Abstract
Recently, there have been several reports using various superparamagnetic iron oxide (SPIO) nanoparticles to label mammalian cells for monitoring their temporal and spatial migration in vivo by magnetic resonance imaging (MRI). The purpose of this study was to evaluate the efficiency and toxicity of labeling cells using 2 commercially available Food and Drug Administration (FDA)-approved agents, ferumoxides, a suspension of dextran-coated SPIO used as an MRI contrast agent, and protamine sulfate, conventionally used to reverse heparin anticoagulation but also used ex vivo as a cationic transfection agent. After labeling of human mesenchymal stem cells (MSCs) and hematopoietic (CD34+) stem cells and other mammalian cells with ferumoxides-protamine sulfate complexes (FE-Pro), cellular toxicity, functional capacity, and quantitative cellular iron incorporation were determined. FE-Pro-labeled cells demonstrated no short- or long-term toxicity, changes in differentiation capacity of the stem cells, or changes in phenotype when compared with unlabeled cells. Efficient labeling with FE-Pro was observed with iron content per cell varying between 2.01 ± 0.1 pg for CD34+ cells and 10.94 ± 1.86 pg for MSCs with 100% of cells labeled. Cell labeling using these agents should facilitate the translation of this method to clinical trials for evaluation of trafficking of infused or transplanted cells by MRI. (Blood. 2004;104:1217-1223)
Introduction
Previous studies of in vivo cell trafficking were dependent on use of radionuclide labels such as 111In, but application of those methods to all cell types and clinical disease states may be limited by toxicity of the label to individual cells or to the patient.1,2 There is increasing interest in using magnetic resonance imaging (MRI) to monitor the in vivo behavior of stem and other cells labeled with superparamagnetic iron oxide (SPIO) nanoparticles. Such cell trafficking studies would be a valuable tool for development and evaluation of cell-based repair, replacement, or treatment strategies.3-12 Ferumoxides (FE), a suspension consisting of dextran-coated SPIO, is approved for in vivo human use by the US Food and Drug Administration (FDA) as an MRI contrast agent. When used for hepatic MRI, the iron is phagocytosed and accumulates in endosomes of Kupffer cells and reticuloendothelial cells.13 The particles are biodegradable in that they are metabolized by cells and enter into normal whole body iron metabolism, as evidenced by transient increase in serum iron values within 1 day and an increase in serum ferritin values 7 days after administration.14 The iron from nanoparticles is ultimately incorporated into hemoglobin in red cells within 30 to 40 days or used for other metabolic processes.14 Ferumoxides have a negative zeta potential and when used alone do not efficiently label nonphagocytic or non-rapidly dividing mammalian cells in vitro.10,15
Complexing of polycationic transfection agents (TAs; eg, poly-L-lysine [PLL]) to ferumoxides occurs through electrostatic interactions and is an efficient and effective technique for incorporating the SPIO nanoparticles within endosomes, thereby labeling cells that can be detected by MRI.9-11,16-18 Iron oxide-labeled cells appear as hypointense areas in tissues with an associated susceptibility artifact or amplification of the decreased signal intensity on iron-sensitive T2-weighted and T2*-weighted gradient echo images.7,8,10-12,17-23
The majority of polydisperse polycationic TAs are not approved by the FDA for clinical use, and when PLL is combined with ferumoxides large complexes can form if the mixture is not monitored. Moreover, most TAs are toxic to cells when used alone and not complexed to DNA.24 For example, PLL has a relatively narrow tolerated concentration of 10 μg/mL or less in media before causing significant cell death.24,25 Ferumoxides complexed to PLL have been shown to effectively label cells, but residual FE-PLL complexes may remain on the surface of the cells or clump cells together in the final cell preparation prior to infusion.
Protamine sulfate is a low-molecular-weight (∼4000 Da), naturally occurring polycationic peptide that is FDA approved as an antidote to heparin anticoagulation.26,27 Protamine sulfate (Pro) is well-tolerated by cells, with a high therapeutic window of more than 50 mg/mL.28 Sorgi et al28 have shown that when protamine sulfate is complexed to DNA it is about 100 times more efficient in transfecting cells compared with PLL. Protamine sulfate is commonly used in gene therapy protocols to facilitate the ex vivo gene transfection of cells. Therefore, it may be possible to combine protamine sulfate with ferumoxides and use the resulting FE-Pro complex to label cells with SPIO nanoparticles for MRI.
The purpose of this study was to determine the optimum ratio of ferumoxides to protamine sulfate that can be used for magnetically labeling cells through endosomal incorporation and to evaluate the labeling efficiency, toxicity, and effect on cellular function and differentiation capacity of this labeling method. Using the FE-Pro combination for magnetic cellular labeling should facilitate translation of this approach into clinical trials.
Materials and methods
Cells
Both freshly collected and cryopreserved mammalian cells were used for labeling with FE-Pro complexes. Human cells included mesenchymal stem cells (MSCs; Cambrex, Baltimore, MD) and CD34+ hematopoietic stem cells (HSCs) obtained by immunomagnetic selection of peripheral blood leukapheresis collections from granulocyte colony-stimulating factor (GCSF)-mobilized healthy volunteers. Animal cells included mouse macrophages (American Type Culture Collection [ATCC], Manassas, VA), mouse lymphoblast cells (LADMACs; ATCC), and mouse splenocytes collected from the spleen of SJL mice (Harlan, Fredrick, MD) induced with proteolipid protein (PLP) and complete Freund adjuvant who subsequently developed experimental allergic encephalitis (EAE). Experiments with mouse macrophages and MSCs were performed when they formed 80% confluence and concentration of LADMACs, mouse splenocytes, and human HSC suspensions at concentrations of 4 × 106 cells per mL. All cells were grown in their respective recommended media with growth factors as suggested by the supplier, and mouse splenocytes were grown in RPMI 1640 media containing HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), sodium pyruvate, minimum essential media (MEM) nonessential amino acid, l-glutamine, and interleukin 2 (IL-2). The mouse splenocytes were not stimulated in culture.
Preparation of ferumoxides-protamine sulfate (FE-Pro) complex
The commercially available ferumoxides suspension (Feridex IV; Berlex Laboratories, Wayne, NJ) contains particles approximately 80 to 150 nm in size and has a total iron content of 11.2 mg/mL (11.2 iron μg/μL). Protamine sulfate (American Pharmaceuticals Partner, Schaumburg, IL), supplied at 10 mg/mL, was prepared as a fresh stock solution of 1 mg/mL in distilled water at the time of use. Ferumoxides at a concentration of 100 μg/mL was put into a mixing flask or tube containing serum-free RPMI 1640 medium (Biosource, Camarillo, CA) containing 25 mM HEPES, MEM nonessential amino acid, sodium pyruvate, and L-glutamine. Protamine sulfate was then added to the solution at different concentrations. The solution containing ferumoxides and protamine sulfate was mixed for 5 to 10 minutes with intermittent hand shakings. After 5 to 10 minutes, an equal volume of the solution containing FE-Pro complexes was added to the existing media in the adherent cell culture. For cells grown in suspension, FE-Pro complexes were added directly to the cells, incubated for 2 to 3 hours, and then an equal volume of the respective complete medium was added to the cells for a final concentration of 50 μg ferumoxides/mL of medium. The cell suspension was then incubated overnight.
Biophysical properties of FE-Pro complex
The surface charge (zeta potential) of protamine sulfate alone or of FE-Pro complexes (with ferumoxides at 100 μg/mL but with protamine sulfate ranging from 1 to 20 μg/mL) were measured by a zeta potential (ZP) analyzer (Brookhaven Instruments, Long Island, NY) and reported as millivolts (mV). Nuclear magnetic resonance (NMR) relaxometry was performed to determine relaxation parameters (ie, 1/T1 and 1/T2) of FE-Pro complexes (in 4% gelatin) at different ferumoxides-to-protamine sulfate ratios as previously described.7,10,16 These properties were repeated and there were 2 to 3 samples in each time.
Cellular viability and proliferation capacity
To determine the effect of protamine sulfate alone on cell proliferation, a specific number of cells were grown in 96-well plates and protamine sulfate, at final concentrations of 1 to 100 μg/mL, was added to the cells. Cellular proliferative activity was determined by the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide) assay (Roche Molecular Biochemicals, Indianapolis, IN) at different time points. Proliferation and viability of FE-Pro-labeled and unlabeled cells were also evaluated using the MTT assay and trypan blue dye exclusion, respectively. After labeling and washing, a specific number of unlabeled (control) and FE-Pro-labeled cells were allowed to grow in 96-well plates and the MTT assay was performed at specific time intervals. To determine the toxicity of protamine sulfate alone, cells were not washed before the MTT assay. The absorbance of the formazan product was then measured at a wavelength of 570 nm with 750 nm (subtracted) as reference. MTT assay values for labeled cells were expressed as the percentage of corresponding average values in control cells. There were at least 4 samples in each condition for different types of cells.
Apoptosis and reactive oxygen species (ROS) in FE-Pro-labeled cells
To determine if FE-Pro labeling resulted in changes in the rates of apoptosis, both unlabeled and FE-Pro-labeled cells were collected at different time points, washed twice with ice-cold phosphate-buffered saline (PBS), and resuspended in 1 mL of annexin media (Vybrant apoptosis assay kit no. 2; Molecular Probes, Eugene, OR) at 1 × 106 cells/mL. Ten microliters of fluorescent-labeled annexin V and 2 μL propidium iodide solution were added to 100 μL of cell suspension, which was then kept at room temperature for 15 to 20 minutes, and flow cytometry was performed using a fluorescent activated cell sorter (FACScalibur; Becton Dickinson, Mountain View, CA). Apoptosis rate was measured from a representative sample in each time point.
For the ROS assay, both FE-Pro-labeled and unlabeled cells were collected and resuspended in respective complete media at 1 × 106 cells/mL. The intracellular formation of ROS was detected by using the fluorescent probe CM-H2DCFDA (Molecular Probes). CM-H2DCFDA was added at a final concentration of 10 μM and cells were incubated for 60 minutes at 37°C. CM-H2DCFDA is a nonfluorescent agent that forms fluorescent esters when it reacted with ROS inside cells. For ROS assays, the fluorescence was analyzed in a Wallac Vector 2 Fluorescent Plate Reader (PerkinElmer Life Sciences, Shelton, CT) using 490 to 500 nm wavelength for excitation and 525 nm for emission. Values were normalized to the values obtained from corresponding unlabeled cells. The samples were also analyzed by flow cytometry to determine the intensity of fluorescence in labeled and unlabeled cells. For fluorescent reader, there were at least 4 samples, however, a representative sample was analyzed with flow cytometry at each time point.
Functional and phenotypic analysis of FE-Pro-labeled cells
Both labeled and unlabeled MSCs were subjected to adipogenic, osteogenic, and chondrogenic differentiation according to the methods developed by the supplier of MSCs (Cambrex) to see whether labeling FE-Pro has any adverse effect on their differentiation capacity. Adipogenic differentiated cells were stained with oil red-O (Sigma, St Louis, MO), chondrogenic cells were stained with Safranin-O (Sigma) for glycosaminoglycans, and collagen production was indicated by standard immunohistochemistry using anti-collagen II antibody (Novacastra, Newcastle upon Tyne, United Kingdom). Cellular calcium concentration in control human MSCs and osteogenic differentiated (both labeled and unlabeled) cells were measured according to the method recommended by the supplier of MSCs using a calcium concentration determination kit (Pointe Scientific, Lincoln Park, MI). Three sets of labeled and unlabeled MSCs were subjected to differentiation for each lineage. Different phenotypic markers of mouse splenocytes (such as, CD4, CD8, CD25, CD11a, CD14, and CD19) and human hematopoietic stem cells (such as CD34, CD31, CXCR4, CD20, CD3, and CD14) were analyzed using fluorescent conjugated antibodies (BD Bioscience, San Diego, CA) for the labeled and unlabeled cells at different time points. Flow panels for phenotypic markers of both labeled and unlabeled CD34+ cells were repeated 3 times.
Histology
After incubation with FE-Pro, cells were washed 3 times to remove excess FE-Pro, trypsinized (adherent cells), and transferred to cytospin slides. Cells were fixed with 4% glutaraldehyde, washed, incubated for 20 to 30 minutes with 2% potassium ferrocyanide (Perl reagent for Prussian blue [PB] staining) in 3.7% hydrochloric acid, washed again, and counterstained with nuclear fast red. For diaminobenzide (DAB)-enhanced PB staining, slides after PB staining were put into hydrogen peroxide-activated DAB solution for 5 to 10 minutes, washed with PBS, and counterstained with nuclear fast red.
Determination of labeling efficiency
FE-Pro labeling efficiency was determined by manual counting of PB-stained and unstained cells using a Zeiss microscope (Axioplan Imaging II; Zeiss, Oberkochen, Germany) at × 100 magnification using a × 40/0.75 and × 100/1.30 (oil) immersion objective lens (Figure 1), × 40/0.75 and × 20/0.75 objective lens (Figure 2), and × 2.5/0.12 and × 10/0.50 objective lens (Figure 3), and Axiovision 4 software (Zeiss). The images were processed by Adobe Photoshop 7.0 (San Jose, CA). Cells were considered PB positive if intracytoplasmic blue or brown (DAB enhanced) granules could be detected. The percentage of labeled cells was determined from the average of 5 to 10 high-powered fields.
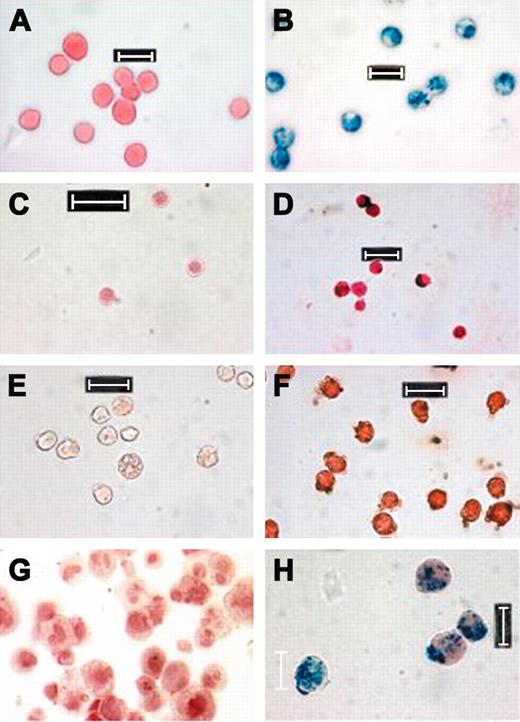
Representative Prussian blue-stained FE-Pro-labeled cells. (A) Unlabeled LADMACs, (B) labeled LADMACs, (C) unlabeled splenocytes, (D) labeled splenocytes, (E) unlabeled CD34+ cells, (F) labeled CD34+ cells, (G) unlabeled mesenchymal stem cells × 40, and (H) labeled mesenchymal stem cells. All labeled cells were incubated with 50:3 μg/mL of FE-Pro. Splenocytes and CD34+ cells were stained with DAB-enhanced Prussian blue. Scale bar represents 10 μm.
Representative Prussian blue-stained FE-Pro-labeled cells. (A) Unlabeled LADMACs, (B) labeled LADMACs, (C) unlabeled splenocytes, (D) labeled splenocytes, (E) unlabeled CD34+ cells, (F) labeled CD34+ cells, (G) unlabeled mesenchymal stem cells × 40, and (H) labeled mesenchymal stem cells. All labeled cells were incubated with 50:3 μg/mL of FE-Pro. Splenocytes and CD34+ cells were stained with DAB-enhanced Prussian blue. Scale bar represents 10 μm.
Differentiation of labeled and unlabeled MSCs. (A) MSC-unlabeled adipogenic changes, (B) MSC-labeled adipogenic changes (note the blue dot of PB-positive iron [arrows]), (C) glycosaminoglycans in unlabeled MSCs, (D) glycosaminoglycans in labeled MSCs, (E) collagen II-positive (brown) in labeled MSCs, and (F) Prussian blue in the consecutive section of labeled MSCs.
Differentiation of labeled and unlabeled MSCs. (A) MSC-unlabeled adipogenic changes, (B) MSC-labeled adipogenic changes (note the blue dot of PB-positive iron [arrows]), (C) glycosaminoglycans in unlabeled MSCs, (D) glycosaminoglycans in labeled MSCs, (E) collagen II-positive (brown) in labeled MSCs, and (F) Prussian blue in the consecutive section of labeled MSCs.
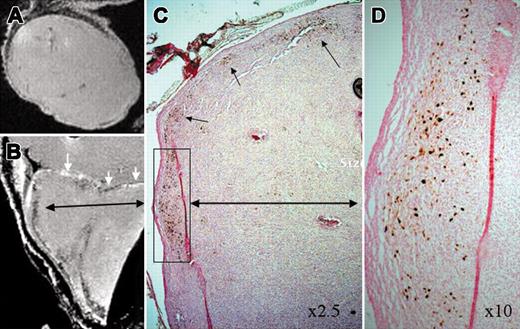
MRI of labeled CD34+cells in tumors. (A) Magnetic resonance imaging (MRI) of tumor with unlabeled CD34+ cells, (B) MRI of tumor with labeled CD 34+ cells, (C) DAB-enhanced Prussian blue staining of tumor with labeled cells. Note low signal intensity along the margin of the tumor, which corresponds with the iron-positive cells on Prussian blue staining. (D) Enlarged inset from panel C, original magnification × 10. White arrows indicate the margin of the tumor and black arrows indicate the accumulation of iron-positive cells.
MRI of labeled CD34+cells in tumors. (A) Magnetic resonance imaging (MRI) of tumor with unlabeled CD34+ cells, (B) MRI of tumor with labeled CD 34+ cells, (C) DAB-enhanced Prussian blue staining of tumor with labeled cells. Note low signal intensity along the margin of the tumor, which corresponds with the iron-positive cells on Prussian blue staining. (D) Enlarged inset from panel C, original magnification × 10. White arrows indicate the margin of the tumor and black arrows indicate the accumulation of iron-positive cells.
Determination of mean iron concentration per cell
Ferumoxides (100 μg/mL) were mixed with different concentrations (3-18 μg/mL) of protamine sulfate and incubated with cells overnight to determine the intracellular iron concentration in relation to the concentration of protamine sulfate and incubation times. Final concentration of ferumoxides was 50 μg/mL and final concentrations of protamine sulfate varied from 1.5 to 9 μg/mL. After labeling, cells were washed twice with PBS and a specific number of unlabeled and labeled cells were collected, centrifuged, and resuspended homogeneously in 0.5 mL of 8% gelatin at 60°C and then chilled at 3°C to allow the suspensions to solidify for NMR relaxometry and quantification of the average iron concentration per cell. Cell suspensions in gelatin with known cell density were first dried at 110°C overnight and then completely digested in a mixture (500 μL) of perchloric and nitric acid at a 3:1 ratio. The samples were digested for at least 3 hours at 60°C using a heating block. For these 500-μL samples, the NMR relaxation rates 1/T1 and 1/T2 (seconds-1) were measured at room temperature at 1.0 T as previously described.7,10,16 Iron concentration in the sample was calculated from a standard curve that was derived from calibration standards of ferumoxides containing 0 to 1 mM Fe in the same acid mixture. Iron concentration was expressed as an average pg iron/cell. There were at least 3 samples for each condition at different time points.
MRI of labeled cells
For MRI of the migration and homing of FE-Pro-labeled CD34+ cells in a xenografted mice tumor model, 2 × 106 FE-Pro-labeled CD34+ cells were injected intravenously through the tail vein soon after subcutaneous (flank) implantation of 1 × 106 rat glioma cells in 4 mice. When the tumor diameter reached 1 cm (usually 14-15 days after implant), the mice were killed and perfused with 4% paraformaldehyde according to the approved Animal Care and Use Committee (ACUC) protocol of Clinical Center at the National Institutes of Health (NIH). The tumors along with surrounding tissues were excised and collected for ex vivo MRI using a 7-tesla, 21-cm horizontal bore MR unit (Bruker, Billerica, MA) with 39-G/cm gradient and 35-mm transmit-receive birdcage volume coil. Fixed tumors were imaged in fomblin perfluoropolyether (Ausimont, Thorofare, NJ). Three-dimensional (3D) gradient echo (GRE) images were obtained using TR = 280 ms, TE = 5.3 ms, number of excitation (NEX) = 4 ms, 512 × 256 × 256 matrix with a field of view (FOV) of 3.2 × 2.1 × 2.1 cm, which produced an image resolution of 80 × 80 × 62 to 90 × 90 × 68 μm. Total time required to acquire the high-resolution 3D images was 20 hours. After MRI, part of the tumor was subjected to histopathology for Prussian blue staining to detect iron-labeled cells. For controls, unlabeled cells were also injected in 4 tumor-implanted mice. Three tumors from mice administered with labeled CD34+ cells and 2 tumors from mice administered with unlabeled CD34+ cells were subjected to MRI.
Data analysis
Data are expressed as mean ± SD. MTT-based toxicity or proliferation data are expressed as percentage of average values of the corresponding control unlabeled cells, and significant test was performed by analysis of variance (ANOVA; Statview 4.51; Abacus Concept, Berkeley, CA) followed by a posthoc test (Fisher projected least significant difference [PLSD]). A P value of less than .05 was considered significant.
Results
Biophysical properties of FE-Pro complex
Similar to other commercially available transfection agents, protamine sulfate has a measured zeta potential of 7.07 ± 0.01 mV. Addition of increasing concentrations of protamine sulfate from 1 to 20 μg/mL to a fixed concentration of ferumoxides (100 μg/mL) resulted in a gradual increase in the FE-Pro zeta potentials from -32.24 ± 0.67 mV (ferumoxides alone) to 20.26 ± 0.34 mV (Table 1). When protamine sulfate was complexed to ferumoxides at increasing concentrations in the media there was a remarkable significant shortening (P ≤ .01) of the 1/T1 relaxation rate from 6.64 ± 0.53 second-1 for ferumoxides alone to 2.34 ± 0.53 second-1 at an FE-Pro ratio of 100:5 μg/mL with a return to the baseline relaxation rate when the concentration of protamine sulfate was at least 8 μg/mL. Protamine sulfate binding to ferumoxides had a greater effect of shortening the 1/T2 relaxation rate when the FE-Pro ratio was 100:5 μg/mL with an increase above baseline at concentrations of protamine sulfate of at least 8μg/mL in solution. This increase in 1/T2 is currently under investigation. These results indicate an electrostatic interaction between the carboxyl groups on the dextran-coated SPIO nanoparticle and the polycation protamine sulfate and coating by protamine sulfate of the paramagnetic sites on the surface of the iron core.
Protamine sulfate and FE-Pro complex on proliferative capacity of cells
There was essentially no significant loss of proliferative activity on MTT assay for HSCs, splenocytes, and LADMACs when incubated with protamine sulfate at concentrations up to 50 μg/mL for 24 and 72 hours (Tables 2-3). Proliferative activity of FE-Pro-labeled (overnight incubation) cells at various ratios of ferumoxides (100 μg/mL) to protamine sulfate (3-8 μg/mL) showed no significant changes in the MTT proliferative assay compared with that of corresponding unlabeled cells at different days after labeling (Tables 2-3). Trypan blue dye exclusion test showed no significant increase in cell death compared with that of corresponding control cells for all cell types at the end of overnight incubation (data not shown).
Labeling efficiency of FE-Pro and iron content per cell
All cell lines evaluated demonstrated approximately 100% labeling efficiency with FE-Pro at ratios of ferumoxides 100 μg/mL to protamine sulfate 3 to 9 μg/mL (Figure 1). The average iron content per cell following FE-Pro labeling of cells varied from 1.47 ± 0.03 pg iron/cell for splenocytes to 17.90 ± 1.85 pg iron/cell for macrophages, with variable endosomal capture of FE-Pro complex depending on the incubation time, cell membrane surface area, and whether cells were grown in suspension or adherent to culture plate (Table 4). There was also iron, ranging from 0.05 to 0.4 pg/cell, seen in unlabeled cells depending on the cell types, culture conditions, and technique to acquire or isolate cell lines. However, the labeling technique always showed at least 5 times increased iron concentration in the labeled cells compared with that of control unlabeled cells.
Long-term toxicity and differential capacity
There were no increases in ROS production observed in the labeled LADMACs, MSCs, and mouse macrophages at different time points (day 1 to day 28) when compared with unlabeled cells. Similarly, there were no significant differences between labeled and unlabeled control cells in the rate of apoptosis for LADMACs and splenocytes (data not shown). Both labeled and unlabeled MSCs showed similar differentiation to adipogenic and chondrogenic lineages when grown in culture with appropriate growth factors (Figure 2). There was also similar osteogenic differentiation, determined by measuring intracellular calcium using a method recommended by the supplier of MSCs. The values of intracellular calcium were as follows: control undifferentiated, 4.87 ± 0.04 mg/dL; unlabeled differentiated, 6.33 ± 0.21 mg/dL; labeled differentiated, 6.70 ± 0.46 mg/dL. Differentiated cells (both labeled and unlabeled) showed significant increased intracellular calcium (P < .001) compared with that of control undifferentiated cells, however, no significant difference was observed between the labeled and unlabeled differentiated cells (n = 6).
Phenotypic analysis of mouse splenocytes and human hematopoietic CD34+ cells showed no significant changes between the labeled and unlabeled cells at different time points (Tables 5-6). Although there were increased numbers of IL-2 receptor-positive cells on day 8 in the labeled splenocytes compared with unlabeled cells, the number of possible activated T lymphocytes (CD11c) remained the same.
Tracking of labeled CD34+ cells in mouse tumor
Ex vivo MR imaging of mouse tumor (1 cm diameter) with or without intravenous administration of labeled CD34+ cells and corresponding PB staining are shown in Figure 3. MR images showed areas of low signal intensities in the tumor, which are the active site of iron-positive CD34+ migration and homing, and similar low signal intensity areas were not observed within tumors in control mice. DAB-enhanced Prussian blue staining showed multiple iron-positive cells in tumors of mice injected with labeled CD34+ cells. Although quantitative analysis of the signal intensity changes of the tumors (with labeled and unlabeled cells) was not attempted, there was a general consensus that all the tumors with administered labeled CD34+ cells showed low signal intensity areas along the periphery of the tumors, which corresponds with PB-positive cells. Current high-resolution 3D image protocol using an FOV of 3.2 × 2.1 × 2.1 cm needed about 20 hours for image acquisition; however, 2D images with satisfactory resolution can be obtained in 20 minutes.
Discussion
Two commercially available, FDA-approved agents, ferumoxides and protamine sulfate, can be combined to create stable complexed nanoparticles capable of effective labeling of cells through endosomal capture. Neither protamine sulfate alone nor in combination with ferumoxides impaired the cellular viability, proliferative capacity, apoptotic rate, ROS production, activation, phenotypic surface marker expression, or capacity to differentiate along various lineages.
Ferumoxides are highly negatively charged SPIO nanoparticles that do not adhere to the cell membrane without modification of the nanoparticle surface charges. Polycationic TAs bind to the dextran coat through electrostatic interactions, thereby modifying the ferumoxides' distribution of positive and negative surface charges that can adhere to the cell membrane. The ability to complex TAs to ferumoxides has been well studied9,10,15 and the effect on the NMR relaxation properties of ferumoxides indicates that TAs coat the surface of the nanoparticles interfering with the ability of water protons to relax faster as a result of interactions with the paramagnetic surface iron.9,10,15 Depending on the size and zeta potential of the TAs, cross-linking can occur between TAs and multiple ferumoxides crystals, transforming these nanoparticles into large clusters observable using light microscopy.
We have previously used poly-L-lysine complexed to ferumoxides to magnetically label stem cells and other mammalian cells with no demonstrable short- or long-term toxicity when the ratio of FE-PLL mixed in culture media is controlled.11,16 The incubation time of PLL with ferumoxides affects the growth and size of the FE-PLL complex, ultimately forming macroscopically visible particles that usually do not get incorporated into the endosomes in cells. Sorgi et al28 have shown that protamine sulfate is superior to PLL for making compact and suitably sized DNA for transfection. We have found that depending on the ratio of ferumoxides to protamine sulfate, microscopically visible particles can be observed. There is a possibility that as the size of the FE-PLL particle increases, the potential for overestimation of the total amount of iron per cell due to adherence of complexes to cell membrane surfaces without incorporation into endosomes also increases. The current method, using protamine sulfate as the transfection agent, showed very clean labeling with minimum extracellular iron complexes and incorporated iron remaining at stable levels despite use of a higher ratio of protamine sulfate to ferumoxides (Table 4). Of note, heparin sulfate will compete with ferumoxides for the complexing to protamine sulfate; therefore, addition of heparin to cell washes after labeling can completely dissolve surface-bound microscopic particles of FE-Pro (G.T.Y., experimental observation). Protamine sulfate has the additional advantage of being a clinical-grade reagent, while PLL is available only as a laboratory-grade reagent and is not FDA approved for human use. For translation of an ex vivo cell labeling method from bench-to-bedside, it would be highly desirable to use agents that are FDA approved for in vivo human use, thereby meeting the highest possible standards for reagent safety, potency, and purity.
Magnetic labeling of cells with FE-Pro complexes is comparable or superior to other FE-TA complexes.10,24 Ratios of ferumoxides to protamine sulfate of 50:1.5 μg/mL to 50:4.5 μg/mL resulted in iron incorporation into cells similar to quantities incorporated after labeling with FE-PLL,10,16 and when FE-Pro-labeled cells were used in conjunction with MRI, they displayed similar properties within tissues as FE-PLL-labeled cells. Of note, suspension cells were efficiently labeled with FE-Pro and appeared to reach threshold of uptake of iron (Table 4). The FE-Pro labeling is similarly effective to that of FE-PLL labeling in tracking the cells' migration to tumors.11
In conclusion, the combination of 2 commercially available, FDA-approved agents, ferumoxides and protamine sulfate, was used to effectively label a variety of cells with no short- or long-term effects on cell viability, proliferation, and differentiation. Clinical experience with use of both agents should allow translation of this method from the experimental setting to clinical trials, after review and approval of clinical protocols by appropriate institutional review boards and regulatory bodies. Magnetic labeling of cells with FE-Pro holds promise for monitoring the temporal and spatial migration of stem cells and other cells into tissues and is likely to enhance the development of cell-based strategies for the repair or replacement of tissues and other novel therapies.
Prepublished online as Blood First Edition Paper, April 20, 2004; DOI 10.1182/blood-2004-02-0655.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 2. Differentiation of labeled and unlabeled MSCs. (A) MSC-unlabeled adipogenic changes, (B) MSC-labeled adipogenic changes (note the blue dot of PB-positive iron [arrows]), (C) glycosaminoglycans in unlabeled MSCs, (D) glycosaminoglycans in labeled MSCs, (E) collagen II-positive (brown) in labeled MSCs, and (F) Prussian blue in the consecutive section of labeled MSCs.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/4/10.1182_blood-2004-02-0655/6/m_zh80160465240002.jpeg?Expires=1763545494&Signature=Yv8OnwQUAuU4R9RxmKuqdtW1jNZmNxZGgfUa6XIMZJR~zuxjb-M4zGulNIfnm29xS0-n91vVwO9zcikeuAZpg2pnsCbFInRe73IIb8Zb1WMS9FietMP4HNyRVcM7XRm4k~43350wIOBs6uKjb8e9Y4i3m7H4Im0vvt6UDhnW-BI3MiI7kIyeEQF0cYin4WRLkLpLluw4NbDDazSuhQ5K0uUaATRYRqe4oqt0FqiHmlckPD2da769pdBOzaQimf97yt-LbYbKqilFy6NUGcp3gsTpV2w8Op9RoTzkAU-rrmv9AL2RfyBBA1vAdCIEX7XCERPoWF2pGs8IXjntR1m-7Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)