Abstract
Although extracellular nucleotides support a wide range of biologic responses of mature blood cells, little is known about their effect on blood cell progenitor cells. In this study, we assessed whether receptors for extracellular nucleotides (P2 receptors [P2Rs]) are expressed on human hematopoietic stem cells (HSCs), and whether activation by their natural ligands, adenosine triphosphate (ATP) and uridine triphosphate (UTP), induces HSC proliferation in vitro and in vivo. Our results demonstrated that CD34+ HSCs express functional P2XRs and P2YRs of several subtypes. Furthermore, stimulation of CD34+ cells with extracellular nucleotides caused a fast release of Ca2+ from intracellular stores and an increase in ion fluxes across the plasma membrane. Functionally, ATP and, to a higher extent, UTP acted as potent early acting growth factors for HSCs, in vitro, because they strongly enhanced the stimulatory activity of several cytokines on clonogenic CD34+ and lineage-negative CD34- progenitors and expanded more primitive CD34+-derived long-term culture-initiating cells. Furthermore, xenogenic transplantation studies showed that short-term preincubation with UTP significantly expanded the number of marrow-repopulating HSCs in nonobese diabetic/severe combined immunodeficiency mice. Our data suggest that extracellular nucleotides may provide a novel and powerful tool to modulate HSC functions. (Blood. 2004;104:1662-1670)
Introduction
During the last few years the biologic activity of extracellular nucleotides has been the focus of increasing attention. Responses to extracellular adenosine triphosphate (ATP) or uridine triphosphate (UTP) as different as cell proliferation, differentiation, chemotaxis, cytokine secretion, release of lysosomal constituents, generation of reactive oxygen or nitrogen species, and cell death have been reported by different groups.1-9 The effects of extracellular nucleotides are mediated by specific plasma membrane receptors, which are classified into 2 families: metabotropic P2Y receptors (P2YRs)10 and ionotropic P2X receptors (P2XRs).11-13
P2YRs are typical G protein-coupled receptors with 7 transmembrane domains. Eight P2YRs have been cloned so far. Signal transduction occurs via activation of phospholipase C or stimulation/inhibition of adenylate cyclase. ATP is an agonist at all P2Y subtypes, with the exception of P2Y12 and P2Y14, where adenosine diphosphate (ADP) and uridine diphosphate (UDP)-glucose are the preferred agonists, respectively, and ATP is either ineffective or an antagonist. UTP is a better agonist than ATP at P2Y4 and P2Y6, whereas at P2Y2 ATP and UTP are equipotent. P2XRs are multimeric plasma membrane ion channels directly gated by ATP that mediate fast permeability changes to monovalent and divalent cations (Na+, K+, and Ca2+). Seven P2X subunits have been cloned so far. P2Rs are widely expressed on mature blood cells.14-20 Moreover, the expression and function of the P2X7R on B-cell chronic lymphocytic leukemia (B-CLL) cells correlate with the severity of the disease, and targeting of such receptors has been proposed as a novel form of treatment for chronic leukemia.21
No information is currently available on the expression and function of P2Rs on human hematopoietic stem cells (HSCs). HSCs are identified by the expression of the CD34 antigen, a cell membrane phosphoglycoprotein present on human bone marrow (BM), peripheral blood (PB), and cord blood progenitors.22 Animal models23 and clinical transplantation studies24 using highly enriched cell populations have demonstrated the capacity of CD34+ cells to behave as true stem cells because they engraft lethally irradiated allogeneic hosts. Moreover, retrovirally marked autologous CD34+ grafts sustain long-term hematopoiesis.25 Recent studies have challenged the dogma that all HSCs express the CD34 antigen.26-30 Sato and colleagues31 have demonstrated that only one class of murine stem cell exists, although in 2 functional states distinguishable by CD34 expression. According to this model, CD34- stem cells are quiescent and can be activated by different stimuli to generate a CD34+ cell population with high engraftment potential.32,33
In this study, we assessed the expression of P2Rs on highly purified CD34+ HSCs and investigated the functional responses of ATP/UTP-stimulated HSCs, in vitro, and in vivo in immunocompromised mice. Our results demonstrate that P2Rs are expressed on CD34+ cells and mediate fast changes in the intracellular ion homeostasis. Functionally, extracellular nucleotides enhanced the stimulatory activity of several cytokines on clonogenic CD34+ and lineage-negative CD34- (Lin-CD34-) progenitors and expanded more primitive CD34+-derived long-term culture-initiating cells (LTC-ICs) and the number of human BM-repopulating CD34+ cells in nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice.
Materials and methods
Reagents
ATP, UTP, cytidine triphosphate (CTP), guanosine triphosphate (GTP), and ADP were purchased from Boehringer Mannheim (Mannheim, Germany). Digitonin, 2′,3′-(4-benzoyl)-benzoyl-ATP, periodate-oxidized ATP (oATP), and ionomycin were purchased from Sigma-Aldrich (Milan, Italy), and KN-62 was from Calbiochem-Novabiochem (La Jolla, CA).
Solutions
Fluorescence measurements were performed in a saline solution containing 125 mM NaCl, 5 mM KCl, 1 mM MgSO4, 1 mM Na2HPO4, 5.5 mM glucose, 5 mM NaHCO3, 1 mM CaCl2, and 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; pH 7.4 with NaOH), hereafter also referred to as standard saline solution.
Cell preparation and HSC purification
Normal adult hematopoietic CD34+ HSCs were obtained from 18 allogeneic PB and 3 BM transplant donors. Healthy donors received glycosylated recombinant human granulocyte colony-stimulating factor (G-CSF; lenograstim; Rhone-Poulenc Rorer, Milan, Italy) administered subcutaneously at 10 μg/kg/d for 5 to 6 days. Leukaphereses were performed on days 5 and 6 as previously described.34 HSC purification and subsequent studies were always performed using day-5 collections at peak time of PB CD34+ cells. The protocol was approved by the ethics committee of the University Hospital and each donor gave written informed consent. Mononuclear cells (MNCs) of light density (< 1.077 g/mL) were enriched using Fycoll-Paque (Pharmacia, Uppsala, Sweden), resuspended in 1% bovine serum albumin (BSA; Sigma Chemical, St Louis, MO), and then processed by MiniMacs high-gradient magnetic separation column (Miltenyi Biotec, Bergisch Gladbach, Germany) to obtain highly purified CD34+ cells as already reported.34 To assess the percentage of CD34+ elements, aliquots of the CD34+ target cells were restained with a monoclonal antibody (mAb; HPCA-2-fluorescein isothiocyanate [FITC]; Becton Dickinson, Mountain View, CA) directed to a different epitope of CD34 antigen than that (Qbend 10) used with the magnetic system. Propidium iodide (2 μg/mL) was added for the detection of nonviable cells, which were excluded from the analyses. Cytometric analysis was performed on a gated population set on scatter properties by using FACScan equipment (Becton Dickinson). A minimum of 10 000 events was collected in list mode on FACScan software. The percentage of CD34+ cells in PB samples was 0.9% ± 0.3% of the MNC fraction. After magnetic separation, the percentage of CD34+ was 94% ± 5%. In separate experiments Lin-CD34- HSCs were isolated as already reported.35 Briefly, MNCs were stained for 30 minutes at 4°C with a mixture of lineage-specific antibodies (CD2, CD3, CD14, CD16, CD19, CD24, CD56, CD66b, CD41, and glycophorin A) provided by StemCell Technologies (Vancouver, BC, Canada), followed by addition of secondary antibodies conjugated to magnetic dextran-iron particles for a further 30 minutes at 4°C.35 Cells were then eluted through a magnetized column (StemSep, Stem Cell Technologies) to remove Lin+ cells. Lin- cells were then collected according to manufacturer's instructions, incubated with anti-human CD34-FITC mAb (HPCA-2; Becton Dickinson), and sorted on FACStar Plus equipment (Becton Dickinson). The sorting gates were set on isotype control antibody-stained samples. Sort windows were adjusted to minimize the chance that weakly positive cells would contaminate the CD34- cell population. Aliquots of sorted Lin-CD34+ and Lin-CD34- cells were reanalyzed by a FACScan to assess their purities, which were 99.5% ± 0.6% and 99.7% ± 0.1%, respectively.
Progenitor cell assays
Human colony-forming unit cells were cultured in methylcellulose as previously described.34,35 Briefly, 1 × 104 CD34+ or Lin-CD34- cells were plated, with or without nucleotides, in duplicate in culture medium consisting of 1 mL Iscove modified Dulbecco medium (IMDM) supplemented with 24% fetal calf serum (FCS; Sera Lab, Crawley Down, Sussex, United Kingdom), 10-4 M 2-mercaptoethanol (Sigma), 0.2 mM bovine hemin (Sigma), 0.8% BSA (Sigma), 5 U/mL recombinant human erythropoietin (EPO; Dompè Biotec, Milan, Italy), 50 ng/mL stem cell factor (SCF; Amgen, Thousand Oaks, CA), 10 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF; Immunex, Seattle, WA), and 50 ng/mL interleukin 3 (IL-3; Immunex). The final concentration of methylcellulose was 1.32%. Granulocyte-macrophage CFUs (CFU-GMs), erythroid burst-forming units (BFU-Es), and multilineage colonies (CFU-Mix), together referred to as CFU-Cs, were scored after 14 days of incubation at 37°C in a fully humidified 5% CO2 atmosphere.34 Where indicated, HSCs were cultured in serum-free medium (MethoCult SFBIT H4236; StemCell Technologies) supplemented with cytokines and nucleotides (ATP, 1 nM; UTP, 10 μM) as shown in “Results.” Blocking experiments were performed by growing CD34+ cells in methylcellulose with or without ATP (1 nM), in the presence of apyrase (0.4 U/mL) or adenosine (1 nM). Controls were run in the presence of denatured apyrase (0.4 U/mL). For LTC-IC assays, 10 000 highly purified CD34+ cells/mL medium were plated onto irradiated murine stromal cells (M2-10B4) genetically engineered to produce G-CSF and IL-3 with weekly half-medium change.34 Nucleotides were added to the culture at each medium change (ATP, 1 nM; UTP, 10 μM). After 5 weeks at 37°C in humidified 5% CO2 atmosphere, the cells were then evaluated for their secondary CFU-C activity, and the number of LTC-ICs was calculated as earlier reported.34
Liquid cultures
Four to 10 × 104 hematopoietic CD34+ cells/mL serum-free medium (IMDM PLUS 10% BIT 9500; StemCell Technologies) were cultured for up to 24 hours with or without nucleotides (ATP, 1 nM; UTP, 10 μM). All cultures were maintained at 37°C in humidified 5% CO2 atmosphere. After 15 and 30 minutes and 1, 6, and 24 hours of culture, aliquots of the CD34+ cell population were washed free of nucleotides, collected, and plated in methylcellulose to assay their clonogenic activity.
Cytoplasmic measurements of free Ca2+
Changes in the intracellular free Ca2+ concentration ([Ca2+]i) were measured with the fluorescent indicator fura-2/am, in an LS50 Perkin Elmer fluorometer (Perkin Elmer, Beaconsfield, United Kingdom), as previously described.15 For fura-2/am loading, cells (1 × 107/mL) were resuspended in standard saline solution in the presence of 4 μM fura-2/am and 250 μM sulfinpyrazone (Sigma-Aldrich). Incubation was performed at 37°C for 15 minutes. Cells were then washed in the same solution and [Ca2+]i changes were measured in a thermostated, magnetically stirred cuvette, with the 340/380 excitation ratio at an emission wavelength of 509 nm.
Measurement of plasma membrane potential
Changes in plasma membrane potential were measured with the fluorescent dye bis1,3-diethylthiobarbiturate trimethineoxonal (bisoxonol; Molecular Probes, Leiden, The Netherlands), at the wavelength pair 540/580 nm, as previously described.19 Experiments were performed in a spectrofluorometer (model LS50, Perkin Elmer) equipped with a thermostat-controlled (37°C) cuvette holder and magnetic stirrer.
Western blot analysis
Cells were lysed in a lysis buffer containing 300 mM sucrose, 1 mM MgSO4, 1 mM K2HPO4, 5.5 mM glucose, 20 mM HEPES (pH 7.4), 1 mM benzamidine, 1 mM phenylmethylsulfonyl fluoride (PMSF), by repeated freeze/thawing (3 cycles). Proteins were separated on 4% to 12% BT minigels (Invitrogen, Carlsbad, CA) and blotted overnight on nitrocellulose paper (Schleicher and Schull Italia, Legnano, Italy). The rabbit polyclonal anti-P2X7 serum was raised against the synthetic peptide corresponding to the last 20 amino acids of the P2X7 protein (KIRKEFPKTQGQYSGFKYPY), and was kindly provided by Dr Gary Buell (Ares-Serono Research Laboratories, Geneva, Switzerland). Anti-P2X7 serum was diluted 1:100 in Tris (tris(hydroxymethyl)aminomethane)-buffered saline-Tween (TBS-T). Membranes were then incubated with alkaline phosphatase-conjugated protein A. The anti-P2Y1 antibody was from Alomone Laboratories (Jerusalem, Israel). The primary antibodies were used at a dilution of 1:200 in TBS-T buffer (10 mM Tris-Cl,150 mM NaCl, pH 8.0). The secondary antibody was a goat anti-rabbit antibody conjugated to horseradish peroxidase (HRP).
RT-PCR
Total RNA was isolated by using RNAzol-B (TEL-TEST, Friendswood, TX). RNA (100 ng) was reversed transcribed using the ACCESS reverse transcription-polymerase chain reaction (RT-PCR) kit (Promega, Madison, WI), then amplified by PCR (35 cycles). Then, 10 μL of the PCR products for P2Rs and 5 μL for β-actin were loaded and separated in a 2% agarose, ethidium bromide-containing gel. Control reactions in the absence of reverse transcriptase were also carried out. The sequences of specific primers used for P2Rs and β-actin (positive control) were reported previously.19
Animal models
NOD/SCID mice, aged 6 to 8 weeks, were sublethally irradiated with 300 cGy and injected intravenously with increasing numbers (1000-20 000) of highly purified CD34+ cells that had been preincubated in serum-free medium with 10 μM UTP or mock medium for 1, 6, or 24 hours at 37°C. Sixty to 90 days after transplantation, mice were humanely killed and their BM collected for the evaluation of human hematopoietic engraftment.36,37 All procedures involving animals were done in accordance with national and international laws and policies.
Measurement of human hematopoietic engraftment by flow cytometry
Marrow samples of NOD/SCID mice were evaluated as previously described by 4-color flow cytometry using a panel of mAbs reacting with human CD19, CD31, CD34, and CD45.36,37 After red cell lysis, cell suspensions were evaluated by a FACSCalibur (Becton Dickinson) using analysis gates designed to exclude dead cells, platelets, and debris. After acquisition of at least 100 000 cells/sample, analyses were considered as informative when adequate numbers of events (ie, > 100; typically 100-200) were collected in the human cell enumeration gates. Percentages of stained cells were determined and compared with appropriate negative controls. The expression of human CD45 in the BM of mice that had received transplants was confirmed by RT-PCR and Southern blotting as previously reported.35,36 For SCID-repopulating cell (SRC) evaluation, mice were considered engrafted if the frequency of human CD45+ cells was more than 0.1% and human lymphoid (CD45+CD19+), myeloid (CD45+CD19-), and endothelial (CD45-CD31+) cells were detected.
Statistical analysis
The results are expressed as the mean ± SEM of at least 3 different experiments. In transplantation experiments, the frequency of SRCs was calculated as previously reported38 by limiting dilution analysis using the single-hit Poisson model and the maximum likelihood estimator. The χ2 analysis was used to validate the Poisson statistics. Results of in vitro studies were analyzed with the paired nonparametric Wilcoxon rank sum test. Two-sided P < .05 was considered as statistically significant.
Results
CD34+ cells express functional P2Rs
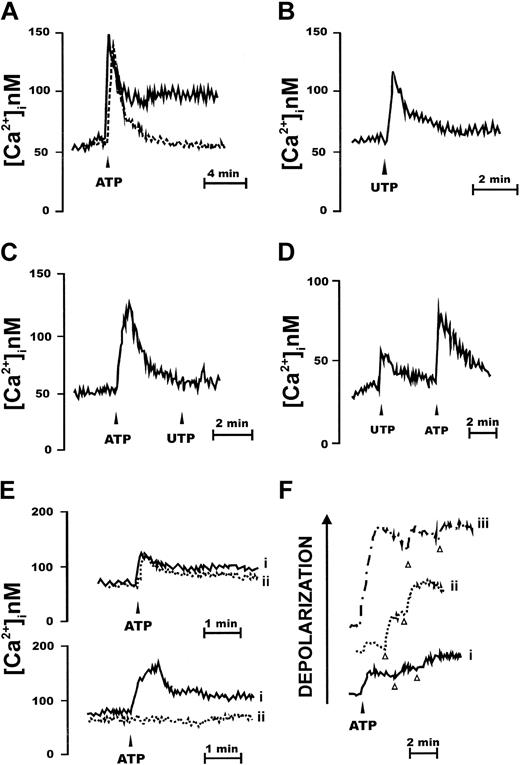
Like almost any hematopoietic cell type, CD34+ cells were responsive to stimulation with extracellular nucleotides. Among nucleotides tested, best response was elicited by ATP, but UTP was also an active stimulant (Figure 1A-B). Two patterns of responses were elicited in different subjects: in the majority (n = 19) ATP caused a transient spiky increase in [Ca2+]i followed by a lower long-lasting plateau (Figure 1A, continuous line). In a few subjects (n = 2) only the fast initial [Ca2+]i increase was present (Figure 1A, dashed line). All the 21 subjects screened responded to both nucleotides, at the concentration of 1 mM. When UTP was the stimulus, the delayed plateau was almost invariably absent or greatly reduced (Figure 1B). We measured an early average ± SEM increase in [Ca2+]i over resting level (peak increase) of 78 ± 12 nM (ATP) and a delayed average [Ca2+]i increase of 28 ± 5 nM (ATP; n = 19). The average ± SEM [Ca2+]i increase over basal induced by UTP was 39 ± 4 nM (n = 21). Chelation of extracellular Ca2+ marginally affected the fast early [Ca2+]i spike due to Ca2+ release from the intracellular stores and abrogated the delayed plateau (Figure 1C). Previous incubation with ATP abrogated the response to a subsequent stimulation with UTP, but not vice versa (Figure 1C-D), suggesting the expression of receptors selective for ATP alone and receptors with mixed (ATP/UTP) selectivity. Among other nucleotides, ADP, α,β-methylene ATP, and ATPγS were effective stimuli (not shown). Benzoylbenzoic ATP (BzATP) also triggered a [Ca2+]i increase, with the exception of those samples that did not show the delayed plateau following ATP stimulation. No response was observed to UDP-glucose, the preferred agonist at the newly cloned P2Y14R. Inhibition by 2 widely used P2XR blockers, oATP and KN-62, was highly variable from sample to sample. In some cases (Figure 1E, upper traces) the ATP-stimulated [Ca2+]i rise was not affected by pretreatment with oATP, whereas in other cases the [Ca2+]i rise was fully abrogated (Figure 1E lower traces). Most of the nucleotide-dependent increase in [Ca2+]i was due to release of Ca2+ from intracellular stores; however, ATP and BzATP (but not UTP) also activated a large ion influx across the plasma membrane, as shown by (1) the sustained plateau following the fast [Ca2+]i transient, and (2) depolarization of the plasma membrane (Figure 1F). We never observed a hyperpolarization in response to nucleotide stimulation.
