Comment on Lee et al, page 1559
Approaches to eliminate interleukin-2 receptor alpha (CD25+) cells have been widely pursued to selectively eliminate alloreactive cells that cause GVHD after allogeneic stem cell transplantation. In this issue, Lee and colleagues report paradoxical findings involving treatment with daclizumab, an anti-CD25 monoclonal antibody, possibly due to effects on CD4+CD25+ immunoregulatory cells.
The narrator in Robert Burns' 1785 poem “To a Mouse” laments to the murine protagonist: But, Mousie, thou art no thy lane, / In proving foresight may be vain; / The best-laid schemes o' mice an' men / Gang aft agley [often go astray] / An' lea'e us nought but grief an' pain.1
The ability to humanize a monoclonal antibody (MAb) produced by a mouse has proved to be one of the best schemes developed by scientists in human cancer therapy, as evidenced by the successes of rituximab and trastuzumab. Diseases of immune dysregulation are logical targets for MAb therapies, as resting and activated lymphocytes are amongst the best-characterized cells with respect to MAb binding.FIG1
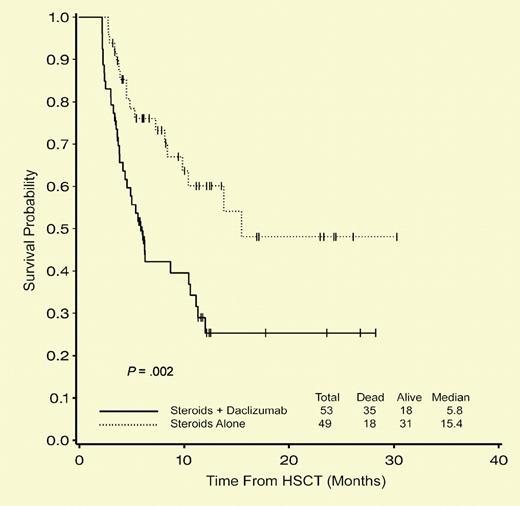
Probability of overall survival is better in the corticosteroids alone arm at 100 days and 1 year. See the complete figure in the article beginning on page 1559.
Probability of overall survival is better in the corticosteroids alone arm at 100 days and 1 year. See the complete figure in the article beginning on page 1559.
Acute graft-versus-host disease (GVHD) is largely mediated by alloreactive T lymphocytes. Interleukin-2 receptor alpha chain (IL-2Rα, CD25) is upregulated on activated human T cells in vitro and in human disease states. The anti-CD25 monoclonal antibody, daclizumab, has been successfully used for treatment of steroid-resistant acute GVHD.2 In this issue of Blood, Lee and colleagues hypothesize that the addition of the CD25-specific MAb daclizumab would increase the efficacy of standard therapy for acute GVHD. Their double-blinded and randomized multi-institutional trial was well designed to determine whether the addition of daclizumab to initial corticosteroid therapy for acute GVHD would improve clinical outcomes. Unfortunately, a planned interim analysis demonstrated that subjects receiving daclizumab experienced significantly worsened 100-day survival (77% vs 94%; P = .02) and 1-year overall survival (29% vs 60%; P = .002). These findings appropriately led to premature termination of the trial and the authors' conclusion that daclizumab should not be added to corticosteroids for the initial treatment of acute GVHD.
Some results were surprising, including the fact that while the response rate in the combined arm was similar to that in the steroid-only arm (53% vs 51% at day +42; P = not significant), GVHD-related and disease-related deaths occurred more frequently in the daclizumab arm. Given the established association between GVHD incidence and severity with decreases in relapse, the fact that relapses also occurred more frequently in the daclizumab group is counter-intuitive. Furthermore, the cumulative incidence of chronic GVHD in the daclizumab group was not higher than in the steroid-alone arm, even when death was considered as a competing risk.
The immunologic mechanisms responsible for the effects observed are not immediately clear, as the authors acknowledge. CD25 is also expressed on CD4+CD25+ immunoregulatory cells, which have been shown to inhibit GVHD in animal models.3 It is tempting to lay the blame at the feet of these regulatory T cells that might have been depleted by daclizumab, thereby aggravating GVHD. Indeed, approaches to expand regulatory T cells hold significant clinical promise.4 However, 2 studies recently published in Blood found that increased frequencies of CD4+CD25+ T cells were present in the donor grafts of recipients who experienced acute GVHD5 and in the peripheral blood of stem cell transplant (SCT) recipients with chronic GVHD.6 In aggregate, these results highlight our need to identify unique surface markers that may differentiate activated and regulatory CD25-expressing T cells so we may better design MAb-based and adoptive cellular therapies for hematopoietic transplantation. With better foresight, and more studies in both mice and men, we might finally advance the therapy for acute GVHD beyond the corticosteroid era.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal