Comment on Diaconescu et al, page 1550
Reduced intensity, or nonablative, conditioning regimens have made allogeneic hematopoietic transplantation available to patients ineligible for conventional ablative regimens.
The rationale behind the nonablative approach rests on the theory that the curative aspect of allogeneic transplantation is mediated by a graft-versus-tumor effect rather than a high-dose cytotoxic effect. Since high-dose therapy is avoided, this approach was predicted to be safer for patients who were ineligible for conventional myeloablative regimens because of age or comorbidities. Investigators hypothesized that the early toxicity of high-dose regimens is caused by a combination of direct organ damage from the conditioning regimen as well as graft-versus-host disease (GVHD) provoked by a “cytokine storm” released from these damaged tissues. Although initial reports showed that successful donor engraftment is safely achievable with nonablative regimens, longer-term results of toxicities have thus far been lacking.1-3 FIG1
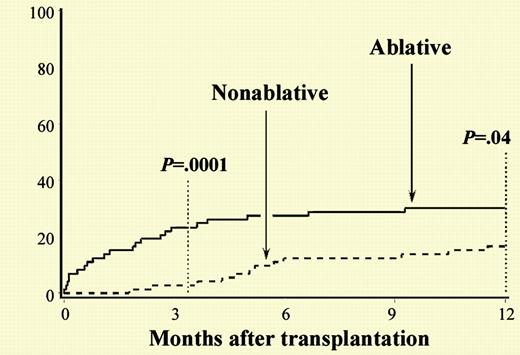
Cumulative incidences of 1-year nonrelapse mortality among HC transplant recipients given nonablative versus ablative conditioning. See the complete figure in the article beginning on page 1550.
Cumulative incidences of 1-year nonrelapse mortality among HC transplant recipients given nonablative versus ablative conditioning. See the complete figure in the article beginning on page 1550.
In this issue of Blood, Diaconescu and colleagues present a comparison of results using nonablative conditioning regimens with historic controls receiving conventional myeloablative transplants. Nonablative conditioning regimens consisted of either low-dose total body irradiation (TBI) only or the combination of fludarabine and TBI. Comorbidities were retrospectively measured by the Charlson comorbidity index, which was developed and validated as a measurement of mortality risk in breast cancer patients.4 This appears to be the first use of this scale to quantitate risk factors in recipients of hematopoietic cell transplants. Because of the incorporation of organ-specific functional assessments, this tool may provide additional power beyond nonspecific performance status assessments, such as the Eastern Cooperative Oncology Group score and Karnofsky scores. The authors present a systematic analysis of toxicities and the nonablative cohort experienced similar or lower toxicity rates for all parameters, with the exception of graft rejection. The authors conclude that despite the presence of higher levels of medical comorbidities, patients treated with nonablative allogeneic transplantation had significantly lower treatment-related mortality at 100 days and at 1 year after transplantation.
A few cautionary notes are warranted. First, over half of the nonablative patients received TBI-only conditioning, a regimen not widely used in other centers. Second, data regarding longer-term follow-up are needed to determine if rates of disease control are similar with ablative and nonablative conditioning regimens; results of disease-related response, morbidity, and mortality are not included. It is possible that certain malignancies are more responsive to the allogeneic antitumor effect than others. Finally, although the onset of acute GVHD was delayed in the nonablative cohort, overall rates of severe acute GVHD were not lower, contrary to reports from other clinical investigators.5 The authors suggest this may have been due, in part, to a shorter course of cyclosporine GVHD prophylaxis in the nonablative cohort.
Nonablative conditioning regimens will clearly retain a place among the available treatment options in allogeneic hematopoietic transplantation. Further work is needed to directly compare the results of nonablative and conventional conditioning regimens and to define the diseases for which the allogeneic graft-versus-malignancy effect is adequate for disease cure. As nonablative allogeneic transplantation becomes more common, formal systems of risk assessment such as the Charlson index might become an important tool for treatment decisions and patient selection, if these results are confirmed in prospective studies.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal