleles comparing donor and recipient.25 Therefore, we also tested CFU-S formation by injecting WEPO BM cells into recipients expressing a weak c-Kit allele (c-KitW/Wv). Under none of these conditions could we observe spleen colonies from WEPO mice. This was true for the standard dose of transplanted BM cells (2 × 105) and for double the amount of progenitor cells.26 This demonstrates that a known deficiency in c-KitW/W HSC/progenitors has not been rescued or modified by introducing the EPO-tg.
Serum EPO level in WEPO mice
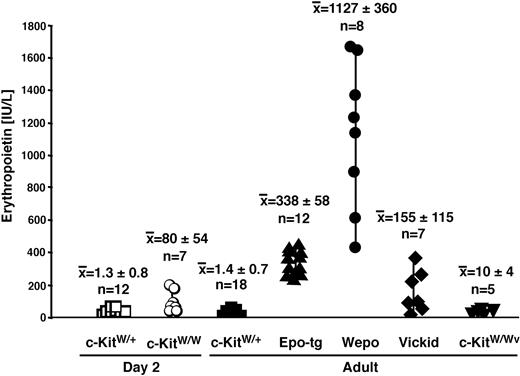
Levels of Epo are subject to tight regulation by oxygen availability (for reviews, see Fisher27 and Hopfl et al28 ). Hence, anemia and hypoxia enhance the expression and stability of key regulators such as hypoxia-inducible factor 1 (Hif-1). Hif-1 increases Epo expression, which, in turn, results in augmented erythropoiesis (for reviews, see Fisher,27 Semenza,29 and Hopfl et al30 ). To assess Epo levels in c-Kit mutant mice of various genotypes and ages, we measured serum EPO concentrations using ELISA (Figure 3). As expected, EPO levels in 2-day-old c-KitW/W mice were elevated (mean, 80 ± 54 IU/L) compared with their wild-type littermates (mean, 1.3 ± 0.8 IU/L). This suggested that c-KitW/W mice responded to the anemia but that an approximately 60-fold increase of serum Epo was insufficient for the survival of c-KitW/W mutants. An important reference strain is the widely used viable anemic hypomorphic c-KitW/Wv mouse. Adult c-KitW/Wv mice (mean, 10 ± 4 IU/L) expressed approximately 7-fold more Epo than adult c-KitW/+ mice (mean, 1.4 ± 0.7 IU/L). In EPO-tg mice (mean, 338 ± 58 IU/L), EPO levels were increased approximately 250-fold compared with wild-type mice. This increase reflected hypoxia-independent, transgene-enforced overexpression19 rather than hypoxia-regulated EPO synthesis. Interestingly, EPO levels were even further elevated in WEPO mice (mean, 1127 ± 360 IU/L). Thus, EPO levels were increased by approximately 800-fold in WEPO compared with wild-type mice and by approximately 3-fold in WEPO compared with c-KitW/+EPO-tg mice. It should be noted that the ELISA does not discriminate between transgenic and intrinsic EPO; hence, detected EPO reflects the sum of intrinsic (murine) and transgenic (human) EPO protein. Finally, Vickid mice only showed an approximately 100-fold increase of Epo compared with wild-type mice, suggesting that this survival variant followed a different molecular rescue mechanism than WEPO mice.
High serum EPO levels in WEPO mice. Serum EPO levels in 2-day-old wild-type (□), 2-day-old c-KitW/W mice (○), and adult wild-type (▪), adult EPO-tg (▴), adult WEPO (•), adult Vickid (♦), and adult c-KitW/Wv (▾) mice were determined using sandwich ELISA.
High serum EPO levels in WEPO mice. Serum EPO levels in 2-day-old wild-type (□), 2-day-old c-KitW/W mice (○), and adult wild-type (▪), adult EPO-tg (▴), adult WEPO (•), adult Vickid (♦), and adult c-KitW/Wv (▾) mice were determined using sandwich ELISA.
Adult c-KitW/W mice define a viability threshold for anemia
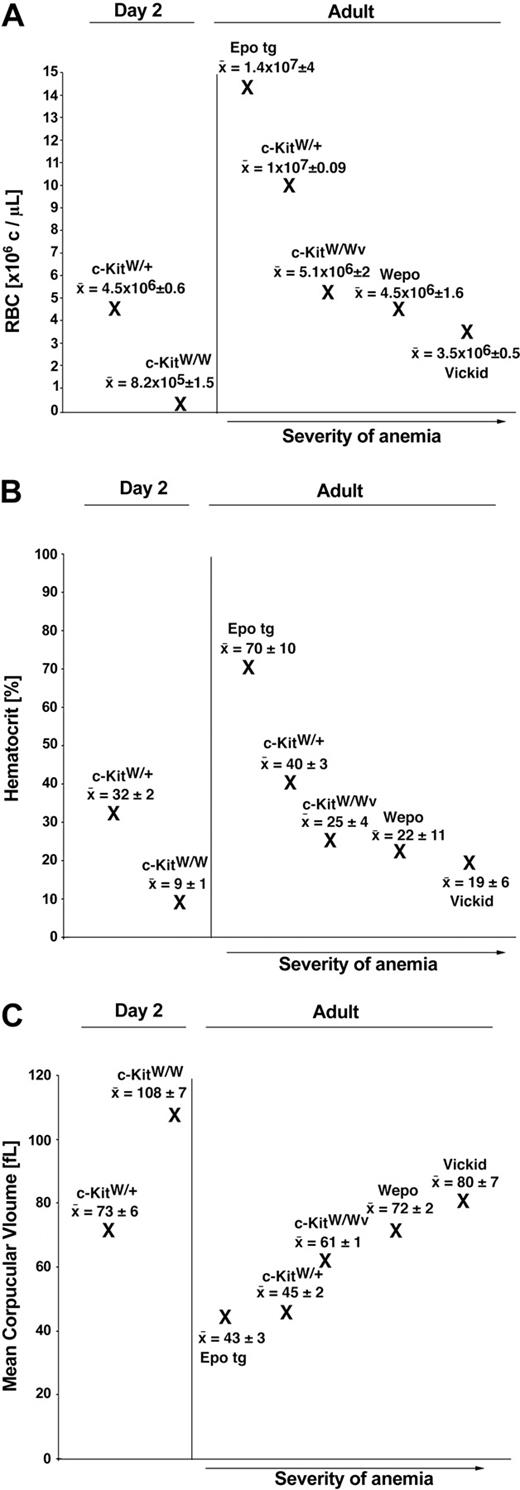
A phenotypic hallmark of c-Kit mutant mice is macrocytic anemia (for a review, see Russell5 ). In c-KitW/Wv mice, the anemia is permissive for survival. To assess the severity of anemia in newly developed WEPO mice compared with wild-type, adult EPO-tg, c-KitW/Wv, and Vickid mice, RBC parameters (RBC numbers [counts/μL]; hematocrit (Hct) [%]; mean corpuscular volume (MCV) [fl]) were determined for all mice (Figure 4). As another important reference, we included postnatal day 2 c-KitW/+ and lethal c-KitW/W mice in the analysis.
RBC parameters in WEPO mice. RBC counts (A), hematocrit (B), and MCV (C) were compared in 2-day-old wild-type (c-KitW/+) and mutant mice (c-KitW/W) and in adult EPO-tg, wild-type (c-KitW/+), and various c-Kit mutant mice (c-KitW/Wv, WEPO, and Vickid). For RBC and MCV values, mice were analyzed as follows: 2-day-old: wild-type (n = 8), c-KitW/W (n = 7); adult: EPO-tg (n = 6), wild-type (n = 16), c-KitW/Wv (n = 4), WEPO (n = 7), and Vickid (n = 6). For hematocrit values, mice were analyzed as follows: 2-day-old: wild-type (n = 7), c-KitW/W (n = 7); adult: EPO-tg (n = 55), wild-type (n = 16), c-KitW/Wv (n = 9), WEPO (n = 17), and Vickid (n = 7).
RBC parameters in WEPO mice. RBC counts (A), hematocrit (B), and MCV (C) were compared in 2-day-old wild-type (c-KitW/+) and mutant mice (c-KitW/W) and in adult EPO-tg, wild-type (c-KitW/+), and various c-Kit mutant mice (c-KitW/Wv, WEPO, and Vickid). For RBC and MCV values, mice were analyzed as follows: 2-day-old: wild-type (n = 8), c-KitW/W (n = 7); adult: EPO-tg (n = 6), wild-type (n = 16), c-KitW/Wv (n = 4), WEPO (n = 7), and Vickid (n = 6). For hematocrit values, mice were analyzed as follows: 2-day-old: wild-type (n = 7), c-KitW/W (n = 7); adult: EPO-tg (n = 55), wild-type (n = 16), c-KitW/Wv (n = 9), WEPO (n = 17), and Vickid (n = 7).