CD34+ HSCs express functional P2XRs. CD34+ cells were loaded with the Ca2+ indicator fura-2/AM, as detailed in “Materials and methods,” and then stimulated with nucleotides in a Ca2+-containing (A-B) or in a Ca2+-free medium (C-D) supplemented with 0.5 mM ethylene glycol tetraacetic acid (EGTA). Nucleotides were added at the concentration of 1 mM. Traces are from a single experiment representative of 5 similar ones. Cells were also pretreated with the P2X inhibitor oATP (600 μM for 2 hours at 37°C), rinsed, and then challenged with 1 mM ATP (E; continuous line (i), ATP; dashed line (ii), oATP + ATP). P2R stimulation induces plasma membrane depolarization (F). Cells were loaded with the fluorescent dye bisoxonol as reported in “Materials and methods” and then challenged with increasing ATP concentrations (trace i, 300 μM; trace ii, 1 mM; trace iii, 3 mM). KCl (arrowhead) was 30 mM. Traces are from a single experiment representative of 5 similar ones.
CD34+ HSCs express functional P2XRs. CD34+ cells were loaded with the Ca2+ indicator fura-2/AM, as detailed in “Materials and methods,” and then stimulated with nucleotides in a Ca2+-containing (A-B) or in a Ca2+-free medium (C-D) supplemented with 0.5 mM ethylene glycol tetraacetic acid (EGTA). Nucleotides were added at the concentration of 1 mM. Traces are from a single experiment representative of 5 similar ones. Cells were also pretreated with the P2X inhibitor oATP (600 μM for 2 hours at 37°C), rinsed, and then challenged with 1 mM ATP (E; continuous line (i), ATP; dashed line (ii), oATP + ATP). P2R stimulation induces plasma membrane depolarization (F). Cells were loaded with the fluorescent dye bisoxonol as reported in “Materials and methods” and then challenged with increasing ATP concentrations (trace i, 300 μM; trace ii, 1 mM; trace iii, 3 mM). KCl (arrowhead) was 30 mM. Traces are from a single experiment representative of 5 similar ones.
These responses are compatible with the expression of several P2 subtypes, from both the P2Y and P2X families. RT-PCR analysis showed that all P2X subtypes known were expressed, whereas among P2YRs, only P2Y1 and P2Y2 were unambiguously detected (Figure 2). CD34+ cells were negative for P2Y4, P2Y6, P2Y11; the same amount of mRNA from human fibroblasts (Fibr) and monocyte-derived dendritic cells (DCs) was used as a positive control (Figure 2B). CD34+ cells were not screened for P2Y12, P2Y13, and P2Y14. Expression of the P2X7R and P2Y1R was also confirmed by Western blotting (Figure 2C-D). Cell lysates from human fibroblasts and human embryonic kidney cells expressing the P2X7R (HEK293-P2X7) were used as a positive control. As previously reported by others,39 we found that the P2Y1R ran as a 50-kDa monomer and a 100-kDa dimer.
CD34+ cells' expression of mRNA for P2XRs and P2YRs and Western blot analysis for P2X7R and P2Y1R. P2XR (A) and P2YR (B) subtypes were expressed by HSCs. RT-PCR and Western blot were performed as described in “Materials and methods”: P2X1 (248 bp), P2X2 (355 bp), P2X3 (437 bp), P2X4 (521 bp), P2X5 (614 bp), P2X6 (520 bp), P2X7 (399 bp), P2Y1 (260 bp), P2Y2 (367 bp), P2Y4 (433 bp), P2Y6 (459 bp), P2Y11 (238 bp). The expression of P2X7 and P2Y1 was confirmed, at the protein level, in panels C and D, respectively. MW indicates molecular weight marker; HSC, hematopoietic stem cells; Fibr, human fibroblasts; DC, human monocyte-derived dendritic cells; HEK293-P2X7, human embryonic kidney cells expressing P2X7R.
CD34+ cells' expression of mRNA for P2XRs and P2YRs and Western blot analysis for P2X7R and P2Y1R. P2XR (A) and P2YR (B) subtypes were expressed by HSCs. RT-PCR and Western blot were performed as described in “Materials and methods”: P2X1 (248 bp), P2X2 (355 bp), P2X3 (437 bp), P2X4 (521 bp), P2X5 (614 bp), P2X6 (520 bp), P2X7 (399 bp), P2Y1 (260 bp), P2Y2 (367 bp), P2Y4 (433 bp), P2Y6 (459 bp), P2Y11 (238 bp). The expression of P2X7 and P2Y1 was confirmed, at the protein level, in panels C and D, respectively. MW indicates molecular weight marker; HSC, hematopoietic stem cells; Fibr, human fibroblasts; DC, human monocyte-derived dendritic cells; HEK293-P2X7, human embryonic kidney cells expressing P2X7R.
Effects of extracellular nucleotides on the clonogenic growth of CD34+ cells
Previous experiments showed that CD34+ cells express functional P2Rs. We next asked whether their ligation with their natural ligands ATP or UTP may affect the proliferation of CD34+ cells. In the first set of experiments, increasing concentrations (from 0.1 nM up to 10 mM) of ATP were added to the culture medium to evaluate its activity on the clonogenic growth of mobilized CD34+ cells. Hematopoietic progenitor cells were stimulated with a cocktail of cytokines containing SCF, EPO, GM-CSF, and IL-3 in serum-repleted cultures. As shown in Figure 3C, the total colony formation potential of CD34+ cells was significantly enhanced by the addition of 1 nM to 1 μM ATP, with the fold increase ranging from 1.45 ± 0.45 (100 nM; P < .05) to 1.95 ± 0.7 (1 nM; P < .05). Concentrations greater than 1 μM ATP inhibited CD34+ cells in a dose-dependent fashion. We did not find a differential effect of ATP on growth of CFU-GMs (Figure 3A) and BFU-Es (Figure 3B). To determine the specificity of the stimulatory activity of ATP, HSCs were incubated with a fixed dose of the nucleotide (1 nM) with or without the inhibitory agent apyrase (Figure 3D). These experiments confirmed the stimulatory activity of ATP on hematopoietic progenitors (P < .04), which was abolished by apyrase and restored when denatured apyrase was used.
ATP and UTP stimulate the proliferation of CD34+ HSCs in serum-repleted cultures. CD34+ cells (1 × 104) were plated in methylcellulose in the presence of GM-CSF, IL-3, SCF, EPO, and increasing concentrations of ATP (A-D) and UTP (E-G). CFU-GMs (A,E), BFU-Es (B,F), and total CFU-Cs (C,G) were scored after 14 days of culture and the results represent the mean ± SEM of 6 different experiments. The number of colonies in control cultures was 157 ± 18 SEM. In panel D, the stimulatory activity of a fixed dose of ATP (1 nM) was inhibited by the blocking agent apyrase and restored when denatured apyrase was used. *P < .05.
ATP and UTP stimulate the proliferation of CD34+ HSCs in serum-repleted cultures. CD34+ cells (1 × 104) were plated in methylcellulose in the presence of GM-CSF, IL-3, SCF, EPO, and increasing concentrations of ATP (A-D) and UTP (E-G). CFU-GMs (A,E), BFU-Es (B,F), and total CFU-Cs (C,G) were scored after 14 days of culture and the results represent the mean ± SEM of 6 different experiments. The number of colonies in control cultures was 157 ± 18 SEM. In panel D, the stimulatory activity of a fixed dose of ATP (1 nM) was inhibited by the blocking agent apyrase and restored when denatured apyrase was used. *P < .05.
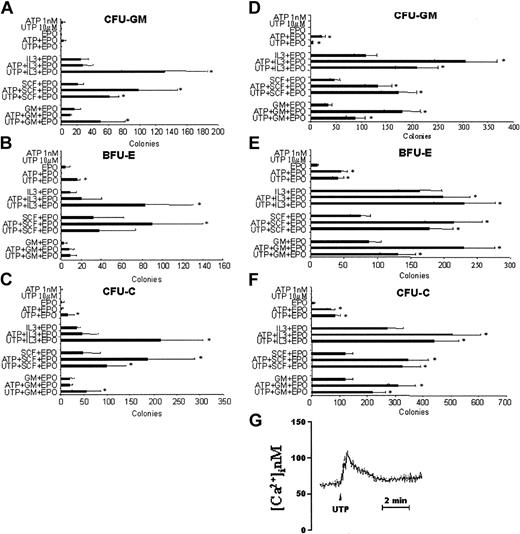
A more profound effect on CD34+ cells was exerted by UTP at concentrations ranging from 100 nM to 1 mM (Figure 3G). All P values in this range of concentration were significant with the most relevant fold increase of CFU-Cs (Figure 3G) achieved at 10 μM (2.85 ± 0.9; P < .02). Also, UTP at the highest concentration tested induced the complete inhibition of colony formation of CD34+ cells. When we analyzed separately CFU-GM (Figure 3E) and BFU-E (Figure 3F) growth, we found that 10 μM UTP induced a 2.8 ± 0.8-fold increase of erythroid progenitors, whereas the addition to the culture of 1 μM nucleotide resulted in a 6.4 ± 3.7-fold increase of CFU-GMs.
Taken together, these results suggest that extracellular nucleotides may have a stimulatory activity on hematopoietic CD34+ progenitor cells. However, because the addition of FCS to the culture medium might interfere with the activity of the nucleotides, we next examined the colony-forming ability of enriched CD34+ cells in serum-free conditions. Moreover, based on our previous results, we assayed optimal doses of ATP (1 nM) and UTP (10 μM) with and without GM-CSF, IL-3, and SCF, as single CSFs, in the presence of EPO. ATP alone showed a negligible colony-forming activity in serum-free medium (Figure 4C). However, the nucleotide did enhance synergistically the CSF activity of SCF in the presence of EPO. The stimulatory activity of ATP was equally exerted on CFU-GMs and BFU-Es (Figure 4A-B). Similarly, UTP alone did not show any stimulatory ability. However, it significantly increased the number of CFU-Cs induced by EPO alone, GM-CSF, IL-3, and SCF in the presence of EPO (Figure 4C). Specifically, UTP showed a synergistic activity with IL-3 on both CFU-GMs (Figure 4A) and BFU-Es (Figure 4B), with EPO on BFU-Es, and with SCF and GM-CSF on CFU-GMs (Figure 4A). At this low concentration (10 μM) UTP was still able to induce a Ca2+ rise in CD34+ cells (Figure 4G). In parallel experiments, we assayed steady-state BM CD34+ cells to rule out that the biologic activity of extracellular nucleotides may be limited to G-CSF-primed HSCs. Our results (Figure 4D-F) confirmed the minimal, if any, proliferative activity of ATP and UTP alone. However, in serum-free conditions both nucleotides demonstrated a remarkable synergistic activity with all cytokines tested. Furthermore, similar results were observed when extracellular nucleotides were assayed with cord blood CD34+ cells (data not shown). Thus, G-CSF treatment does not significantly alter the responsiveness of CD34+ cells to ATP and UTP and, therefore, the following experiments were performed with PB cells.
Extracellular nucleotides enhance the colony growth of G-CSF-primed and steady-state BM CD34+ cells stimulated by single cytokines, in presence of EPO, in serum-free cultures. A total of 1 × 104 PB (A-C) or BM (D-F) CD34+ cells were plated in semisolid medium under serum-free conditions. Optimal doses of ATP (1 nM) or UTP (10 μM) were added to the EPO-, GM-CSF/EPO-, IL-3/EPO-, and SCF/EPO-stimulated cultures. CFU-GMs (A,D), BFU-Es (B,E), and total CFU-Cs (C,F) were scored after 14 days of culture and the results represent the mean ± SEM of 4 different experiments. *P < .05. Cells were also loaded with fura-2/AM as reported in “Materials and methods” and stimulated with 10 μM UTP (G).
Extracellular nucleotides enhance the colony growth of G-CSF-primed and steady-state BM CD34+ cells stimulated by single cytokines, in presence of EPO, in serum-free cultures. A total of 1 × 104 PB (A-C) or BM (D-F) CD34+ cells were plated in semisolid medium under serum-free conditions. Optimal doses of ATP (1 nM) or UTP (10 μM) were added to the EPO-, GM-CSF/EPO-, IL-3/EPO-, and SCF/EPO-stimulated cultures. CFU-GMs (A,D), BFU-Es (B,E), and total CFU-Cs (C,F) were scored after 14 days of culture and the results represent the mean ± SEM of 4 different experiments. *P < .05. Cells were also loaded with fura-2/AM as reported in “Materials and methods” and stimulated with 10 μM UTP (G).
UTP enhances the colony growth of CD34+ cells stimulated with suboptimal doses of CSF
Extracellular nucleotides act synergistically with optimal doses of CSFs on the colony-forming activity of CD34+ cells. We next tested whether the addition of a fixed dose of UTP (10 μM) may counterbalance the use of suboptimal concentrations of GM-CSF and SCF in serum-free cultures. The addition of UTP compensated a 5-fold decrease of the concentration of GM-CSF/EPO and SCF/EPO for both CFU-GM and BFU-E colony formation (Figure 5A-C).
Extracellular nucleotides counteract a 5-fold decrease of cytokine concentrations to stimulate the optimal growth of CD34+ HSCs and synergize with other cytokines to induce colony formation of Lin-CD34- HSCs. CD34+ cells were plated in serum-free medium with suboptimal concentrations of GM-CSF/EPO and SCF/EPO combinations with and without a fixed dose of UTP (10 μM). The results (A-C) represent the mean ± SEM of 4 different experiments. (D-F) Cells (1 × 104 Lin-CD34-) were plated in methylcellulose under serum-depleted conditions and stimulated with optimal concentrations of ATP or UTP alone, GM-CSF/IL-3/SCF/EPO (together referred to as CSFs), and the combination of the cytokine cocktail plus UTP or ATP. The results represent the mean ± SEM of 3 different experiments. The addition of ATP or UTP makes HSCs significantly more responsive to additional growth factors. *P < .05.
Extracellular nucleotides counteract a 5-fold decrease of cytokine concentrations to stimulate the optimal growth of CD34+ HSCs and synergize with other cytokines to induce colony formation of Lin-CD34- HSCs. CD34+ cells were plated in serum-free medium with suboptimal concentrations of GM-CSF/EPO and SCF/EPO combinations with and without a fixed dose of UTP (10 μM). The results (A-C) represent the mean ± SEM of 4 different experiments. (D-F) Cells (1 × 104 Lin-CD34-) were plated in methylcellulose under serum-depleted conditions and stimulated with optimal concentrations of ATP or UTP alone, GM-CSF/IL-3/SCF/EPO (together referred to as CSFs), and the combination of the cytokine cocktail plus UTP or ATP. The results represent the mean ± SEM of 3 different experiments. The addition of ATP or UTP makes HSCs significantly more responsive to additional growth factors. *P < .05.
Extracellular nucleotides enhance the cytokine-mediated proliferation of CD34- HSCs
A novel subset of HSCs, lacking the CD34 antigen, has been recently isolated.28 This cell population is characterized by kinetic quiescence and very low or absent clonogenic potential and engraftment capacity in immunocompromised animals.35 We thus asked whether ATP and UTP may be capable of stimulating the colony-forming ability of CD34- progenitors. The results (Figure 5D-F) demonstrate no stimulatory effects of extracellular nucleotides alone. However, the addition of ATP and UTP to the cytokines cocktail used induced a more than 2- and 4-fold increase of CFU-Cs (P < .01; Figure 5F), respectively, with no significant differences between CFU-GMs (Figure 5 D) and BFU-Es (Figure 5E).
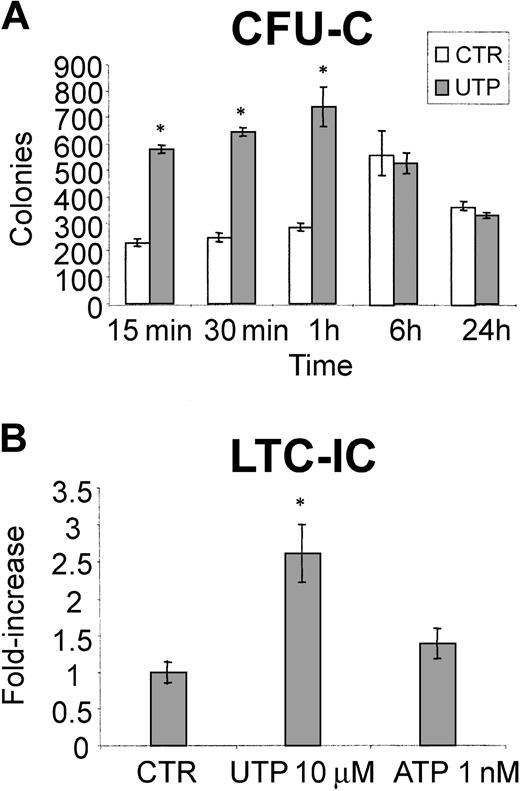
UTP exerts its activity within 1 hour of incubation and stimulates LTC-ICs
Previous experiments (Figure 1) have shown that extracellular nucleotides trigger fast intracellular second-messenger generation. To further explore the kinetics of the stimulatory activity of UTP on hematopoietic progenitors, CD34+ cells were incubated in serum-free liquid cultures and primed for different lengths of time with this nucleotide. As shown in Figure 6A, as little as 15 minutes of incubation with UTP was sufficient to cause a remarkable stimulation of CD34-derived CFU-Cs and the enhancement of colony growth induced by UTP remained statistically significant up to 60 minutes from the beginning of UTP treatment. Furthermore, along with clonogenic progenitors, we also assessed whether the addition of ATP and UTP to long-term liquid cultures of CD34+ cells may influence the number of more primitive LTC-ICs. As shown in Figure 6B, the number of LTC-ICs evaluated after 5 weeks of culture showed a 2.6 ± 0.4-fold (P = .01) and 1.4 ± 0.2-fold (P = .06) increase in the presence of UTP and ATP, respectively.
UTP exerts its activity on HSCs after short-term exposure and induces the expansion of primitive CD34-derived LTC-ICs. CD34+ cells were incubated in liquid culture in serum-free medium, with and without UTP, for up to 24 hours and then plated in methylcellulose. Addition of UTP up to 1 hour induced a remarkable stimulatory activity on CD34-derived CFU-Cs (A). In panel B, 1 × 104 highly purified CD34+ cells/mL medium were plated onto irradiated murine stromal cells (M2-10B4) genetically engineered to produce G-CSF and IL-3 with weekly half-medium change. Nucleotides were added to the culture at each medium change (ATP, 1 nM; UTP, 10 μM). After 5 weeks at 37°C in a humidified 5% CO2 atmosphere, the cells were then evaluated for their LTC-IC content. The results represent the mean ± SEM of 3 different experiments. The addition of extracellular UTP results in the significant expansion of early LTC-ICs. *P < .05.
UTP exerts its activity on HSCs after short-term exposure and induces the expansion of primitive CD34-derived LTC-ICs. CD34+ cells were incubated in liquid culture in serum-free medium, with and without UTP, for up to 24 hours and then plated in methylcellulose. Addition of UTP up to 1 hour induced a remarkable stimulatory activity on CD34-derived CFU-Cs (A). In panel B, 1 × 104 highly purified CD34+ cells/mL medium were plated onto irradiated murine stromal cells (M2-10B4) genetically engineered to produce G-CSF and IL-3 with weekly half-medium change. Nucleotides were added to the culture at each medium change (ATP, 1 nM; UTP, 10 μM). After 5 weeks at 37°C in a humidified 5% CO2 atmosphere, the cells were then evaluated for their LTC-IC content. The results represent the mean ± SEM of 3 different experiments. The addition of extracellular UTP results in the significant expansion of early LTC-ICs. *P < .05.
Taken together, our in vitro data demonstrate that ATP and, to a higher extent, UTP are potent early acting growth factors that, albeit per se unable to support proliferation, strongly enhance the stimulatory activity of several cytokines on different subsets of HSCs (ie, CD34+ and CD34- progenitors), with different levels of commitment (ie, clonogenic cells and LTC-ICs).
Short-term incubation with UTP expands SRCs
The last set of experiments was designed to assess whether the stimulatory effects of UTP on hematopoietic progenitors, in vitro, could be also observed on the most primitive HSCs with repopulating capacity in vivo. UTP was chosen for its stronger in vitro activity. Figure 7 shows the level (Figure 7E-F) of multilineage (Figure 7C-D; CD45+/CD19+, human B cells; CD45+/CD19-, human myeloid cells; CD31+, human endothelial cells) engraftment of HSCs in sublethally irradiated NOD/SCID mice (n = 6/study group) injected with increasing numbers of CD34+ cells cultured with (Figure 7E) or without UTP (Figure 7F). From the data presented in Figure 7, panels E and F, we calculated that as short as 1 hour of incubation with UTP increased the frequency of SRCs approximately 2.5-fold from 1/9049 (95% CI, 1/6581-1/14 478) to 1/3666 (95% CI, 1/2931-1/4895; P = .007). Similar data were observed when the exposure of HSCs to UTP was extended to 6 and 24 hours (data not shown). Although this study does not formally assess the effect of UTP on HSC self-renewal (eg, we did not perform secondary transplantation assays), our experimental observations demonstrate that UTP can significantly expand the number of marrow-repopulating cells.
Quantitative SRC analysis in sublethally irradiated NOD/SCID mice injected with CD34+ cells cultured with UTP or control medium. (A) The gate used to exclude platelets, dead cells, and debris. (B) The negative control. (C) A representative evaluation of the presence of human lymphoid (CD45+CD19+) and myeloid (CD45+CD19-) cells in mice given transplants. (D) A representative evaluation of the presence of human endothelial (CD45-CD31+) cells in mice receiving transplants. Panels E and F show the percentage of human CD45+ cells in the marrow of mice given injections with increasing numbers of purified CD34+ cells (ranging from 1000 to 20 000) cultured as described in “Materials and methods” with UTP (E) or control medium (F; n = 6/study group). Each symbol represents the data from an individual mouse evaluated 60 to 90 days after transplantation. A limiting dilution analysis using the single-hit Poisson model and the maximum likelihood estimator38 was used to calculate SRC frequency in mice given transplants with CD34+ cells cultured with UTP or control medium. The Poisson statistic was further validated by χ2 analysis.
Quantitative SRC analysis in sublethally irradiated NOD/SCID mice injected with CD34+ cells cultured with UTP or control medium. (A) The gate used to exclude platelets, dead cells, and debris. (B) The negative control. (C) A representative evaluation of the presence of human lymphoid (CD45+CD19+) and myeloid (CD45+CD19-) cells in mice given transplants. (D) A representative evaluation of the presence of human endothelial (CD45-CD31+) cells in mice receiving transplants. Panels E and F show the percentage of human CD45+ cells in the marrow of mice given injections with increasing numbers of purified CD34+ cells (ranging from 1000 to 20 000) cultured as described in “Materials and methods” with UTP (E) or control medium (F; n = 6/study group). Each symbol represents the data from an individual mouse evaluated 60 to 90 days after transplantation. A limiting dilution analysis using the single-hit Poisson model and the maximum likelihood estimator38 was used to calculate SRC frequency in mice given transplants with CD34+ cells cultured with UTP or control medium. The Poisson statistic was further validated by χ2 analysis.
Discussion
Functional P2Rs for extracellular nucleotides have been found in many mature blood cells and their activation has been associated with stimulation of responses as different as chemotaxis, cytokine secretion, proliferation, and cell death.14 More recently, P2X7R has also been identified on B-CLL cells and its expression was found to correlate with an aggressive course of the disease.21 In these cells, P2X7 appears to play 2 opposite roles: as a growth-promoting receptor under conditions of low-level stimulation, and as a cytotoxic receptor if overactivated.40
Although the interest for nucleotides as potential regulators of blood cell functions has steadily increased, so far no studies have addressed the issue of whether P2Rs are expressed on HSCs and whether their ligation induces the functional activation of HSCs. Recently, cyclic ADP-ribose, which shares with nucleotides the capacity to release calcium from intracellular stores, has been shown to induce proliferation of hematopoietic progenitors, but the mechanism of this effect and the plasma receptors involved are unknown.41
In this study, we extensively investigated, in vitro and in vivo, the stimulatory effects of ATP and UTP on different subsets of HSCs with different degrees of commitment. HSCs were isolated from 3 sources: steady-state BM, cord blood, and mobilized PB. Molecular analysis of CD34+ HSCs demonstrated the expression of mRNA for all known P2XRs, in addition to P2Y1R and P2Y2R. We also confirmed the expression of P2Y1 and P2X7 at the protein level.
Functionally, low concentrations of ATP and, to a higher extent, UTP, acted as potent early acting growth factors for HSCs, in vitro, because they did not show any proliferative activity per se but synergistically enhanced the stimulatory effects of several cytokines on clonogenic CD34+ and Lin- CD34- cells and expanded more primitive LTC-ICs. Stimulation of CD34+ cells was kinetically paralleled by the increase in [Ca2+]i. Taken together, these results indicate a direct proliferative effect of nucleotides on HSCs, likely mediated by their intracellular calcium-releasing activity. Blocking experiments with specific inhibitors of ATP signaling and the use of highly purified HSC populations cultured in serum-free medium provided support for the conclusion that nucleotides are themselves responsible for these effects and their activity is not mediated by accessory cells or soluble factors present in FCS. In this view, previous studies have shown that adenosine monophosphate (AMP), a degradation product of ATP, stimulates murine hematopoietic progenitors in clonogenic assays and liquid cultures.42 Thus, it may be argued that the activity of ATP is mediated by AMP derived in culture from enzymatic digestion. However, AMP does not link to P2Rs whose activation is associated, in this study, with the biologic effects of extracellular nucleotides.
More importantly, in vivo studies showed that short-term preincubation with UTP alone modestly, but significantly, increased the number of SRCs. This finding is relevant because cell purification and gene marking studies have demonstrated that SRCs are biologically distinct and more primitive than CFU-Cs and LTC-ICs evaluated after 5 weeks of liquid culture.43 Further experiments of competitive repopulation are in progress to evaluate the synergistic activity of UTP with other cytokines and the optimal time of incubation to achieve the maximum expansion of SRCs within the CD34+/CD38- cell population. However, the present results are promising because previous studies have shown that none of the other early acting cytokines tested alone could expand or even maintain human HSC.44 Furthermore, we are actively investigating the effects of extracellular nucleotides on cycling of HSCs and their activity on cell-cycle molecular regulators.
Not surprisingly, CD34+ cell proliferation was stimulated by low and inhibited by high concentrations of ATP and UTP. The bifunctional capacity of P2Rs has been already observed in CLL cells21 and provides the likely explanation for the previously reported toxicity of high ATP doses on murine hematopoietic precursors and macrophages. Thus, in view of future applications in clinical oncology, it would be important to assess the expression and function of P2Rs on a wide range of tumor cells as well as the modulatory effect of nucleotides on the balance inhibition/stimulation. Furthermore, different concentrations of nucleotides may have a physiologic relevance as extracellular mediators of activation/inhibition of HSCs. In our study, 1 nM and 10 μM were the optimal doses of ATP and UTP, respectively, that were found to stimulate HSCs. These concentrations are well below the intracellular concentrations in the cytosol (5-10 mM) and in the intracellular compartment of platelets (100-1000 mM) and are also significantly lower than the high micromolar concentrations of ATP that were shown to affect the function of immunocompetent cells and HSCs19,20 (and present results). Thus, it may be hypothesized that very low physiologic concentrations of extracellular nucleotides stimulate HSCs, whereas during inflammation the release of high concentrations of ATP from the damaged tissue represents an inhibitory signal for hematopoietic progenitors.
In summary, our results suggest that extracellular nucleotides may hold promise to be novel and powerful tools to modulate HSC functions ex vivo to increase the number of transplantable cells and in vivo in case of BM failure.
Prepublished online as Blood First Edition Paper, May 25, 2004; DOI 10.1182/blood-2004-03-0834.
Supported by grants from the Italian Ministry of Education, University and Scientific Research (MIUR), the National Research Council of Italy, the Italian Association for Cancer Research (AIRC), the Italian Space Agency (ASI), the University of Ferrara and the University of Bologna (funds for selected topics), Italy.
R.M.L. and D.F. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank Dr Carmelo Carlo-Stella (National Cancer Institute, Milan, Italy) for critically reviewing the manuscript and Mrs Katia Vitali for editing.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal