Abstract
The events that regulate engraftment and long-term repopulating ability of hematopoietic stem cells (HSCs) after transplantation are not well defined. We report for the first time that major histocompatibility complex (MHC) class I K plays a critical role in HSC engraftment via interaction with recipient natural killer (NK) cells. Durable engraftment of purified HSCs requires MHC class I K matching between HSC donor and recipient. In the absence of MHC class I K matching, HSCs exhibit impaired long-term engraftment (P = .01). Dependence on MHC class I K matching is eliminated in B6 beige mice that lack NK cell function, as well as in wild-type mice depleted of NK cells, implicating a possible regulatory role of NK cells for HSC engraftment. The coadministration of CD8+/T-cell receptor–negative (TCR-) graft facilitating cells (FCs) matched at MHC class I K to the HSC donor overcomes the requirement for MHC class I K matching between HSCs and recipient. These data demonstrate that FCs inhibit NK cell effects on the HSCs. Notably, FCs do not suppress the cytotoxic activity of activated NK cells. Enhanced green fluorescent protein–positive (EGFP+) FCs persist for one month following allogeneic transplantation, making cold target inhibition an unlikely mechanism. Therefore, MHC class I may play a critical role in the initiating events that dictate HSC engraftment and/or NK-mediated rejection following allogeneic transplantation.
Introduction
Hematopoietic stem cells (HSCs) are responsible for the continuous generation of lineage-committed progenitor cells, which in turn give rise to mature blood cells. Successful bone marrow transplantation requires that HSCs with the ability to self-renew survive in the transplant recipient. After bone marrow transplantation, some HSCs retain self-renewal, while others retain only short-term repopulating potential and terminally differentiate to produce progeny. The mechanism controlling HSC fate has not been defined but is clearly influenced by the hematopoietic microenvironment. In the present studies, we have evaluated the influence of the major histocompatibility complex (MHC) on HSC engraftment.
Transplantation of purified HSCs across MHC barriers encounters greater resistance than whole bone marrow cells.1,2 If the HSC donor and recipient are MHC matched, irrespective of minor antigen matching, long-term engraftment of HSCs occurs reliably.1,3 In contrast, if donor and recipient are MHC disparate, purified HSCs engraft less readily and much higher cell doses are required. In addition, often only short-term radioprotection is observed, even when syngeneic marrow is coadministered concomitantly with the allogeneic HSCs.3 This graft failure has been attributed by some to natural killer (NK)–mediated rejection.4-6 However, NK cells do not directly reject purified HSCs,7,8 and the fact that a similar outcome is observed in recipients deficient in perforin and granzyme B, as well as those deficient in Fas and Fas ligand, strongly suggests that cytotoxic mechanisms are not responsible for failure of durable HSC engraftment.9-11 The kinetic for graft failure of MHC-disparate purified HSCs also differs significantly from the relatively rapid NK-mediated rejection observed in bone marrow transplants from class I–deficient donors.4,12 NK cell activity is regulated by MHC molecules through activating or inhibiting receptors.1,2 In the mouse, 2 types of receptors have been identified: the Ly49 and the CD94/NKG2 families,13 which are both expressed on the same cell. As a result, NK cells can be cytolytic under certain conditions and inhibitory under others.
We have previously shown that CD8+/T-cell receptor–negative (TCR-) facilitating cells (FCs) enhance engraftment of purified HSCs in allogeneic recipients.1 To function, FCs must be MHC matched with the HSCs. In the present study, we therefore evaluated the role of MHC class I and class II molecules in the engraftment of purified HSCs in allogeneic recipients. We demonstrate for the first time that matching between recipient and donor HSCs at class I K is critical to durable HSC engraftment. Strikingly, mice deficient in production of NK cells or depleted of NK cells in vivo engraft MHC class I K disparate HSCs, implicating recipient NK cells as regulators of early hematopoiesis. Most notably, the addition of FCs matched to the HSCs at class I K overcomes the requirement for MHC class I K matching between HSC donor and recipient. Taken together, these data demonstrate that the MHC class I K molecule influences engraftment of allogeneic HSCs and may contribute to regulation of self-renewal and/or survival via NK interaction. It is likely that in allogeneic HSC transplantation the FC serves as a regulatory cell to inhibit recipient NK cells. An understanding of the cellular interactions that take place early after HSC transplantation will allow development of therapeutic strategies to promote suppression of host-versus-graft reactivity and enhance engraftment.
Materials and methods
Mice
Male (4-5 weeks old) B10.BR, AKR, C57BL/10 (B10), B10.AKM, B10.MBR, B10.A(2R), B10.A(4R), B10.A(5R), B10.D2, C57BL/6 (B6), and B6/beige mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Animals were housed in the barrier animal facility at the Institute for Cellular Therapeutics, University of Louisville (Louisville, KY) and cared for according to National Institutes of Health animal care guidelines.
Antibodies
All of the monoclonal antibodies (mAbs) used in this study were directly conjugated (PharMingen, San Diego, CA). For HSC sorting, the following mAbs were used: stem cell antigen (Sca)–1 phycoerythrin (PE; E13-161.7; rat immunoglobulin G2a [IgG2a]), c-Kit allophycocyanin (APCs; 2B8; IgG2b), CD8α fluorescein isothiocyanate (FITC; 53-6.7; rat IgG2a), Mac-1 FITC (CD11b) (M1/70; IgG2b), B220 FITC (RA3-6B2; rat IgG2a), Gr-1 FITC (RB6-8C5; rat IgG2b), and β-TCR FITC (H57-597; Armenian hamster IgG). Facilitating cells were sorted using β-TCR FITC (H57-597; Armenian hamster IgG); γδ-TCR FITC (GL3; Armenian hamster IgG); and CD8α PE (53-6.7; IgG2a).
H-2Kk FITC (AF3-12.1; mouse IgG1 ), H-2Kb PE (AF6-88.5; mouse IgG2a), H-2Dd PE (34-2-12; mouse IgG2a), H-2Db PE (KH95; mouse IgG2a), CD8α PE (53-6.7; rat IgG2a), CD4 PE (RM4-5; rat IgG2a), β-TCR PE (H57-597; Armenian hamster IgG), NK1.1 PE (PK136; IgG2a), B220 PE (RA3-6B2; rat IgG2a), Gr-1 PE (RB6-8C5; rat IgG2b), and CD11b PE (M1/70; IgG2b) mAbs (from PharMingen) were used for assessment of chimerism.
Anti-NK1.1 (produced in our laboratory) was used for in vivo depletion of NK cells. The antibody was titrated for use, and depletion was confirmed by flow cytometry.
Purification of c-Kit+/Sca-1+/Lin- (KSL) hematopoietic stem cells and facilitating cells (CD8+/TCR-)
KSL HSCs and FCs were isolated from bone marrow by multiparameter, live sterile cell sorting (FACSVantage SE; Becton Dickinson, Mountain-view, CA), as previously described.1 Briefly, whole bone marrow was isolated in a single cell suspension at a concentration of 100 × 106 cells/mL in sterile cell sort media (CSM), composed of sterile 1 × Hanks balanced salt solution without phenol red (GIBCO, Grand Island, NY), 2% heat-inactivated fetal calf serum (FCS; GIBCO), 10 mM/mL HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer (GIBCO), and 30 μg/mL Gentamicin (GIBCO). mAbs were added at saturating concentrations and the cells were incubated for 30 minutes on ice and washed twice, then resuspended in CSM at 2.5 × 106 cells/mL.
Hematopoietic stem cell and/or FC transplantation
Recipient AKR, B10.A(2R), B10.A(4R), B10.A(5R), B10.MBR, C57BL/10, and B10.BR mice were conditioned with 950 cGy total body irradiation (TBI) and reconstituted with 5000 purified HSCs of donor B10.BR or B10.AKM mice by tail vein injection at least 4 hours after irradiation. For recipients of FCs and HSCs, mixing was performed prior to injection and a single injection was administered.
Assessment of chimerism
Recipients were characterized for allogeneic engraftment using 2-color flow cytometry, as previously described.1 Briefly, whole blood from recipients was collected in heparinized tubes, and aliquots of 100 μL were stained with anti–H-2Kk FITC and/or anti–H-2Kb PE, anti–H-2Dd PE, and anti–H-2Db PE for 30 minutes. Red blood cells were lysed with ammonium chloride lysing buffer for 5 minutes at room temperature, then washed twice and fixed in 1% paraformaldehyde. Multilineage analysis was performed by staining peripheral blood lymphocytes with anti–donor class I mAbs versus lineage-specific mAbs for T cells (β-TCR PE, CD8α PE, CD4 PE); B cells (CD45R/B220 PE); NK cells (NK1.1 PE); granulocytes (Gr-1 PE); and macrophages (CD11b PE).
White blood cell measurement
White blood cell (WBC) counts (number × 106/mL) were determined using the MASCOT Multispecies Hematology Analyzer (CDC Technologies, Oxford, CT) on EDTA (ethylenediaminetetraacetic acid)–treated peripheral blood 6 months after transplantation.
Analysis of the MHC molecules expressed on hematopoietic stem cells
HSCs were sorted and then stained with one of the following class I or class II monoclonal antibodies: H-2Kk FITC, H-2Dk FITC (15-5-5; mouse IgG2a; PharMingen), class II I-Ak FITC (11-5.2; mouse IgG2b; PharMingen), or I-Ek FITC (14-4-4S; mouse IgG2a; PharMingen). Isotype staining served as controls.
Transplantation of purified hematopoietic stem cells into B6/beige or NK cell–depleted allogeneic recipients
NK cells were depleted in recipient animals by administering ascites containing anti-NK1.1 mAb (PK13614 ) intraperitoneally on days -3 and -1. NK cell depletion was assessed by direct and indirect flow cytometric analysis of splenocytes against a variety of NK cell markers.
Activated NK cell (aNK)–specific killing using the JAM test
Bone marrow cells were harvested from C3H (H-2k) mice, and T cells were removed using biotinylated mAbs against β- and γδ-TCR with Dynabeads M-280 Streptavidin (Dynal ASA, Oslo, Norway). The cells were then resuspended at 2 to 4 × 106/mL in RPMI 1640 + 10% fetal bovine serum (FBS; HyClone Laboratories, Logan, UT), 100 U/mL penicillin, 100 μg/mL streptomycin (GIBCO), 2 mM l-glutadmin (GIBCO), 10 mM HEPES (GIBCO), 1 mM sodium pyruvate (GIBCO), and 10-5 2-mecaptoethanol (GIBCO), and cultured in the presence of 1000 U/mL recombinant human (rhu) interleukin-2 (IL-2; Genzyme, Cambridge, MA) for 6 days at 37°C 5% CO2. The target YAC-1 cells were labeled with 5 μCi/mL (0.185 MBq/mL) [3H]-thymidine (Life Sciences, Boston, MA) and incubated in a humidified atmosphere with 5% CO2 at 37°C for 12 to 16 hours. Excess [3H]-thymidine was removed by washing, and 5 × 103 labeled YAC-1 target cells were mixed with varying numbers of NK cells in 96-well round-bottom plates (Becton Dickinson Labware, Franklin Lakes, NJ). The plates were incubated at 37°C for 4.5 hours, cells were harvested on the filter, and radioactivity was counted in a beta counter (Financial Services, Doylestown, PA). The mean percentage of specific killing was calculated from triplicate culture using the following formula: % specific killing = (S - E)/S × 100, where S is retained DNA in the absence of killers (spontaneous) and E is experimentally retained DNA in the presence of killers. NK cells (5 × 105) were incubated in the absence or presence of increasing numbers of sorted FCs or T cells from B6 mice at 37°C for 16 hours. After incubation, 100 μL supernatant was removed from each well and 100 μL media containing 5 × 103 YAC-1 cells was added. The plates were incubated at 37°C for 4.5 hours, cells were harvested on the filter, and radioactivity was counted in a beta counter.
Statistical analysis
Experimental data were evaluated for significant differences using the Independent-Samples test; P < .05 was considered significant. Graft survival was calculated according to the Kaplan-Meier method.
Results
MHC classIKis brightly expressed on HSCs
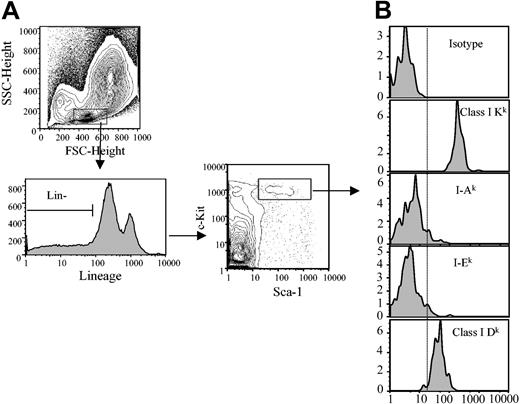
Different loci of the MHC encode 2 general types of antigens: class I and class II. In the mouse, the MHC or H-2 consists of 9 genetic loci: class I composed of K, D, and L; and class II composed of I-A and I-E.15 We first analyzed the level of expression of MHC class I K and D molecules on HSCs (Figure 1). ClassIKis expressed with very high intensity, while class I D is intermediate in intensity on HSCs. In contrast, MHC class II markers, I-A and I-E, are only weakly expressed on HSCs.
Analysis of the MHC class I and class II molecule expression on HSCs. (A) B10.BR bone marrow cells were stained (Sca-1+/c-Kit+/Lin-) and gated for HSCs as lineage marker negative (lower left panel) and Sca-1+/c-Kit+ (lower right panel). (B) Expression of class I or class II MHC markers on HSCs was determined. Vertical dashed line indicates negative staining from isotype control. A minimum of 500 000 total events was collected for each histogram. FSC indicates forward scatter; SSC, side scatter.
Analysis of the MHC class I and class II molecule expression on HSCs. (A) B10.BR bone marrow cells were stained (Sca-1+/c-Kit+/Lin-) and gated for HSCs as lineage marker negative (lower left panel) and Sca-1+/c-Kit+ (lower right panel). (B) Expression of class I or class II MHC markers on HSCs was determined. Vertical dashed line indicates negative staining from isotype control. A minimum of 500 000 total events was collected for each histogram. FSC indicates forward scatter; SSC, side scatter.
Matching at MHC classIKis important to HSC engraftment
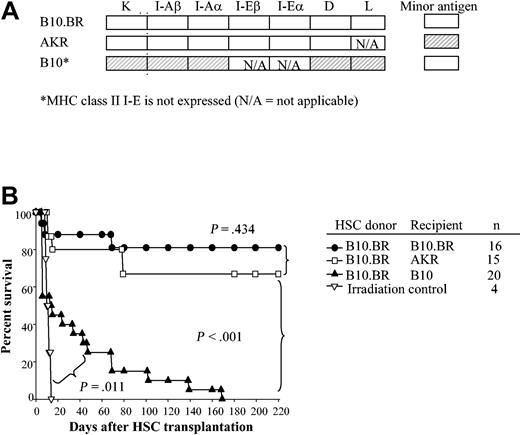
To determine which MHC molecules are important to engraftment of HSCs, recipient B10.BR, AKR, C57BL/10, B10.A(2R), B10.A(4R), B10.A(5R), B10.AKM, and B10.MBR mice were conditioned with 950 cGy and received transplants of 5000 Sca-1+/c-Kit+/Lin- HSCs from B10.BR donors. The MHC pedigree of these mouse strains is displayed in Table 1, and the disparities between donor and recipient are shaded (Figure 2A). As expected, mice matched at MHC (Figure 2A-B, B10.BR → B10.BR; B10.BR → AKR) exhibited durable engraftment. In contrast, HSCs provided only short-term radioprotection in MHC-disparate allogeneic recipients (Figure 2A-B, B10.BR → B10) (P < .001). However, engraftment of allogeneic HSCs alone was significantly prolonged compared with irradiation controls (Figure 2B, P = .01).
HSCs engraft long term in MHC-matched but not MHC-disparate recipients. (A) Degree of MHC matching between donor and recipient is shown. The shading denotes the genetic disparity between B10.BR HSC donor and recipients (□ indicates k haplotype; ▨, b haplotype). (B) Kaplan-Meier survival curves of recipients of 5000 syngeneic HSCs (B10.BR → B10.BR); MHC-matched but minor antigen–mismatched (B10.BR → AKR); and MHC-disparate but minor antigen–matched (B10.BR → C57BL/10) HSCs following conditioning with 950 cGy TBI. P values denote significance estimates.
HSCs engraft long term in MHC-matched but not MHC-disparate recipients. (A) Degree of MHC matching between donor and recipient is shown. The shading denotes the genetic disparity between B10.BR HSC donor and recipients (□ indicates k haplotype; ▨, b haplotype). (B) Kaplan-Meier survival curves of recipients of 5000 syngeneic HSCs (B10.BR → B10.BR); MHC-matched but minor antigen–mismatched (B10.BR → AKR); and MHC-disparate but minor antigen–matched (B10.BR → C57BL/10) HSCs following conditioning with 950 cGy TBI. P values denote significance estimates.
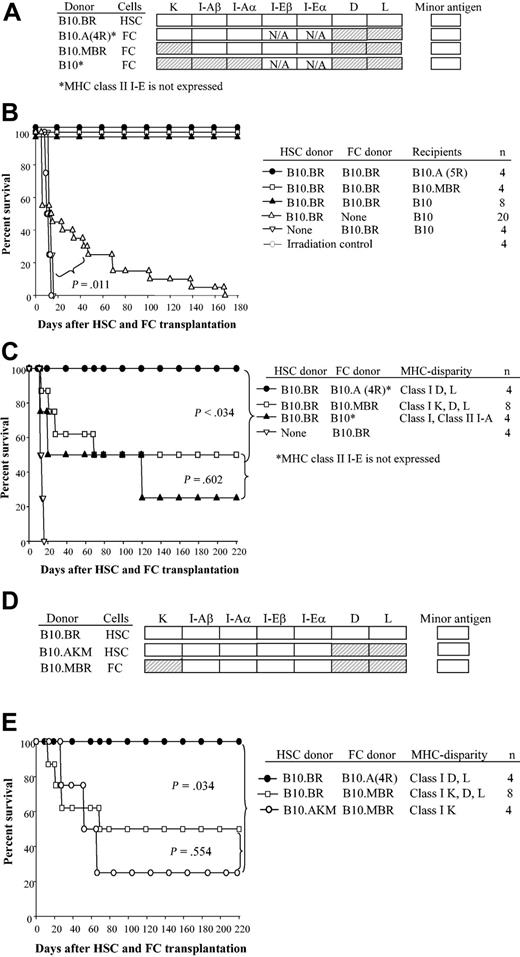
In order to define which MHC molecules were important to long-term HSC engraftment, transplantations were performed with specific loci mismatched between HSC donor and recipient. Figure 3A indicates the disparities between donor and recipient as shaded boxes. Transplantation of class I K, D, and L disparate HSCs offered early radioprotection compared with radiation controls, but recipients expired from late graft failure up to 180 days following transplantation. For example, only 3 (25%) of 12 recipients in which the HSC was mismatched with the recipient at class I K, D, and L and class II I-A survived up to 180 days (Figure 3B, B10.BR → B10.A[5R]) and only 1 (14%) of 7 recipients of HSCs disparate at class I K, D, and L (Figure 3B, B10.BR → B10.MBR) engrafted. In striking contrast, 100% and 83% of HSCs durably engrafted when recipients were matched at class I K but disparate at class I D and L, with or without class II I-E expression, respectively (Figure 3B, B10.BR → B10.A[2R]; B10.BR → B10.A[4R]). Class II I-E is not expressed in B10.A(4R) mice.15,17 Taken together, these data indicate that neither matching at MHC class I D and L nor expression of I-E is essential for durable HSC engraftment.
Matching at the class I K locus between HSCs and recipient is critical for long-term self-renewal. HSCs (5000) were sorted from donors and transplanted into recipients that were disparate at selected MHC loci. (A) Shading shows the MHC disparity relative to B10.BR. (B) Survival curves of recipients disparate at class I D and L (B10.A[2R]); class I D and L with no class II I-E expression (B10.A[4R]), class I K, D, and L and class II I-A (B10.A[5R]), and class I K plus D and L (B10.MBR). (C) Survival of recipients of HSCs disparate at class I K plus class I D and L (B10.BR → B10.MBR) versus class I K only (B10.AKM → B10.MBR).
Matching at the class I K locus between HSCs and recipient is critical for long-term self-renewal. HSCs (5000) were sorted from donors and transplanted into recipients that were disparate at selected MHC loci. (A) Shading shows the MHC disparity relative to B10.BR. (B) Survival curves of recipients disparate at class I D and L (B10.A[2R]); class I D and L with no class II I-E expression (B10.A[4R]), class I K, D, and L and class II I-A (B10.A[5R]), and class I K plus D and L (B10.MBR). (C) Survival of recipients of HSCs disparate at class I K plus class I D and L (B10.BR → B10.MBR) versus class I K only (B10.AKM → B10.MBR).
We next focused on class I K. If the recipient mice and donor HSCs were mismatched at class I K, D, and L plus class II I-A or at class I K plus class I D and L, engraftment of HSCs was significantly impaired (Figure 3B, B10.BR → B10.A[5R]; Figure 3B, B10.BR → B10.MBR: P < .001). To define whether matching at class I K was truly critical, we used a strain combination disparate only at class I K and congenic for all other MHC loci. HSCs from B10.AKM donors were transplanted into B10.MBR recipients (Figure 3C). Just as the previous strain combination with mismatch at class I K failed, long-term engraftment was significantly impaired when only class I K was disparate (P < .001), while short-term repopulation was achieved as reflected by a significantly prolonged survival compared with radiation controls. Taken together, these data show that matching at the MHC class I K molecule is critical for durable engraftment of purified HSCs. That is, purified donor HSC and recipient mice must be matched at class I K, but no other locus, for long-term engraftment to occur readily.
Facilitating cells matched to the HSC donor replace the requirement for class I K matching between HSCs and recipient
Facilitating cells are a rare CD8+/TCR- cell population in bone marrow that enhances engraftment of purified HSCs in allogeneic recipients. The biologic effect occurs only when FCs and HSCs are MHC matched.1,2,18 FCs express a unique 33-kDa protein (FCp33) associated with a TCR-β chain homodimer that has been hypothesized to mediate HSC/FC recognition.18 To define the mechanism of action of FC-mediated HSC engraftment, HSCs and FCs obtained from mice congenic at specific MHC loci were transplanted into MHC-disparate recipients (Figure 4A). First, as a control to demonstrate the effectiveness of FCs in the chosen strain combinations, 5000 HSCs plus 30 000 FCs from B10.BR donors were transplanted into ablated recipients disparate at both class I K, D, and L and class II (B10.A[5R]); class I K, D, and L (B10.MBR); and fully MHC disparate (B10) (Figure 4B). As expected, all recipients engrafted and exhibited durable chimerism 180 days or more after transplantation. Control recipients of B10.BR FCs alone expired at the time of radiation controls (median survival time [MST] = 14 days) (Figure 4B).
FCs matched to HSCs at class I K enable engraftment in allogeneic recipients. HSCs (5000) and FCs (30 000) were sorted from donors disparate at the indicated MHC loci, mixed, and transplanted into allogeneic B10 recipients. (A) Shading shows the MHC disparity between FC donor and HSC donor. (B) Survival of recipients of HSCs plus FCs or FCs alone from B10.BR. (C) Survival of recipients given B10.BR HSCs (H-2k) plus FCs from B10.A(4R) donors (MHC disparate to HSCs at class I D and L and also lacking class II I-E expression), from B10.MBR donors (MHC disparate to HSCs at class I K, D, and L), and from H-2 disparate C57BL/10 (H-2b) donors. (D) MHC disparity between HSC and FC donors used in experiments shown in panel E. (E) Survival curve of B10 recipients given HSCs and FCs that were mismatched only at class I K (B10.AKM HSCs plus B10.MBR FCs).
FCs matched to HSCs at class I K enable engraftment in allogeneic recipients. HSCs (5000) and FCs (30 000) were sorted from donors disparate at the indicated MHC loci, mixed, and transplanted into allogeneic B10 recipients. (A) Shading shows the MHC disparity between FC donor and HSC donor. (B) Survival of recipients of HSCs plus FCs or FCs alone from B10.BR. (C) Survival of recipients given B10.BR HSCs (H-2k) plus FCs from B10.A(4R) donors (MHC disparate to HSCs at class I D and L and also lacking class II I-E expression), from B10.MBR donors (MHC disparate to HSCs at class I K, D, and L), and from H-2 disparate C57BL/10 (H-2b) donors. (D) MHC disparity between HSC and FC donors used in experiments shown in panel E. (E) Survival curve of B10 recipients given HSCs and FCs that were mismatched only at class I K (B10.AKM HSCs plus B10.MBR FCs).
We next tested which MHC loci must be matched between FCs and HSCs for facilitation to occur. FCs and HSCs were sorted from donors matched only at indicated MHC loci (Figure 4A). Fully MHC-disparate recipient B10 mice were conditioned with 950 cGy TBI and received transplants of 5000 HSCs from B10.BR mice and 30 000 FCs from either B10.A(4R), B10.MBR, or B10 mice. When HSCs and FCs were MHC disparate (Figure 4C, B10.BR HSCs + B10 FCs → B10) or disparate only at the class I K, D, and L locus (Figure 4C, B10.BR HSCs + B10.MBR FCs → B10), 2 (50%) of 4 or 5 (62%) of 8 animals engrafted, respectively. When the HSCs and FCs were disparate at only class I D and L (Figure 4C, B10.BR HSCs + B10.A[4R] FCs → B10), facilitation resulted in mismatched recipients as evidenced by durable engraftment. In striking contrast, when FCs and HSCs were disparate at only class I K, long-term engraftment was impaired in allogeneic recipients (Figure 4D-E, B10.AKM HSCs + B10.MBR FCs → B10). Thus, long-term engraftment is most efficient in transplantations for which HSCs and FCs are matched at class I K, and the coadministration of FCs can overcome the requirement for class I matching between HSC donor and normal recipients. Unfortunately, mice congenic only at class I K are not available.
Recipients of HSCs that engrafted were monitored for the level and durability of donor chimerism over time (Figure 5A). The absolute WBC count was higher in animals with MHC or class I K matching between FCs and HSCs compared with recipients of FCs and HSCs mismatched at class I K (Figure 5B). This pattern showed that true alloengraftment had occurred and that it depended upon class I K compatible FCs and HSCs.
Assessment of chimerism and WBC counts. (A) The percentage donor chimerism in the peripheral blood over time for each of the groups shown in Figure 4C and 4E. The number of animals surviving is shown at the top of each bar. (B) WBC counts in recipients after 180 days. Error bars indicate standard deviation.
Assessment of chimerism and WBC counts. (A) The percentage donor chimerism in the peripheral blood over time for each of the groups shown in Figure 4C and 4E. The number of animals surviving is shown at the top of each bar. (B) WBC counts in recipients after 180 days. Error bars indicate standard deviation.
Mice deficient in NK cells more readily accept purified allogeneic HSCs
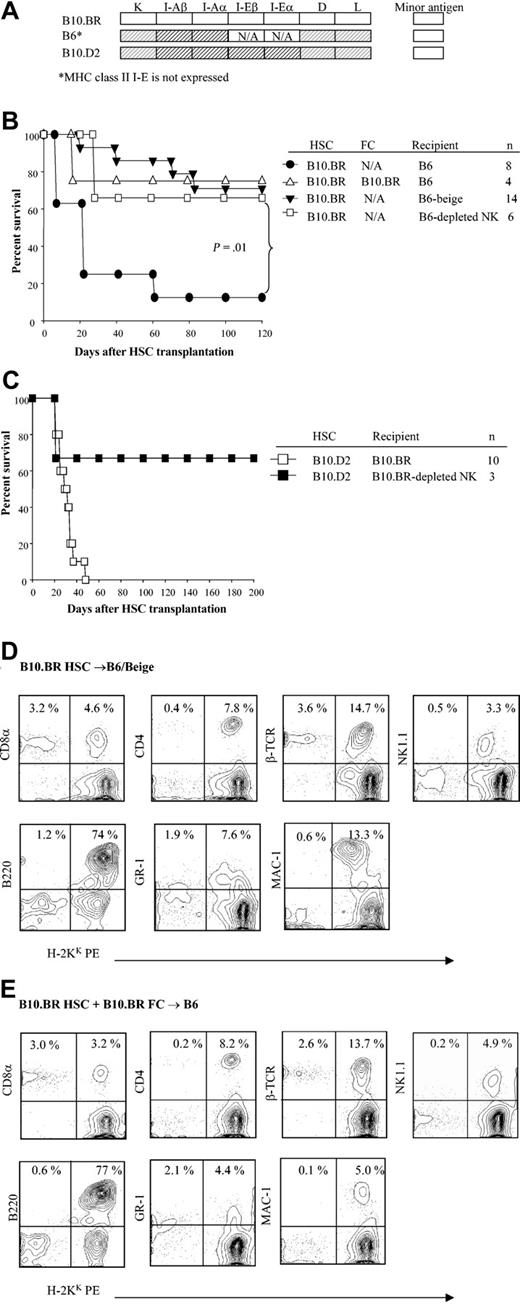
We next examined whether recipient NK cells played a role regulating HSC engraftment using B6/Beige recipients that have a severe deficiency in NK cell and cytolytic T-cell function. Purified B10.BR HSCs were administered to B6/Beige recipients conditioned with 950 cGy TBI with or without B10.BR facilitating cells. In striking contrast with wild-type recipients, facilitating cells were not required for engraftment of allogeneic HSCs to occur in B6/Beige recipients (Figure 6A-B; P = .01). The chimerism established was durable and multilineage (Figure 6D-E). In order to distinguish the role of host NK cells and class I–dependent CD8+ T cells in rejection of donor HSCs, wild-type B6 and B10.BR mice were specifically depleted in vivo of NK cells also using anti-NK1.1 mAb treatment before transplantation. Evaluation of the marrow and spleen of anti-NK1.1–treated mice determined that NK cells, but not CD4+ or CD8+ cells, were depleted from the recipients prior to HSC transplantation (data not shown). As shown in Figure 6, removal of NK cells overcame the requirement for class I K matching between HSCs and recipient in different strain combinations (B10.BR → B6; B10.D2 → B10.BR).
The role of NK cells in engraftment of purified HSCs. (A) Shading represents MHC disparity between donor and recipients. (B) Kaplan-Meier curves show the survival of B6, B6/Beige, or NK cell–depleted B6 recipients of B10.BR HSCs with or without B10.BR FCs. (C) Survival curve of B10.D2 HSCs into B10.BR or NK cell–depleted B10.BR recipients. (D) Multilineage typing of representative B6/Beige recipients of B10.BR HSCs alone compared with (E) normal B6 recipients of B10.BR HSCs plus B10.BR FCs. Multilineage data are from peripheral blood 3 months after transplantation and analyzed based on the lymphoid gate. Data shown are from one representative recipient. A total of 3 to 4 recipients were analyzed per group.
The role of NK cells in engraftment of purified HSCs. (A) Shading represents MHC disparity between donor and recipients. (B) Kaplan-Meier curves show the survival of B6, B6/Beige, or NK cell–depleted B6 recipients of B10.BR HSCs with or without B10.BR FCs. (C) Survival curve of B10.D2 HSCs into B10.BR or NK cell–depleted B10.BR recipients. (D) Multilineage typing of representative B6/Beige recipients of B10.BR HSCs alone compared with (E) normal B6 recipients of B10.BR HSCs plus B10.BR FCs. Multilineage data are from peripheral blood 3 months after transplantation and analyzed based on the lymphoid gate. Data shown are from one representative recipient. A total of 3 to 4 recipients were analyzed per group.
Facilitating cells do not suppress aNK cytolytic activity in vitro
Since either the inclusion of FCs or the depletion of NK cell function eliminates the requirement for class I K matching between HSCs and recipient, we assessed the ability of purified FCs to directly affect NK cell–mediated cytolysis. To this end, bone marrow was T-cell–depleted and the cells incubated with IL-2 for 6 days to produce aNK. aNK cells were harvested and cultured overnight in the absence and presence of sorted FCs or CD8+ T cells. The NK cytolytic activity against [3H]-thymidine–labeled YAC-1 cells (JAM assay) was then assessed.
As shown in Figure 7A, cultured NK cells exhibit dose-dependent killing of target YAC-1 cells. When either FCs or CD8+ T cells were included in the culture with the NK cells at a 10:100 ratio for 16 hours, no effect on cytolysis was observed (Figure 7B). In addition, FCs did not affect the NK-mediated cytolysis of YAC-1 cells after longer incubation (20 hours) nor of various tumor cell lines including P815 (H-2d) and EL4 (H-2b) or R1.G1 (H-2k) (data not shown). These results indicate that aNK can lyse even H2-matched targets (R1.G1: H-2k) and that the FCs do not function to directly inhibit aNK cell cytolysis in vitro.
FCs do not affect cytolytic activity of NK cells on YAC-1 cells. To evaluate whether FCs affect NK cytolytic activity as a potential mechanism of action, YAC-1 targets were cocultured with NK cells to which varying ratios of FCs or CD8+ T cells were added. This experiment is representative of a total of 3 performed. (A) Cytolytic activity of C3H NK cells was determined by incubating 5 × 103 [3H]-thymidine–labeled YAC-1 cells with increasing numbers of NK cells to determine the optimal effector-target (E/T) ratio for the suppression assays. To determine maximal killing percentage, 2N HCl was added to YAC-1 cells in the absence of NK cells. (B) NK cells (500 000 per well) were preincubated in triplicate with increasing ratios of FCs or CD8+ T cells for 16 hours. After the coculture, 5 × 103 labeled [3H]-labeled YAC-1 target cells (constant effector to target ratio of 100:1) was then added to each well. Function of NK cells incubated in the absence of FCs is indicated by the open bar. FC or T-cell–mediated cytolysis was measured, but no specific killing was detected (n.d. = not detected). Error bars indicate standard deviation.
FCs do not affect cytolytic activity of NK cells on YAC-1 cells. To evaluate whether FCs affect NK cytolytic activity as a potential mechanism of action, YAC-1 targets were cocultured with NK cells to which varying ratios of FCs or CD8+ T cells were added. This experiment is representative of a total of 3 performed. (A) Cytolytic activity of C3H NK cells was determined by incubating 5 × 103 [3H]-thymidine–labeled YAC-1 cells with increasing numbers of NK cells to determine the optimal effector-target (E/T) ratio for the suppression assays. To determine maximal killing percentage, 2N HCl was added to YAC-1 cells in the absence of NK cells. (B) NK cells (500 000 per well) were preincubated in triplicate with increasing ratios of FCs or CD8+ T cells for 16 hours. After the coculture, 5 × 103 labeled [3H]-labeled YAC-1 target cells (constant effector to target ratio of 100:1) was then added to each well. Function of NK cells incubated in the absence of FCs is indicated by the open bar. FC or T-cell–mediated cytolysis was measured, but no specific killing was detected (n.d. = not detected). Error bars indicate standard deviation.
Facilitating cells persist after transplantation in allogeneic recipients
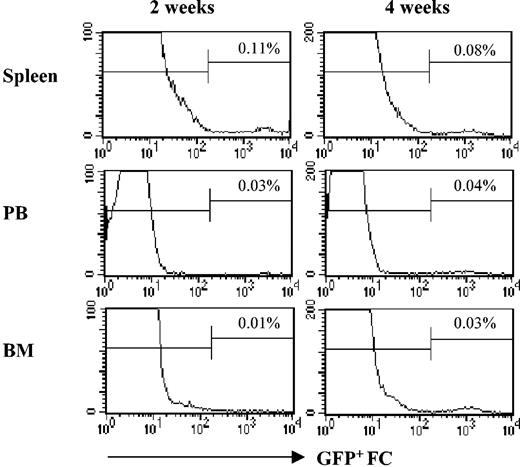
To examine the fate of FCs after transplantation, C3H recipients were conditioned with 950 cGy TBI and received transplants of 5000 B6 HSCs with or without 30 000 enhanced green fluorescent protein–positive (EGFP+) B6 FCs. At 2 and 4 weeks, the peripheral blood, spleen, and bone marrow were analyzed for EGPF+ FCs. At 2 weeks, FCs were detectable in all compartments but the majority were in the spleen (Figure 8). At 4 weeks, FCs were detectable in peripheral blood, spleen, and bone marrow and in greater numbers in the bone marrow compartment compared with 2 weeks (Figure 8).
FCs persist in allogeneic animals after transplantation. C3H mice were conditioned with 950 cGy TBI and received transplants of 5000 KSL HSCs with or without 30 000 EGFP+ FCs. Mice were killed at 2 and 4 weeks, and EGFP+ FCs (CD8+/TCR-) were enumerated in spleen, peripheral blood (PB), and bone marrow. A minimum of 1 × 106 events was analyzed. % reflects percent of GFP+ FCs in lymphoid gate. A minimum of 3 mice were analyzed per time point.
FCs persist in allogeneic animals after transplantation. C3H mice were conditioned with 950 cGy TBI and received transplants of 5000 KSL HSCs with or without 30 000 EGFP+ FCs. Mice were killed at 2 and 4 weeks, and EGFP+ FCs (CD8+/TCR-) were enumerated in spleen, peripheral blood (PB), and bone marrow. A minimum of 1 × 106 events was analyzed. % reflects percent of GFP+ FCs in lymphoid gate. A minimum of 3 mice were analyzed per time point.
Discussion
HSCs are responsible for the continuous production of lineage-committed progenitor cells, which are capable of increasing the production of their progeny dramatically in response to various stimuli, including bone marrow transplantation (BMT).19 Despite the dynamic proliferative nature of HSCs, the incidence of malignant transformation and bone marrow failure is very low, suggesting that HSCs are under tight regulation. There are convincing data to support the fact that all pluripotent HSCs undergo intermittent cycling.20 After transplantation, it is hypothesized that HSCs must enter into cycle after homing into the appropriate niche21 in order to repopulate different lineages.22 Little is known about mechanisms influencing the homing of HSCs to where hematopoiesis will become established, or the changes that take place within the HSC population after they arrive in the new microenvironment. We show for the first time that the MHC class I K contributes to stem cell fate after transplantation in that matching between HSCs and the hematopoietic microenvironment at MHC class I K is required for durable engraftment of HSCs.
The MHC functions as the identification card of an individual, controlling the initiation of specific immunologic responses of T and NK cells and self/nonself discrimination. In the innate immune system, NK cells can detect and be activated by soluble mediators (cytokines, MHC class I heavy chains), as well as through cell-cell contact when MHC class I expression is altered or low.23 Murine HSCs have been reported to express high levels of MHC class I.24-26 A role for this high level expression has not been defined to date. In the present studies, we found that HSCs exhibit very bright expression of MHC class I K and, to a much lesser extent, class I D. Notably, we found that when purified HSCs are transplanted into recipients with an MHC class I K disparity, long-term engraftment is significantly impaired. Strikingly, if the HSC donor is matched to the recipient at class I K, engraftment potential is restored. One could hypothesize that HSCs express high levels of class I in order to avoid elimination by innate immunity and therefore function properly, suggesting a role for NK cells in homeostasis of HSCs. After transplantation, HSCs would theoretically become a target for host NK cells. Our findings that MHC class I K expression is higher on HSCs and that matching at class I K between recipient and donor HSCs is necessary for HSC engraftment suggest a regulatory role for this molecule in NK function. Class I K, or possibly a K-linked gene, appears to be required for durable engraftment after transplantation. The contribution of MHC molecules to the process of HSC engraftment and self-renewal or lineage commitment has not been previously evaluated. The artificial situation in allogeneic transplantation allows these mechanisms to be defined.
Over the past 20 years, NK cells have been shown to play a major role in regulating early engraftment of allogeneic marrow both by activation as well as inhibition of immune responses.27 The rejection of parental hematopoietic cell grafts by NK cells from F1 and MHC-disparate recipients, or hybrid resistance, was first described in the late 1950s.28-37 This barrier can also be overcome by pretreatment of the conditioned recipient with antibody directed against NK cell–associated antigens.38-40 The cytotoxic activity of NK cells is promoted by the absence of matching at MHC class I molecules.41,42 Rejection of MHC class I–deficient marrow occurs rapidly in syngeneic wild-type recipients, usually by 8 to 16 days4 (and our own unpublished observations: Y.H., F.R., P.M.C., S.T.I., Hong Xu, Isabelle Fugier, and Carrie Schanie, October 2002). In contrast, bone marrow from class II–deficient donors behaves in a fashion similar to that for normal bone marrow donors.6 The presence of the class I molecule on the HSCs is hypothesized to offer it partial protection from NK cell attack, while syngeneic class I molecules provide full protection from NK cell–mediated rejection of bone marrow.
To evaluate whether NK cells were involved in regulating HSC engraftment, we performed allogeneic HSC transplantations using B6 beige mice deficient in NK cells, but not NK/T cells, as recipients. Notably, these mice accepted purified HSCs much more readily, as did wild-type mice depleted of NK cells, further pointing to an interaction between HSCs and NK cells in vivo. However, it is unlikely that NK-mediated rejection is the mechanism underlying the requirement for MHC class I K matching between HSC and recipient since the kinetic for graft failure is significantly protracted compared with NK-mediated rejection, in which survival approximates that for radiation controls. Moreover, the fact that FCs persist and even increase over time after transplantation in allogeneic recipients would make cold target inhibition a less likely mechanism. Furthermore, a noncytolytic mechanism is supported by observations of others that host NK inhibition of allogeneic bone marrow engraftment is independent of granzyme, perforin, and Fas activity.8,43 NK cell activity could be to trigger apoptotic or proapoptotic pathways on HSCs, resulting in cell death. NK cells may therefore also function as regulators of early hematopoiesis.
The regulatory or inhibitory functions mediated by NK cells are just being defined (reviewed in Raulet et al26 ). Murine NK cells express receptors belonging to the Ly49 family, many of which transmit inhibitory signals to NK cells through interaction with MHC class I.44,45 NK cells express inhibitory receptors specific for MHC class I proteins and stimulatory receptors with diverse specificities. Grigoriadou et al demonstrated a role for MHC class I D in regulating NK cells in bone marrow transplantation.46 Engagement of Ly49 receptors of the inhibitory receptor superfamily (IRS) on NK cells by MHC prevents the lysis of healthy autologous cells, a process critical to self-discrimination/non–self-discrimination in innate immune responses.47 Similarly, in humans IRS engagement with HLA class I delivers an inhibitory signal that down-regulates several T and NK functions such as cytokine production, proliferation, and cytolytic activity. Thus, NK cells are kept silent under normal conditions by appropriate combinations and levels of ligated inhibitory receptors. There are some examples to indicate that positive recognition may also occur in MHC class I/NK cell interactions,48-50 suggesting that NK cells contain both positive and negative signaling receptors and/or that donor bone marrow cells (BMCs) may contain genes that regulate expression of certain class I epitopes.51 One might hypothesize that the requirement for MHC class I K matching for HSC engraftment may therefore occur via inhibition and or regulation by recipient NK cells in that if the HSC is matched to the recipient at this locus, NK activation is inhibited and engraftment is permitted. Moreover, the fact that the requirement for matching at class I K between HSC and recipient is overcome if the recipients are depleted of NK cells further supports a direct role for NK cells in regulating HSC engraftment. One could hypothesize that a direct interaction between MHC classIKon HSCs and inhibitory receptors on NK cells is necessary for durable engraftment. In a system as central to existence as regulation of HSC survival and function, where loss of control could result in malignancy or graft failure, a feedback system to discriminate self from nonself and control production of progeny would be important.
Interestingly, the requirement for MHC class I K matching between HSC donor and recipient is completely overcome also if CD8+/TCR- graft facilitating cells are coadministered with the HSCs. FCs may directly protect HSCs from NK-mediated graft rejection. Although only speculative, an alternative explanation for the role of FCs in allogeneic HSC engraftment would be for the FCs to prevent NK-mediated effects on allogeneic HSCs indirectly via CD8/FC receptor interaction with host NK cells since class I molecules recognize TCR, CD8, and NK Ly49 receptors,41 via cytokines, or directly by inhibiting NK activation and/or increasing inhibitory receptor expression. The fact that FCs do not suppress NK cytolytic function on YAC-1 cells as well as other cell lines expressing different MHCs may support a regulatory mechanism for FCs in vivo that may involve cytokine production and/or cell-cell interaction between HSCs, FCs, and NK cells and makes a veto mechanism of action less likely. Studies are in progress to evaluate each of these possibilities. Finally, the fact that FCs allow HSCs to bypass the MHC matching requirement with the recipient points to a role for FCs on innate immunity and possibly the HSC microenvironment.
It is interesting to note that purified HSCs exhibit a significantly prolonged survival when transplanted into allogeneic recipients compared with radiation controls, perhaps reflecting short-term repopulating capability. Notably, the coadministration of graft FCs restores long-term engraftment potential. We have strong data that FCs restore or maintain quiescence and self-renewal in newly transplanted HSCs as well as in vitro (manuscript in preparation). It is conceivable therefore that class I K on the HSCs contributes to a ligand for the FCp33 receptor on FCs,18 and that in the absence of appropriately matched FCs, purified HSCs become committed progenitors and/or enter into apoptosis. Whether this effect of FCs involves NK cells or is independent remains to be determined.
In summary, we demonstrate for the first time that the MHC class I K locus, or genes linked to it, plays a critical role in regulating durable engraftment of purified HSCs in allogeneic recipients via interaction with host NK cells. In a system as highly regulated as hematopoiesis, MHC class I may play a critical role in the initiating events that dictate HSC self-renewal following allogeneic transplantation. As such, cell-based therapies to enhance engraftment and/or inhibit residual host NK cells may be used to potentiate engraftment and avoid graft-versus-host disease (GVHD), promoting a tolerogenic environment for hematopoietic graft acceptance.
Prepublished online as Blood First Edition Paper, March 30, 2004; DOI 10.1182/blood-2003-11-3910.
Supported in part by grants from the National Institutes of Health (grants DK52294 and R01 HL 63442; S.T.I.), The Jewish Hospital Foundation, The Commonwealth of Kentucky Research Challenge Trust Fund, and the University of Louisville Hospital.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Drs Rolf M. Zinkernagel, Gene M. Shearer, Thomas C. Mitchell, and Isabelle Fugier for review of the manuscript and helpful comments; Theresa Perry for technical assistance; Ms Carolyn DeLautre for manuscript preparation; and the staff of the animal facility for outstanding animal care.

![Figure 3. Matching at the class I K locus between HSCs and recipient is critical for long-term self-renewal. HSCs (5000) were sorted from donors and transplanted into recipients that were disparate at selected MHC loci. (A) Shading shows the MHC disparity relative to B10.BR. (B) Survival curves of recipients disparate at class I D and L (B10.A[2R]); class I D and L with no class II I-E expression (B10.A[4R]), class I K, D, and L and class II I-A (B10.A[5R]), and class I K plus D and L (B10.MBR). (C) Survival of recipients of HSCs disparate at class I K plus class I D and L (B10.BR → B10.MBR) versus class I K only (B10.AKM → B10.MBR).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/3/10.1182_blood-2003-11-3910/6/m_zh80150464440003.jpeg?Expires=1765916173&Signature=0rVO4bpvdlKt5WCk2dEn3rx6snzT-~feKzz7r3Gqwl0p7UD8UxW3pRBzt5~spC-p1eROePXhPfmsRhOIlZmDCgP4c6VErqsAo9DgbJkf2HeZiKtwFNXFzFZOwz9f-gbA6bDHj0njvzTFXBlw4JfxkOjzLKSM57~hETS0kly6WEZEVxLR~gzSY29wXJTJYVmO38debrg8LOS9HbV5ozfWjnkzMOHMt74H6XYojr-vwB3~4gOHEA9V0XnqknctaIXzEj~3jOfWcVmRlJ1rbKuqAQj~e7MCepbCkiQLM22EkXtXU7Eyu8SrJweU8-7WkWzLjlRIqnxjBimruJ1U3E1Iyw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 7. FCs do not affect cytolytic activity of NK cells on YAC-1 cells. To evaluate whether FCs affect NK cytolytic activity as a potential mechanism of action, YAC-1 targets were cocultured with NK cells to which varying ratios of FCs or CD8+ T cells were added. This experiment is representative of a total of 3 performed. (A) Cytolytic activity of C3H NK cells was determined by incubating 5 × 103 [3H]-thymidine–labeled YAC-1 cells with increasing numbers of NK cells to determine the optimal effector-target (E/T) ratio for the suppression assays. To determine maximal killing percentage, 2N HCl was added to YAC-1 cells in the absence of NK cells. (B) NK cells (500 000 per well) were preincubated in triplicate with increasing ratios of FCs or CD8+ T cells for 16 hours. After the coculture, 5 × 103 labeled [3H]-labeled YAC-1 target cells (constant effector to target ratio of 100:1) was then added to each well. Function of NK cells incubated in the absence of FCs is indicated by the open bar. FC or T-cell–mediated cytolysis was measured, but no specific killing was detected (n.d. = not detected). Error bars indicate standard deviation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/3/10.1182_blood-2003-11-3910/6/m_zh80150464440007.jpeg?Expires=1765916173&Signature=vy0LVu-8aYkMwYYk6zXecxJMscINljoJYPF6wcC8~RGBNM-LgnozZAv0Vfb2Vhvz5dEYGgH6eSE0QMCXPqEwRz4XfSgXOlsph70HX~CY20lQT83CtaT8t-Hj1HkT0P2QsdmNiHd50KydmKa0tG3z7pmSF3ExXQpYBcRaaMhkDBjLtNr4X2ZOK8rcm11Ufup1vxu9bido-ZmqG5ZffbCxQvO1FLtTZx3lwT6TTxyJjTCY8DYGicISDrqYPxNwCs9JmE0smgaJhbFgQLmdiMB1p9BKJ4LFG6~Pb4jc2NfPjx~xnlFEMpUePNp2yLSvg6BYVXKOjmAnC5NP0Gs3FYNmCQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)