At this early age, c-KitW/W mice have 5.5-fold fewer RBCs than wild-type littermates (Figure 4A). The Hct of c-KitW/W mice is reduced more than 3-fold (Figure 4B). This anemia is so severe that c-KitW/W mice die by day 10.
Adult WEPO mice have 2.2-fold fewer RBCs than wild-type mice (Figure 4). The 1.6-fold increase in RBC volume in adult WEPO mice partially compensates for this reduction in cell number; hence, their hematocrit is reduced only 1.8-fold. Only Vickid, the second independently derived adult c-KitW/W mouse line, has fewer RBCs (2.9-fold less than wild type). This decrease in numbers is associated with an even larger erythrocyte size (1.8-fold compared with wild type) and a lower hematocrit (2.1-fold reduced compared with wild-type mice). A further decrease of RBC numbers is apparently lethal because c-KitW/W mice do not survive if they have 5.5-fold fewer RBCs than wild-type littermates. Conversely, RBCs and hematocrit are increased 1.4-fold and 1.7-fold, respectively, when comparing EPO-tg mice on a c-Kit+ background with wild-type mice (Figure 4).16
These results show that the anemia threshold for viability is at or below 3.5 × 106 RBCs per milliliter or at a hematocrit of 19% (Figure 4; data from Vickid mice). Despite the fact that the EPO transgene pushes the RBC numbers in the absence of c-Kit above the lethality threshold, RBC parameters in WEPO mice are not restored to wild-type levels.
These RBC data reflect the mature erythrocyte compartment. To gain information on immature erythrocytes in peripheral blood, numbers of reticulocytes were determined (Table 2). In 2-day-old c-KitW/W mice, absolute reticulocyte numbers were reduced approximately 9-fold. In addition, the relative percentages of reticulocytes per blood cells were reduced from 22% (wild type) to 13% (c-KitW/W), suggesting that impaired de novo RBC production is a cause of severe anemia in nonviable, early postnatal c-KitW/W mice.
Peripheral blood analysis of 2-day-old c-KitW/+, c-KitW/W, and adult EPO-tg, wild-type, and various c-Kit mutant (c-KitW/Wv, WEPO, Vickid) mice
. | Day 2 . | . | Adult . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | W/+, n = 8 . | W/W, n = 7 . | EPO-tg, n = 6 . | W/+, n = 16 . | W/Wv, n = 4 . | WEPO, n = 7 . | Vickid, n = 6 . | |||||
| Reticulocytes, × 109/L | 995 ± 125 | 109 ± 30 | 1002 ± 600 | 425 ± 82 | 354 ± 143 | 353 ± 106 | 592 ± 355 | |||||
| Reticulocytes, % | 22.4 ± 2 | 13.1 ± 3.3 | 6.3 ± 2.3 | 4 ± 0.8 | 6.8 ± 0.7 | 8.3 ± 1.6 | 18.9 ± 14 | |||||
| Hemoglobin, mM | 6.5 ± 0.5 | 2 ± 0.7 | 11.9 ± 1.9 | 9.4 ± 0.6 | 4.5 ± 3.6 | 5.9 ± 2.8 | 5.1 ± 0.6 | |||||
| Cellular hemoglobin, pg | 22 ± 1.6 | 36.1 ± 2.6 | 13.1 ± 1.1 | 13.5 ± 0.4 | 18.6 ± 0.4 | 21.4 ± 0.9 | 21.4 ± 1.1 | |||||
| Thrombocytes, × 109/L | 380 ± 162 | 375 ± 34 | 813 ± 364 | 1002 ± 137 | 1051 ± 405 | 878 ± 273 | 884 ± 366 | |||||
. | Day 2 . | . | Adult . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | W/+, n = 8 . | W/W, n = 7 . | EPO-tg, n = 6 . | W/+, n = 16 . | W/Wv, n = 4 . | WEPO, n = 7 . | Vickid, n = 6 . | |||||
| Reticulocytes, × 109/L | 995 ± 125 | 109 ± 30 | 1002 ± 600 | 425 ± 82 | 354 ± 143 | 353 ± 106 | 592 ± 355 | |||||
| Reticulocytes, % | 22.4 ± 2 | 13.1 ± 3.3 | 6.3 ± 2.3 | 4 ± 0.8 | 6.8 ± 0.7 | 8.3 ± 1.6 | 18.9 ± 14 | |||||
| Hemoglobin, mM | 6.5 ± 0.5 | 2 ± 0.7 | 11.9 ± 1.9 | 9.4 ± 0.6 | 4.5 ± 3.6 | 5.9 ± 2.8 | 5.1 ± 0.6 | |||||
| Cellular hemoglobin, pg | 22 ± 1.6 | 36.1 ± 2.6 | 13.1 ± 1.1 | 13.5 ± 0.4 | 18.6 ± 0.4 | 21.4 ± 0.9 | 21.4 ± 1.1 | |||||
| Thrombocytes, × 109/L | 380 ± 162 | 375 ± 34 | 813 ± 364 | 1002 ± 137 | 1051 ± 405 | 878 ± 273 | 884 ± 366 | |||||
Analysis of adult mice revealed that WEPO mutants had absolute numbers of reticulocytes similar to those of c-KitW/+ mice. Consistent with the fact that EPO can enhance erythropoiesis, EPO-tg mice had higher numbers of reticulocytes in absolute numbers and in the percentage of all blood cells (Table 2).21 Comparison of adult mice showed that the relative percentages of reticulocytes increased as follows: c-KitW/+ (4.0%) less than c-KitW/Wv (6.8%) less than WEPO (8.3%) less than Vickid (18.9%). These data correlate with the severity of the anemia in each c-Kit mutant line. Serum hemoglobin (Hgb) concentrations were reduced in all anemic mice. Increases in cellular hemoglobin probably reflected the increased cell size of erythrocytes. Thrombocyte numbers were similar in wild-type and c-Kit mutants (Table 2).
Rescue from c-KitW/W lethality by EPO occurs at late stages (CFU-E) of erythropoiesis
Erythropoiesis can be analyzed quantitatively by the formation of hemoglobinized colonies from single progenitor cells after culture in growth factors. BFU-E is an early erythroid progenitor that requires EPO and IL-3 to form large colonies after 7 to 8 days in vitro. In contrast, Epo alone is sufficient for CFU-E colonies that arise after 48 hours from a late erythroid progenitor.31 BFU-E and CFU-E progenitors are found, albeit at reduced frequencies, in Epo receptor (EpoR)-null32 and c-Kit-null33,34 mutant mice, demonstrating that EPO-EPOR and c-Kit-c-Kit-ligand (KitL) interactions are crucial for normal erythropoiesis. Nevertheless, erythrocyte progenitors can develop without EpoR- or c-Kit-mediated signaling. Erythroid colony formation has, until now, not been assessed in adult c-KitW/W mice. Therefore, we compared BFU-E (Figure 5) and CFU-E (Figure 6) frequencies from BM and spleen in 2-day-old c-KitW/W and wild-type mice and in adult WEPO, EPO-tg, and wild-type animals. Frequencies of BFU-E and CFU-E were calculated per 105 Ter119neg/lo cells in each organ because BFU-E and CFU-E activity are included in the Ter119neg/lo population and are excluded from the Ter119+ population, which are later stages of erythropoiesis and mature RBCs.35 In addition, we determined the absolute numbers of BFU-Es and CFU-Es for each organ.
BFU-E in the BM and spleen. BM (A-B) and spleen (C-D) of 2-day-old c-KitW/+ (□) and c-KitW/W (○) and adult c-KitW/+ (▪), EPO-tg (▴), and WEPO (•) mice were analyzed. Frequencies of BFU-Es per 105 Ter119neg/Low cells (enriched for progenitors) (A, C) and total numbers of BFU-Es per each organ are shown. Total BM cells correspond to the cells from 2 femura. Two-tailed P values were determined according to the Mann-Whitney U test. Brackets and corresponding P values are indicated for the compared groups. Horizontal black lines indicate mean colony numbers.
BFU-E in the BM and spleen. BM (A-B) and spleen (C-D) of 2-day-old c-KitW/+ (□) and c-KitW/W (○) and adult c-KitW/+ (▪), EPO-tg (▴), and WEPO (•) mice were analyzed. Frequencies of BFU-Es per 105 Ter119neg/Low cells (enriched for progenitors) (A, C) and total numbers of BFU-Es per each organ are shown. Total BM cells correspond to the cells from 2 femura. Two-tailed P values were determined according to the Mann-Whitney U test. Brackets and corresponding P values are indicated for the compared groups. Horizontal black lines indicate mean colony numbers.
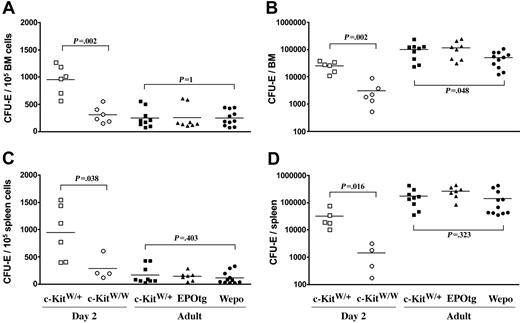
CFU-Es in the BM and spleen. BM (A-B) and spleen (C-D) of 2-day-old c-KitW/+ (□) and c-KitW/W (○) and adult c-KitW/+ (▪), EPO-tg (▴), and WEPO (•) mice were analyzed. Frequencies of CFU-Es per 105 Ter119neg/Low cells (enriched for progenitors) (A, C) and total numbers of CFU-Es per each organ are shown. Total BM cells correspond to the cells from 2 femura. Two-tailed P values were determined according to the Mann-Whitney U test. Brackets and corresponding P values are indicated for the compared groups. Horizontal black bars represent mean colony numbers.
CFU-Es in the BM and spleen. BM (A-B) and spleen (C-D) of 2-day-old c-KitW/+ (□) and c-KitW/W (○) and adult c-KitW/+ (▪), EPO-tg (▴), and WEPO (•) mice were analyzed. Frequencies of CFU-Es per 105 Ter119neg/Low cells (enriched for progenitors) (A, C) and total numbers of CFU-Es per each organ are shown. Total BM cells correspond to the cells from 2 femura. Two-tailed P values were determined according to the Mann-Whitney U test. Brackets and corresponding P values are indicated for the compared groups. Horizontal black bars represent mean colony numbers.
In 2-day-old mice, frequencies of BFU-E in BM and spleen were variable in individual c-KitW/W mice, but overall the frequencies were similar comparing c-KitW/+ and c-KitW/W mice (Figure 5A, C). However, because of the strongly reduced total BM and spleen cellularity in day 2 c-KitW/W mice, absolute numbers of BFU-Es were significantly reduced (Figure 5B, D).
In adult mice, BFU-E frequencies were comparable in BM and spleen from c-KitW/+, EPO-tg, and WEPO mice (Figure 5). Thus, during ontogeny, the generation of BFU-E is c-Kit independent in BM and spleen. The measurable reduction (P < .05) in BFU-E numbers in adult WEPO BM can be attributed to an overall reduced BM cellularity in this mutant. Spleen cellularity was comparable in c-KitW/+ and WEPO mice.
In contrast, we measured a marked c-Kit dependency of CFU-E in BM and spleen in 2 day-old-mice (Figure 6). This was true for frequencies (Figure 6A, C) and for absolute cell numbers (Figure 6B, D). These data are in agreement with earlier reports33,34 and imply that frequencies of CFU-E progenitors are c-Kit dependent, whereas frequencies of BFU-E progenitors are c-Kit independent. In WEPO mice, CFU-E frequencies in BM (Figure 6A) and spleen (Figure 6C) were increased, comparable to frequencies in wild-type BM. Again, corresponding to the slight reduction in overall BM but not spleen cellularity in WEPO mice, absolute CFU-E numbers were reduced in BM but not spleen (Figure 6B, D). Thus, under steady state hematopoiesis, augmented EpoR signaling can compensate for lack of c-Kit signaling at the CFU-E stage in vivo. Finally, EPO overexpression in c-Kit+ mice did not increase the frequencies or absolute numbers of BFU-Es or CFU-Es in BM or spleen (Figures 5, 6).
Discussion
We have shown here that introducing an EPO-encoding transgene mediates adult viability of the otherwise lethal c-KitW/W genotype. Viable c-KitW/WEPO-tg mice have been generated continuously in our laboratory for more than 3 years. Survival depends strictly on the presence of the EPO transgene. In normal mice, Epo expression is regulated by oxygen supply. Reduced oxygenation (hypoxia) and, indirectly, anemia can augment Epo expression. This is the case, for example, in inherited chronic anemia, in acute anemia that can be experimentally induced by phlebotomy, or after RBC lysis by phenylhydrazine treatment (for a review, see Fisher27 ). The macrocytic anemia of c-Kit hypomorphs (c-KitW/Wv) is associated with elevated levels of Epo in the serum, and these mice can respond to hypoxia by producing even more Epo.36 Nevertheless, their anemia cannot be corrected. To achieve increased hematocrits, c-KitW/Wv mice require approximately 150-fold more Epo than wild-type mice.36 This suggests that c-KitW/Wv mice are at least partially refractory in their response to Epo. This long known fact might have argued against the possibility of rescuing c-KitW/W mice through the overexpression of EPOtg. However, our data demonstrate that strongly increased EPO expression can rescue c-KitW/W mice. In WEPO mice, EPO levels are elevated approximately 800-fold compared with wild-type mice and 3-fold compared with c-KitW/+EPO-tg mice. We could not directly distinguish between endogenous and transgenic EPO. Only endogenous Epo is regulated by hypoxia, whereas transgenic EPO is expressed constitutively.21 By comparing EPO levels in WEPO mice with those in c-KitW/+ mice and c-KitW/+EPO-tg mice, we estimate that approximately 300 of 1100 IU EPO is transgene derived. Clearly, endogenous plus transgenic EPO are sufficient to raise RBC numbers in WEPO mice to viable levels. Interestingly, this demonstrates that enforced EpoR-mediated signaling can partially overcome the complete lack of c-Kit-mediated signals in vivo. EPO expression in EPO-tg and WEPO mice is variable. We assume that the premature death of approximately 60% of WEPO mice resulted from insufficient or delayed expression of the transgene in individual mice. The fact that all viable WEPO mice had very high levels of EPO (Figure 3) suggests that only c-KitW/W mice with strong EPO transgene expression are selected for survival.
In cell lines, c-Kit and EpoR interact physically.37 In the fetal liver, the interaction of c-Kit and EpoR occurs before or at the CFU-E stage.38 Moreover, KitL can replace Epo in supporting the growth and survival of erythroid cell lines.37 Our data suggest that this functional substitution can also occur in reverse in that elevated levels of Epo replace an absolute requirement for KitL-mediated signals in vivo.
Erythroid progenitors can be defined morphologically (for example, see Socolovsky et al39 ; for a review, see Koury et al40 ) and functionally.31 Functional analysis includes in vitro colony formation. BFU-Es are early erythroid progenitors that give rise to large colonies in the presence of EPO and IL-3 within 7 days. In contrast, CFU-Es require only EPO to develop into small colonies within 48 hours. Both types of colonies are characterized by hemoglobinization.31 Thus, EPO promotes the growth and differentiation of noncommitted and committed erythroid progenitors in vitro. Mutations in the genes encoding either Epo or EpoR lead to lethality at approximately embryonic day 13.32 Nevertheless, these EPO and EpoR mutants contain BFU-E and CFU-E progenitors in their fetal livers, although frequencies for both colonies are dramatically reduced.32 Interestingly, compared with Epo and EpoR mutants, mice lacking KitL (KitLSteel/Steel(Sl/Sl)) or c-Kit (c-KitW/W) show a different phenotype with regard to colony formation. KitLSl/Sl mice are lethal in utero,41 and frequencies of CFU-E colony formation are reduced in KitLSl/Sl fetal liver. In contrast, BFU-E formation is comparable in KitLSl/Sl and wild-type fetal liver cells.42 This finding indicates an erythroid lineage-specific defect in Steel mutant mice. Consistent with these data from KitLSl/Sl fetal liver, 2 independent analyses of c-KitW/W fetal liver cells revealed a strong reduction in the frequencies of CFU-E colonies.33,34
Data on the c-Kit dependency of BFU-Es are controversial. Human BFU-Es are KitL dependent when cultured in serum-free medium.43 This phenomenon can be partially reversed by high concentrations of EPO.44 In lethal c-KitW/W mice, reduced BFU-E formation was shown in 1 report34 but not in an earlier study.33 We found BFU-E formation in 2-day-old c-KitW/W BM and spleen cells to be, on a per cell basis, comparable to that in wild-type cells. In terms of absolute numbers, the lower overall cellularity of c-KitW/W hematopoietic organs accounts for fewer total BFU-Es from BM and spleen. This finding is supported by earlier reports showing lower CFU-E but equal BFU-E numbers in Steel mutant mice, again pointing to a specific defect at the CFU-E stage. Our parallel analysis of postnatal day 2 and adult mice now shows that overexpression of EPO can rescue exactly the stage of erythropoiesis that is dependent on c-Kit in neonatal mice, the CFU-E stage. This rescue is functionally highly significant because it correlates with the viability of adult c-KitW/W mice.
Short-term injection of recombinant EPO enhances CFU-E numbers in wild-type and c-KitW/Wv mice, indicating that EpoR signaling can augment CFU-E on a c-Kit+ and on a c-KitW/Wv background.45-47 We now show that this effect of EPO is possible even in the complete absence of c-Kit expression. Interestingly, BFU-E and CFU-E numbers are not higher than wild-type levels in EPO-tg mice, possibly because of an adaptation process that limits the overstimulation of erythropoiesis caused by the permanent availability of high EPO concentrations. Alternatively, the effect of EPO overexpression on RBC development may not be entirely reflected by BFU-E or CFU-E assays.
Within the hematopoietic system, the best-documented action of Epo is its effect on erythroid progenitors that express EpoR.48 However, moderate levels of EPOR expression have also been observed in HSCs.49 This raises the possibility that increased erythropoietin levels may affect HSC survival or that the Epo effects may not be limited to erythroid progenitor cells. Is there evidence of a c-Kit-dependent HSC deficiency that has been counteracted by EPO overexpression? Spleen colony formation is a c-Kit-dependent HSC function. EPO overexpression does not rescue the CFU-S deficiency in WEPO mice. In contrast, CFU-E formation in the BM and spleen of WEPO mice is rescued to levels seen in wild-type mice. Absolute numbers of phenotypically defined stem/progenitor cells (Lin-Sca-1+CD34- or Lin-Sca-1+ CD34+; c-Kit is not available as a marker) are comparable in adult Vickid and in WEPO mice. The fact that EPO levels are approximately 7-fold higher in WEPO than in Vickid mice (Figure 3) suggests that EPO levels have no dramatic impact on the size of the stem/progenitor cell compartment in the absence of c-Kit. Whether HSC function, beyond CFU-S formation, is altered by EPO overexpression remains to be determined. Future experiments should focus on the cellular and molecular defects caused by the absence of c-Kit in erythroid progenitors. WEPO mice will be useful for these experiments. In addition, WEPO mice may be instrumental for studying the in vivo role of c-Kit in cellular systems that are not influenced by EPO overexpression. Within hematopoiesis, analysis of WEPO mice already demonstrated an unexpected role for c-Kit in the maintenance of adult lymphopoiesis.8 The availability of adult c-KitW/W mice now allows analyses into the adult function of this receptor tyrosine kinase in other c-Kit-expressing lineages, such as interstitial cells of Cajal and neuronal cells, and in additional hematopoietic cells, such as mast cell progenitors.
Prepublished online as Blood First Edition Paper, June 3, 2004; DOI 10.1182/blood-2004-04-1247.
Supported by grants from the Deutsche Forschungsgemeinschaft (SFB-497-B5) (H.R.R.), by the Landesstiftung Baden-Württemberg (P-LS-AL/11) (C.W.), and by the Swiss National Science Foundation (3100A0-100214) (M.G.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs. H. J. Fehling and T. Borggrefe for discussions, H. Dietrich, G. Heller (Department for Internal Medicine III, Ulm, Germany), and B. Grenacher (VetSuisse Faculty, Zurich, Switzerland) for help with EPO measurements and blood parameter analyses, and K. Merkel for animal care.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal