Comment on Chou et al, page 1498
The erythropoietin gene from Fugu rubripes (pufferfish) is homologous to the human protein but lacks a flanking hypoxia-responsive element that is critical for erythropoietin induction by hypoxia in mammals.
Work by Chou and colleagues in this issue represents the first study of a nonmammalian erythropoietin (Epo) gene in the gourmet's fish delicacy, Fugu rubripes (pufferfish). Using homology with the human EPO protein sequence, the teleost Epo gene was identified in silico from the Fugu genome, indicating similar exon organization but only 32% homology with the human protein.
Hypoxic EPO gene induction in mammals is regulated by a hypoxic response element (HRE) in the 3′ untranslated region (UTR) that binds hypoxia-inducible factor 1 (HIF-1).1 Hypoxia increases HIF-1α stability allowing for nuclear localization, dimerization with HIF-1β to form HIF-1, and transcription activation via HRE binding. The Fugu Epo gene has no functional HRE in the 5′ or 3′ flanking regions, although there is some indication that hypoxia increases appropriate splicing of Epo transcripts. This altered response to hypoxia may reflect the low oxygen availability in the aquatic environment and a significant difference in regulation of erythropoiesis between mammals and fish, or the limitation of available reagents, and suggests important areas for further investigation.FIG1
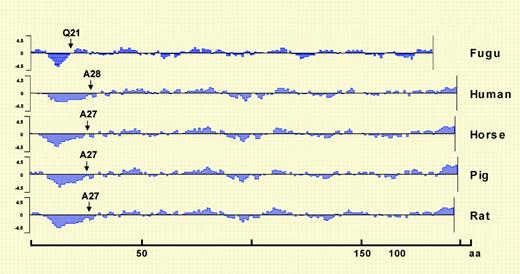
The hydrophilicity plots for Epos from Fugu, human, horse, pig, and rat generated using Kyte & Doolittle hydrophilicity parameters. See the complete figure in the article beginning on page 1498.
The hydrophilicity plots for Epos from Fugu, human, horse, pig, and rat generated using Kyte & Doolittle hydrophilicity parameters. See the complete figure in the article beginning on page 1498.
An important evolutionary development in vertebrates is the delivery of oxygen by hemoglobin-encapsulated erythrocytes. Much is known about hypoxia induction of EPO in the kidney and EPO stimulation of bone marrow erythropoiesis in mammals, especially humans and rodents. In contrast, Fugu Epo is expressed mainly in the heart, with some expression in brain and liver, but not in kidney, and erythropoiesis takes place in the kidney rather than in the bone marrow. Nevertheless, it appears that Epo synthesis away from the site of erythropoiesis and its secretion into the circulation for transport to stimulate red cell production is conserved between mammals and fish. Of note is the similarity of Epo expression in brain and liver. In mammalian model systems, EPO effects extend beyond erythropoiesis and provide protection in the embryo and select adult organs against ischemia or stress. In addition to EPO requirement for erythropoiesis, we observed a developmental defect in brain neuroepithelium and in heart endocardium and myocardium as well as increased neuron sensitivity to hypoxia in mice that lack the erythropoietin receptor.2 Neuroprotection by EPO has been demonstrated in several adult animal models for brain hypoxia and mechanical trauma and includes EPO protection against brain ischemia that reduces hippocampal neuron damage and memory loss.3 The high level of Epo expression in the Fugu heart report by Chou and colleagues also draws attention to recent reports of EPO protection in mammalian models of heart ischemia. For example, a single dose of EPO following myocardial infarction in rats reduced infarct size and functional decline 8 weeks after insult.4 Fugu Epo expression in heart and brain raises the possibility that its neuroprotective and cardioprotective activities require local EPO production, may be evolutionarily conserved, and perhaps were among the original functions of this molecule. Chou and colleagues point out important similarities and differences between mammalian and Fugu Epo. Further understanding of the spatial and temporal expression of teleost Epo may yield important insight into vertebrate erythropoiesis and EPO function in other tissues.