Abstract
Cerebral X-linked adrenoleukodystrophy (X-ALD) is a disorder of very-long-chain fatty acid metabolism, adrenal insufficiency, and cerebral demyelination. Death occurs within 2 to 5 years of clinical onset without hematopoietic cell transplantation (HCT). One hundred twenty-six boys with X-ALD received HCT from 1982 to 1999. Survival, engraftment, and acute graft-versus-host disease were studied. Degree of disability associated with neurologic and neuropsychological function and cerebral demyelination were evaluated before and after HCT. Complete data were available and analyzed for 94 boys with cerebral X-ALD. The estimated 5- and 8-year survival was 56%. The leading cause of death was disease progression. Donor-derived engraftment occurred in 86% of patients. Demyelination involved parietal-occipital lobes in 90%, leading to visual and auditory processing deficits in many boys. Overall 5-year survival of 92% in patients with 0 or 1 neurologic deficits and magnetic resonance imaging (MRI) severity score less than 9 before HCT was superior to survival for all others (45%; P < .01). Baseline neurologic and neuropsychological function, degree of disability, and neuroradiologic status predicted outcomes following HCT. In this first comprehensive report of the international HCT experience for X-ALD, we conclude that boys with early-stage disease benefit from HCT, whereas boys with advanced disease may be candidates for experimental therapies.
Introduction
Hematopoietic cell transplantation (HCT) is the only effective long-term treatment for cerebral X-linked adrenoleukodystrophy (X-ALD), a β-oxidation disorder of very-long-chain fatty acids (VLCFAs).1 Six distinct clinical phenotypes exist independent of biochemical abnormality, mutation, or family history.2 These phenotypes include (1) childhood-onset, (2) adolescent-onset, and (3) adult-onset cerebral disease, (4) adrenomyeloneuropathy (AMN) with cerebral disease, (5) AMN without cerebral disease, and (6) adrenal insufficiency alone. With a median onset of 7 years, childhood cerebral X-ALD is characterized by adrenal insufficiency in more than 90% of boys and neurologic deterioration in all of them.3-6 Disability, dementia, and death typically occur within months to several years after clinical onset.2 We know of 126 boys with X-ALD who received HCT from July 1982 to January 1999. Data suitable for analysis were available for 94 patients with cerebral X-ALD. We report survival, complications, and outcomes for these patients to provide guidelines for HCT.
Patients and methods
Patients, donors, stem cell sources and matching, and preparative and graft-versus-host disease (GVHD) prophylaxis regimens
We received data from 43 transplantation centers (listed in Acknowledgments) regarding their patients with X-ALD who underwent hematopoietic cell (HC) transplant. All patients were males who were younger than 19 years of age with elevated concentrations of fasting plasma VLCFA. All but 2 had evidence of cerebral disease by magnetic resonance imaging (MRI) of the brain. They underwent neurologic examination, neuropsychological testing, and/or ALD–disability rating scale evaluation. HLA compatibility between recipients and their donors was determined according to contemporaneous criteria at the respective transplantation centers; the result was either matched (ie, 6 of 6 HLA A, B, and DR antigen-level match) or mismatched (ie, 5 of 6 or 4 of 6 HLA A, B, and DR antigen-level match). Patients received related donor (RD) or unrelated donor (URD) HC transplants with preparative regimens determined by each of the participating transplantation centers. Patient characteristics are summarized (Table 1). Consent was obtained prior to HCT from patients or their parents; respective institutional review boards approved all treatment protocols. Twenty-four patients have been reported previously.1,7-20
Characteristics of 94 patients with cerebral X-adrenoleukodystrophy (X-ALD) who underwent hematopoietic cell transplant (HC transplant)
. | Related donor (n = 42) . | Unrelated donor (n = 52) . | Total (n = 94) . |
|---|---|---|---|
| Year of HCT, no. (%) | |||
| Before 1990 | 5 (12) | 0 (0) | 5 (5) |
| 1991 to 1995 | 24 (56) | 20 (38) | 44 (46) |
| 1996 to 1999 | 13 (31) | 32 (62) | 45 (48) |
| Median age at HCT, y (range) | 9.1 (5.2-15.6) | 8.9 (4.9-18.6) | 9.0 (4.9-18.6) |
| Reason for diagnosis, no. (%)* | |||
| Family history | 11 (29) | 17 (35) | 28 (33) |
| X-ALD signs and symptoms | 27 (71) | 31 (65) | 58 (67) |
| Unknown | 4 | 4 | 8 |
| Stem cell source and HLA matching | |||
| Marrow/UCB | 42/0 | 40/12 | 82/12 |
| Matched | 33/0 | 27/4 | 60/4 |
| Mismatched | 9/0 | 13/8 | 22/8 |
| Preparative regimen, no. (%) | |||
| Chemotherapy only | 31 (74) | 17 (33) | 48 (51) |
| Bu/Cy | 30 (97) | 17 (100) | 47 (98) |
| Bu only | 1 (3) | 0 | 1 (2) |
| Chemotherapy and radiation | 11 (26) | 35 (67) | 46 (49) |
| Cy/TBI, brain-sparing at 1400 cGy | 5 (45) | 13 (37) | 18 (39) |
| Bu/Cy/TBI at 300-1200 cGy | 1 (9) | 9 (26) | 10 (22) |
| Cy/TBI at 1200-1400 cGy | 1 (9) | 8 (23) | 9 (20) |
| Bu/Cy/TLI at 150-750 cGy | 2 (18) | 1 (3) | 3 (7) |
| Cy/TAI at 800 cGy | 1 (9) | 1 (3) | 2 (4) |
| VP-16/Ara-C/Cy/TBI at 1200 cGy | 1 (9) | 1 (3) | 2 (4) |
| Other | 0 | 2 (6) | 2 (4) |
| GVHD prophylaxis | |||
| CSA/MP, no. (%) | 24 (57) | 29 (56) | 53 (56) |
| No. with/no. without ATG | 3/21 | 12/17 | 15/38 |
| CSA/MTX, no. (%) | 9 (21) | 8 (15) | 17 (18) |
| No. with/no. without ATG | 2/7 | 6/2 | 8/9 |
| Other, no. (%) | 9 (21) | 15 (29) | 24 (26) |
| Graft manipulation by elutriation, no. yes/no. no | 3/39 | 15/37 | 18/76 |
. | Related donor (n = 42) . | Unrelated donor (n = 52) . | Total (n = 94) . |
|---|---|---|---|
| Year of HCT, no. (%) | |||
| Before 1990 | 5 (12) | 0 (0) | 5 (5) |
| 1991 to 1995 | 24 (56) | 20 (38) | 44 (46) |
| 1996 to 1999 | 13 (31) | 32 (62) | 45 (48) |
| Median age at HCT, y (range) | 9.1 (5.2-15.6) | 8.9 (4.9-18.6) | 9.0 (4.9-18.6) |
| Reason for diagnosis, no. (%)* | |||
| Family history | 11 (29) | 17 (35) | 28 (33) |
| X-ALD signs and symptoms | 27 (71) | 31 (65) | 58 (67) |
| Unknown | 4 | 4 | 8 |
| Stem cell source and HLA matching | |||
| Marrow/UCB | 42/0 | 40/12 | 82/12 |
| Matched | 33/0 | 27/4 | 60/4 |
| Mismatched | 9/0 | 13/8 | 22/8 |
| Preparative regimen, no. (%) | |||
| Chemotherapy only | 31 (74) | 17 (33) | 48 (51) |
| Bu/Cy | 30 (97) | 17 (100) | 47 (98) |
| Bu only | 1 (3) | 0 | 1 (2) |
| Chemotherapy and radiation | 11 (26) | 35 (67) | 46 (49) |
| Cy/TBI, brain-sparing at 1400 cGy | 5 (45) | 13 (37) | 18 (39) |
| Bu/Cy/TBI at 300-1200 cGy | 1 (9) | 9 (26) | 10 (22) |
| Cy/TBI at 1200-1400 cGy | 1 (9) | 8 (23) | 9 (20) |
| Bu/Cy/TLI at 150-750 cGy | 2 (18) | 1 (3) | 3 (7) |
| Cy/TAI at 800 cGy | 1 (9) | 1 (3) | 2 (4) |
| VP-16/Ara-C/Cy/TBI at 1200 cGy | 1 (9) | 1 (3) | 2 (4) |
| Other | 0 | 2 (6) | 2 (4) |
| GVHD prophylaxis | |||
| CSA/MP, no. (%) | 24 (57) | 29 (56) | 53 (56) |
| No. with/no. without ATG | 3/21 | 12/17 | 15/38 |
| CSA/MTX, no. (%) | 9 (21) | 8 (15) | 17 (18) |
| No. with/no. without ATG | 2/7 | 6/2 | 8/9 |
| Other, no. (%) | 9 (21) | 15 (29) | 24 (26) |
| Graft manipulation by elutriation, no. yes/no. no | 3/39 | 15/37 | 18/76 |
UCB indicates umbilical cord blood; Bu, busulfan; Cy, cyclophosphamide; TBI, total body irradiation; TLI, total lymphoid irradiation; TAI, total abdominal irradiation; VP-16, etoposide; Ara-C, cytosine arabinoside; CSA, cyclosporin; MP, methylprednisolone; and ATG, antithymocyte globulin.
Percentages were calculated only for patients with a known reason
Hematopoietic recovery, engraftment, and GVHD
Full and partial donor-derived engraftments were defined as hematologic recovery of neutrophils to at least 5 × 108/L and either more than 89% or 10% to 89% donor chimerism, respectively, by DNA-based or sex chromosome analysis. Failed engraftment occurred when neutrophils did not reach 5 × 108/L and/or chimerism was less than 10% donor. Diagnosis of acute GVHD was based on clinical criteria, with histologic confirmation when possible. Overall staging was based on published criteria.21
Neurologic function
Neurologic function was evaluated by neurology consultants prior to and following HCT through assessments of patients in the following areas: vision, hearing, speech, gait, and/or other areas such as fine motor skills and activities of daily living. Abnormality in any of these areas was considered a deficit. Neurologic deficit scores were assigned as 0 (no deficits), 1 (a single deficit), 2 (2 deficits), or more than 2 (> 2 deficits).
Neuropsychological function
Patients were administered the Wechsler Intelligence Scale for Children Third Edition (WISC-III)22 or the Wechsler Preschool and Primary Scale of Intelligence.23 Patients whose disease progression led to cortical blindness but who could take part in the assessment process were assigned a Performance Intelligence Quotient (PIQ) score of 45. This score reflected the patient's inability to meet the visual demands of the Performance Scale subtests, while demonstrating the ability to perform at measurable levels on Verbal Scale subtests (verbal intelligence quotient [VIQ]).
X-adrenoleukodystrophy–disability rating scale (ALD-DRS)
The ALD-DRS was developed at the University of Minnesota to assess functional level as a composite. It addresses requirements for services distinct from the neurologic and neuropsychological examinations; levels range from 0 to IV with increasing disability (Table 2).
X-linked adrenoleukodystrophy-disability rating scale (ALD-DRS)
Scale level . | Description . |
|---|---|
| 0 | No difficulties |
| I | Mild learning or coordination difficulties from ALD; patient does not require support or intervention |
| II | Moderate learning, sensory, and/or neurologic abnormality; patient requires support or intervention in a few areas |
| III | Severe learning, sensory, and/or neurologic abnormality; patient requires support or intervention in many areas |
| IV | Loss of cognitive ability and disorientation; patient requires constant supervision |
Scale level . | Description . |
|---|---|
| 0 | No difficulties |
| I | Mild learning or coordination difficulties from ALD; patient does not require support or intervention |
| II | Moderate learning, sensory, and/or neurologic abnormality; patient requires support or intervention in a few areas |
| III | Severe learning, sensory, and/or neurologic abnormality; patient requires support or intervention in many areas |
| IV | Loss of cognitive ability and disorientation; patient requires constant supervision |
Neuroradiologic assessment
Statistical analysis
A patient's data were deemed complete if the following were verified: (1) vital status as of June 15, 1999, (2) engraftment data at least 6 months after HCT or not evaluable because of death, and (3) neurologic deficits or neuropsychological function at least 1 year after HCT. Although some patients died before 1 year or were too severely affected to be tested, these patients were included in the appropriate analyses. Complete data were available on 94 of 126 patients; statistical analyses were performed on these 94 patients all of whom had cerebral X-ALD. Analyses of acute and chronic GVHD, engraftment, neurologic deficits, and ALD-DRS were performed by taking simple proportions. These variables were analyzed across donor type by the Pearson chi-square test. MRI score and neuropsychological function were analyzed by the general Wilcoxon test. Paired t tests were used to evaluate mean changes in scores after HCT from baseline for the number of neurologic deficits, neuropsychological function, MRI severity, and ALD-DRS scores. Categorical end points were analyzed similarly with the McNemar test for paired data.26 X-ALD disease progression-related mortality (ARM) and transplant-related mortality (TRM) were estimated by using cumulative incidence, treating nonevent deaths as a competing risk.27 The probability of survival was estimated by the Kaplan-Meier method.28 Univariate comparisons of survival across factors were completed by the log-rank test. Cox regression was used to examine the independent effect of all factors on survival.29
Results
From July 2, 1982, to January 15, 1999, 126 boys with X-ALD underwent HC transplant at 43 centers. Of the 122 boys for whom donor type was known, 54 patients received bone marrow or umbilical cord blood from a RD; 68 patients received bone marrow or umbilical cord blood from an URD. We present the 94 patients with cerebral X-ALD and complete, analyzable data (described in “Statistical analysis”). Two centers, the University of Minnesota and Hôpital Saint-Vincent de Paul, cared for 30 and 10 patients; 9 centers cared for 3 or 4 patients; 21 centers cared for 1 or 2 patients.
Vital status, transplant-related mortality (TRM), X-ALD disease progression-related mortality (ARM), Kaplan-Meier analyses, and causes of death
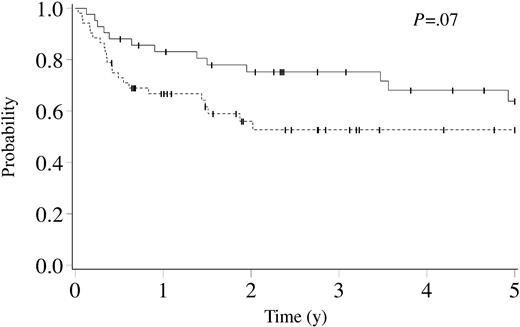
Fifty-nine of 94 patients were alive at a median of 3.1 years (range, 0.4-11.2 years) after HCT. The estimated 5- and 8-year survival probabilities were both 56% (95% confidence interval [CI], 44%-68%). After RD HCT, 29 of 42 patients were alive at a median of 5.0 years (range, 0.5-11.2 years); after URD HCT, 30 of 52 patients were alive at a median of 2.4 years (range, 0.4-7.8 years). The estimated 5-year survival probabilities for RD and URD HCT were 64% (95% CI, 47%-80%) and 53% (95% CI, 38%-68%), respectively (P = .07, Figure 1).
Kaplan-Meier estimate of survival for cerebral X-linked adrenoleukodystrophy following related (solid line, n = 42) and unrelated donor (dashed line, n = 52) hematopoietic cell transplantation. Ticks on probability lines indicate dates of censoring at last follow-up.
Kaplan-Meier estimate of survival for cerebral X-linked adrenoleukodystrophy following related (solid line, n = 42) and unrelated donor (dashed line, n = 52) hematopoietic cell transplantation. Ticks on probability lines indicate dates of censoring at last follow-up.
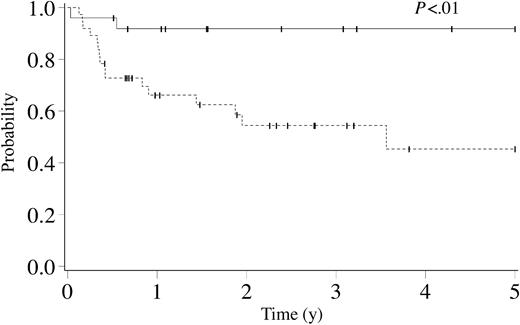
The cumulative incidence at 3 years of TRM was 14% (95% CI, 7%-21%); following RD HCT it was 10% (95% CI, 1%-18%); after URD HCT it was 18% (95% CI, 7%-29%) (P = .22). Overall 5-year survival varied with the number of neurologic deficits before HCT: 0 neurologic deficits (n = 32), 70% (95% CI, 51%-89%); 1 neurologic deficit (n = 28), 67% (95% CI, 45%-89%); 2 or more neurologic deficits (n = 30), 35% (95% CI, 14%-57%; P < .01). Overall 5-year survival varied with ALD-DRS level prior to HCT: level of 0, 93% (95% CI, 79%-100%); level of I, 57% (95% CI, 32%-81%); level of II or greater, 44% (95% CI, 25%-64%; P = .05). Overall 5-year survival varied with MRI severity score before HCT: score less than 9 (n = 32), 84% (95% CI, 71%-97%); score 9 or greater (n = 34), 42% (95% CI, 18%-66%; P < .01). The estimated 5-year survival varied with the number of neurologic deficits and the MRI severity score before HCT: 0 or 1 neurologic deficits and MRI severity score less than 9 (n = 25), 92% (95% CI, 81%-100%); all other patients (n = 37), 45% (95% CI, 23%-67%; P < .01; Figure 2).
Kaplan-Meier estimate of survival for cerebral X-linked adrenoleukodystrophy following hematopoietic cell transplantation by number of neurologic deficits and MRI severity score before transplantation. Solid line indicates patients with 0 to 1 neurologic deficits and MRI severity score less than 9 (n = 25). Dashed line indicates patients with 2 or more neurologic deficits or MRI severity score 9 or greater (n = 37). Ticks on probability lines indicate dates of censoring at last follow-up.
Kaplan-Meier estimate of survival for cerebral X-linked adrenoleukodystrophy following hematopoietic cell transplantation by number of neurologic deficits and MRI severity score before transplantation. Solid line indicates patients with 0 to 1 neurologic deficits and MRI severity score less than 9 (n = 25). Dashed line indicates patients with 2 or more neurologic deficits or MRI severity score 9 or greater (n = 37). Ticks on probability lines indicate dates of censoring at last follow-up.
The leading causes of death were progression of cerebral X-ALD (n = 21) and GVHD (n = 5). The cumulative incidence at 3 years of X-ALD disease progression-related mortality was 22% (95% CI, 13%-32%); following RD HCT it was 15% (95% CI, 4%-26%); after URD HCT it was 29% (95% CI, 15%-43%) (P = .07). Cox regression analysis of survival showed HLA mismatch to be significant for the risk of death: RD and URD fully HLA-matched, relative risk (RR) 1.0 versus URD HLA-mismatched RR 2.3 (95% CI, 1.1-5.0; P = .026). Cox regression analysis of survival showed baseline neurologic status to be significant for the risk of death: 0 to 1 neurologic deficits, RR 1.0 versus 2 or more neurologic deficits, RR 2.8 (95% CI, 1.4-5.6; P = .003).
Engraftment
Full or partial donor-derived engraftment occurred in 80 (86%) of 93 evaluable patients at a median follow-up of 11 months (range, 0.2-97 months). Following RD HCT, 39 (93%) of 42 evaluable patients engrafted at a median follow-up of 13 months (range, 0.9-85 months); after URD HCT 41 (80%) of 51 evaluable patients engrafted at a median follow-up of 7 months (range, 0.2-97 months; P = NS).
Graft-versus-host disease
The incidence of severe (ie, grade III-IV) acute GVHD was 12%. For recipients of RD HCT, the incidence of severe acute GVHD was 17%; for recipients of URD HCT, the incidence was 8% (P = NS).
Neurologic and neuropsychological function, neuroradiologic assessments, and ALD-DRS levels
Assessments of neurologic function with identification of neurologic deficits were performed before and after HCT (Table 3). There were 58 evaluable patients with 0 or 1 neurologic deficit before HCT (2 of the 28 patients with 1 neurologic deficit before HCT did not have available post-HCT neurologic deficit data). After HCT, 31 of these 58 patients demonstrated no neurologic progression, whereas 27 patients progressed. In the group that progressed, a lower percentage of patients had full or partial donor-derived engraftment (ie, 78%) compared with 97% for the group that did not progress (P = .07). Results of neurologic deficits, neuropsychological function, MRI severity, and ALD-DRS scores after HCT according to baseline PIQ are presented (Table 4). As baseline PIQ less than 80 has been previously noted to predict outcome, we separated patients into 2 groups: baseline PIQ 80 or greater and PIQ less than 80. Patients with a baseline PIQ less that 80 were significantly more impaired in both neuropsychological function measured by PIQ and on the ALD–disability rating scale after HCT (both P < .01). However, no differences were found in neurologic deficit or MRI severity scores after HCT between these 2 groups. Assessments of disability (ie, ALD-DRS level, range 0-IV) were performed before and after HCT (Table 5). Patients with higher levels of disability at baseline had an increasingly higher likelihood of progressing to a higher level of disability after HCT, eg, 38% chance for level 0, 63% to 65% for levels I and II, and 91% for level III. Neuroradiologic assessments of T2-weighted images of the brain by MRI were performed before and after HCT to determine the location and severity of demyelination. Eighty-one (90%) of 90 patients demonstrated evidence of demyelination in the parietal-occipital lobes. Comparisons of neuropsychological function (VIQ and PIQ) according to MRI pattern of demyelination (parietal-occipital lobes versus frontal lobes or pyramidal tracts) are presented (Table 6). All survivors were compared with those who died on the basis of baseline neuropsychological testing. Although no difference was found in baseline median VIQ scores (survivors, 98 versus deceased patients, 92), the median baseline PIQ score was significantly lower in patients who died (survivors, 94.5 versus deceased patients, 77.5; P < .01). Patients with a parietal-occipital lobe pattern of demyelination demonstrated a greater mean loss of PIQ points (ie, -21.6) than patients with a frontal lobe pattern of demyelination (ie, 0.4 PIQ points; P = .03).
Neurologic deficit score before and after hematopoietic cell transplantation (HCT) in 94 patients with cerebral X-adrenoleukodystrophy
. | . | After HCT, no. (%) . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before HCT . | No. . | 0 . | 1 . | 2 . | More than 2 . | Unknown or not available . | ||||
| 0 | 32 | 18 (56) | 4 (13) | 1 (3) | 9 (28) | — | ||||
| 1 | 28 | 3 (11) | 10 (36) | 4 (14) | 9 (32) | 2 (7) | ||||
| 2 | 21 | — | 1 (5) | 6 (29) | 14 (67) | — | ||||
| More than 2 | 9 | — | — | — | 9 (100) | — | ||||
| Unknown or not available | 4 | — | — | — | — | 4 (100) | ||||
| Totals | 94 | 21 | 15 | 11 | 41 | 6 | ||||
. | . | After HCT, no. (%) . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before HCT . | No. . | 0 . | 1 . | 2 . | More than 2 . | Unknown or not available . | ||||
| 0 | 32 | 18 (56) | 4 (13) | 1 (3) | 9 (28) | — | ||||
| 1 | 28 | 3 (11) | 10 (36) | 4 (14) | 9 (32) | 2 (7) | ||||
| 2 | 21 | — | 1 (5) | 6 (29) | 14 (67) | — | ||||
| More than 2 | 9 | — | — | — | 9 (100) | — | ||||
| Unknown or not available | 4 | — | — | — | — | 4 (100) | ||||
| Totals | 94 | 21 | 15 | 11 | 41 | 6 | ||||
Text in bold indicates patients for whom neurologic deficit scores were unchanged. — indicates none.
Neurologic deficits, neuropsychological function, MRI severity, and ALD-Disability Rating Scale (ALD-DRS) levels after hematopoietic cell transplantation in patients with cerebral X-ALD according to baseline performance intelligence quotient (PIQ)
. | Baseline PIQ below 80 . | Baseline PIQ of 80 or more . | P . |
|---|---|---|---|
| Neurologic deficits | .29 | ||
| Median, no. (range) | 2 (0-4) | 1 (0-4) | |
| Observations, no. | 32 | 43 | |
| PIQ | < .01 | ||
| Median (range) | 45 (45-63) | 78.5 (45-122.5) | |
| Observations, no. | 6 | 26 | |
| MRI severity score | .25 | ||
| Median (range) | 12.8 (8-19) | 8 (0-23.5) | |
| Observations, no. | 8 | 23 | |
| ALD-DRS level | < .01 | ||
| Median (range) | IV (I-IV) | II (0-IV) | |
| Observations, no. | 28 | 36 |
. | Baseline PIQ below 80 . | Baseline PIQ of 80 or more . | P . |
|---|---|---|---|
| Neurologic deficits | .29 | ||
| Median, no. (range) | 2 (0-4) | 1 (0-4) | |
| Observations, no. | 32 | 43 | |
| PIQ | < .01 | ||
| Median (range) | 45 (45-63) | 78.5 (45-122.5) | |
| Observations, no. | 6 | 26 | |
| MRI severity score | .25 | ||
| Median (range) | 12.8 (8-19) | 8 (0-23.5) | |
| Observations, no. | 8 | 23 | |
| ALD-DRS level | < .01 | ||
| Median (range) | IV (I-IV) | II (0-IV) | |
| Observations, no. | 28 | 36 |
X-adrenoleukodystrophy–disability rating scale levels before and after hematopoietic cell transplantation (HCT) in 94 patients with cerebral X-adrenoleukodystrophy
. | . | Level after HCT, no. (%) . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Level before HCT . | No. . | 0 . | I . | II . | III . | IV . | No data, because of death . | |||||
| 0 | 13 | 8 (62)* | 3 (23) | 1 (7.6) | — | 1 (7.6) | — | |||||
| I | 21 | 1 (4.8) | 6 (28)† | 4 (19)† | 3 (14) | 6 (28)† | 1 (4.8) | |||||
| II | 36 | 1 (2.8) | 1 (2.8) | 10 (2.8)‡ | 1 (2.8) | 19 (53)∥ | 4 (11) | |||||
| III | 12 | — | — | — | 1 (8.3) | 10 (83)§ | 1 (8.3) | |||||
| IV | 1 | — | — | — | — | 1 (100)* | — | |||||
| Missing data | 11 | — | — | 1 (9) | — | — | 5 (45) | |||||
| Totals | 94 | 10 | 10 | 16 | 5 | 37 | 11 | |||||
| Deaths | 35 | 1 | 2 | 5 | 0 | 16 | 11 | |||||
. | . | Level after HCT, no. (%) . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Level before HCT . | No. . | 0 . | I . | II . | III . | IV . | No data, because of death . | |||||
| 0 | 13 | 8 (62)* | 3 (23) | 1 (7.6) | — | 1 (7.6) | — | |||||
| I | 21 | 1 (4.8) | 6 (28)† | 4 (19)† | 3 (14) | 6 (28)† | 1 (4.8) | |||||
| II | 36 | 1 (2.8) | 1 (2.8) | 10 (2.8)‡ | 1 (2.8) | 19 (53)∥ | 4 (11) | |||||
| III | 12 | — | — | — | 1 (8.3) | 10 (83)§ | 1 (8.3) | |||||
| IV | 1 | — | — | — | — | 1 (100)* | — | |||||
| Missing data | 11 | — | — | 1 (9) | — | — | 5 (45) | |||||
| Totals | 94 | 10 | 10 | 16 | 5 | 37 | 11 | |||||
| Deaths | 35 | 1 | 2 | 5 | 0 | 16 | 11 | |||||
Denotes 1 death
Denotes 2 deaths
Denotes 3 deaths
Denotes 4 deaths
Denotes 9 deaths
Comparison of neuropsychological functional outcomes according to MRI pattern of demyelination following hematopoietic cell transplantation for cerebral X-linked adrenoleukodystrophy
. | Parietal-occipital lobes, n = 25 . | Frontal lobes or pyramidal tracts, n = 7 . | Total, n = 32 . | No neuropsychological function assessment after HCT because of death, n = 29 . | P . |
|---|---|---|---|---|---|
| VIQ* | |||||
| Baseline (range) | 99 (59-127) | 89 (70-107) | 98 (59-127) | 92 (74-124) | NS |
| After HCT (range) | 82 (46-123) | 79 (57-113) | 80.5 (46-123) | — | — |
| Mean change (CI) | –13.7 (–20.5 to –6.8) | –6.4 (–17.8 to 4.9) | –12.1 (–17.1 to –7.1) | — | .25 |
| PIQ* | |||||
| Baseline (range) | 96 (45-122.5) | 92 (61-100) | 94.5 (45-122.5) | 77.5 (45-138) | < .01 |
| After HCT (range) | 64 (45-122.5) | 93 (54-108) | 73 (45-122.5) | — | — |
| Mean change (CI) | –21.6 (–30.6 to –12.6) | 0.4 (–6.8 to 7.7) | –16.8 (–24.6 to –8.9) | — | .03 |
. | Parietal-occipital lobes, n = 25 . | Frontal lobes or pyramidal tracts, n = 7 . | Total, n = 32 . | No neuropsychological function assessment after HCT because of death, n = 29 . | P . |
|---|---|---|---|---|---|
| VIQ* | |||||
| Baseline (range) | 99 (59-127) | 89 (70-107) | 98 (59-127) | 92 (74-124) | NS |
| After HCT (range) | 82 (46-123) | 79 (57-113) | 80.5 (46-123) | — | — |
| Mean change (CI) | –13.7 (–20.5 to –6.8) | –6.4 (–17.8 to 4.9) | –12.1 (–17.1 to –7.1) | — | .25 |
| PIQ* | |||||
| Baseline (range) | 96 (45-122.5) | 92 (61-100) | 94.5 (45-122.5) | 77.5 (45-138) | < .01 |
| After HCT (range) | 64 (45-122.5) | 93 (54-108) | 73 (45-122.5) | — | — |
| Mean change (CI) | –21.6 (–30.6 to –12.6) | 0.4 (–6.8 to 7.7) | –16.8 (–24.6 to –8.9) | — | .03 |
VIQ and PIQ baseline evaluations and those after HCT: median (range). Bold denotes statistical comparisons and corresponding P values. VIQ indicates verbal intelligence quotient; PIQ, performance intelligence quotient; CI, 95% confidence interval
Discussion
Cerebral X-ALD is a rapidly progressive demyelinating disease that leads to severe disability, dementia, and death over months to years. In contrast, this first report of the large international HCT experience for cerebral X-ALD clearly demonstrates the excellent likelihood of survival with good neurologic and neuropsychological function in selected boys. We conclude that HCT is the only effective long-term treatment if performed at an early stage of cerebral disease.
To assess the effect of HCT, we reported the probability of survival with good function and contrasted it with the natural history of cerebral X-ALD. The estimate of overall 5-year survival after HCT was 56%; in contrast, the survival at 5 years after presentation in boys with cerebral X-ALD not receiving transplants was no more than 40% and approached 0% at 15 years.30 Boys with early-stage cerebral X-ALD treated with HCT have a clear survival and function advantage over boys not receiving transplants who, therefore, experience the progressive changes associated with the natural history of this devastating disease.
A successful transplantation was due, in part, to achieving donor engraftment while minimizing GVHD. Donor-derived engraftment occurred in 86% of patients, including 82% of patients who received transplants with URD cord blood. The high success rate of achieving donor chimerism compares favorably with that seen in patients with malignant disorders undergoing HC transplant.31 Many factors can affect neurologic outcomes in boys with advanced cerebral X-ALD, including their clinical course following HCT. Severe acute GVHD can be associated with rapid, profound clinical deterioration in these patients (C.P. and W.K., unpublished data, June 1999). The incidence of severe acute GVHD was very low (ie, 12%). This low level of morbidity and favorable donor-derived engraftment profile contributed to the overall success. Additional experience with cord blood, peripheral blood, and haploidentical HCT is needed to assess their safety and efficacy in cerebral X-ALD.20
Disease-specific data were important in evaluating outcomes after HCT for cerebral X-ALD. Early cerebral X-ALD (ie, 0 or 1 neurologic deficit, PIQ ≥ 80, ALD-DRS level of 0 or I, and MRI severity score < 9) must be distinguished from advanced disease. This was underscored by the Kaplan-Meier survival curves presented (Figure 2). Figure 2 included data on 62 patients; for the 32 patients who were excluded from this analysis, MRI scores (n = 28) and neurologic deficits (n = 4) were irretrievable. However, these 2 groups were comparable with respect to disease status before HCT (eg, neurologic deficits, ALD-DRS levels, PIQ scores, percentage of patients with parietal-occipital demyelination) and survival in patients with advanced cerebral X-ALD (ie, 42% versus 45%). Therefore, data presented on 62 patients were deemed to accurately reflect the entire cohort. Patients with 2 or more neurologic deficits had a significantly increased likelihood of death (RR, 2.8; P = .003). Death as a result of progression of cerebral X-ALD was the most common cause of death (ie, 21 of 35 deaths). Because cerebral X-ALD is a progressive disorder, all patients with demyelination can be expected to exhibit further injury to myelin before stabilization occurs after HCT. Even patients with a favorable PIQ score (ie, PIQ ≥ 80) and 0 or 1 neurologic deficit had cerebral disease; when compared with more advanced patients, the former simply had a higher likelihood, although not certainty, of preserving neurocognitive function. Among the 58 patients with 0 or 1 neurologic deficits before HCT, 31 did not show neurologic progression after HCT. Thirty of these 31 (97%) patients had donor engraftment compared with only 21 of 27 (78%) patients who showed neurologic progression (P = .07), thereby underscoring the importance of maintaining donor chimerism after HCT. There were no significant differences between these 2 groups with respect to incidence or grade of acute and chronic GVHD.
Neuropsychological testing identified the functional deficit associated with demyelination. Ninety percent of patients had demyelination in the parietal-occipital lobes, the sites of visual and auditory pathways. Tests comprising the PIQ score were sensitive to changes in visual processing ability. Boys with parietal-occipital lobe demyelination showed a dramatic decrease in their PIQ scores after HCT but did not show significant decline in their VIQ scores, whereas boys with frontal lobe or pyramidal tract disease showed little change in intellectual functioning. Patients who died during HCT were more likely to have a low PIQ (ie, < 80) before HCT.
Historically, the PIQ score before HCT predicted functional outcome in patients with cerebral X-ALD. In the present study, the PIQ score was less than 80 in 32 boys. After HCT, only 6 of these boys were evaluable (median PIQ, 45; range, 45-63), suggesting that disability increased markedly in nearly all of these patients. In contrast, when the PIQ was 80 or greater (n = 48), 26 boys were evaluated after HCT (median PIQ, 79; range, 45-123; P < .01). The leading reasons for these boys being deemed nonevaluable were death and progression of demyelination, resulting in cortical blindness. Thus, PIQ remains an excellent predictor of neurocognitive function after HCT for cerebral X-ALD. Extent of demyelination on MRI and neurologic status after HCT were not associated with baseline PIQ. PIQ may be the most sensitive predicator of quality of life after HCT.
Extent and location of cerebral demyelination also influenced long-term outcome. MRI severity score before HCT was highly predictive of survival (score < 9, 84% versus score ≥ 9, 42%; P < .01) as well as correlating with the degree of neuropsychological dysfunction. Significant injury to the parietal-occipital lobes typically led to impaired visual and auditory processing.
The ALD-DRS, developed at the University of Minnesota to provide insight into the quality of life of boys with cerebral X-ALD, described levels of disability and the assistance required for the disability. Median ALD-DRS levels before and after HCT were II and III; the mean increase was 1.1 level (P < .01). The effect of this degree of disability requires further elucidation both with respect to the patient's need for psychological and physical support and his family's needs because of the profound effects of the disease process and the significant ramifications for changes in lifestyle.
These data on MRI severity and ALD-DRS levels provide strong justification for performing HCT in boys with early cerebral X-ALD. By investigating a family pedigree, this X-linked disorder can be diagnosed biochemically in boys and men before the onset of cerebral demyelination and identified in heterozygous girls and women by genotype analysis. On identification, boys aged 3 to 15 years with X-ALD should undergo comprehensive neurologic, neuropsychological, neuroradiologic, and adrenal function evaluations with subsequent serial monitoring at least every 6 months during the first decade of life with consideration given to annual monitoring in the second decade. The goals of these recommendations are (1) to detect cerebral disease early, prior to the development of neuropsychological and/or neurologic signs and symptoms, and (2) to identify adrenal insufficiency, a potentially life-threatening condition, and to treat it appropriately. Timely and effective HCT should be possible.
Discussion of the role of treatment to biochemically correct VLCFA by dietary measures (eg, Lorenzo oil) and restricted intake of VLCFA is ongoing. Compliant use of Lorenzo oil in boys may reduce but not eliminate their risk for developing childhood cerebral X-ALD.32 The importance of normalizing fasting plasma VLCFA levels in boys with cerebral X-ALD before and after HCT remains questionable. As previously reported by Shapiro et al,1 long-term follow-up of boys who received transplants for early cerebral X-ALD revealed a 55% reduction but not normalization in VLCFA levels. There were no apparent relationships between plasma VLCFA levels and neurologic, neuropsychological, disability, and neuroradiologic outcomes. For this study, data were not collected on plasma VLCFA levels, use of Lorenzo oil before or after HCT, or compliance with dietary restrictions.
Timely HCT is critical for appropriate patients with cerebral X-ALD. On the basis of the natural history of cerebral disease and the significance of gadolinium enhancement of increased signal intensity abnormalities seen on T2-weighted MRI images, guidelines can be deduced. For boys with evidence of demyelination (ie, measurable MRI severity score) and gadolinium enhancement, the likelihood of progression to extensive demyelination with ultimate dementia and death was high (ie, approximately 80%-90%).2 However, only 35% to 40% of boys aged younger than 10 years with the biochemical diagnosis of X-ALD will develop childhoodonset cerebral disease.2 Because 60% to 65% of boys will not develop cerebral disease during childhood, HCT cannot be justified in the absence of MRI evidence of demyelination (ie, MRI score of 0). Two of 126 patients with X-ALD who underwent HC transplant did not have evidence of cerebral disease. Whether HCT should be performed in such patients is a matter of controversy. We oppose it for the following reasons: (1) noninvasive neuroimaging and neuropsychological studies now permit early detection of cerebral involvement in asymptomatic patients; (2) although the risk is low, HCT is still associated with mortality; (3) HCT in boys with early cerebral disease has excellent outcomes; and (4) the certainty that HCT benefits patients without evidence of cerebral disease is lacking.
The mechanism by which HCT halts the process of demyelination and leads to clinical stability is not fully understood. Gadolinium enhancement of the peripheral aspects of the area of demyelination represents the loss of integrity of the blood-brain barrier and, by inference, the sites of active inflammation and attendant CD3-mediated myelin destruction and injury to oligodendrocytes.33 It is postulated that HCT immunosuppressive therapy contributes to the disappearance of gadolinium enhancement in MRI abnormalities within several months after HCT (L.R.C., unpublished data, October 2003) and that with long-term donor-derived engraftment there is no recurrence of this characteristic MRI finding. Primary and secondary graft failures have been associated with progression or resumption of the demyelinating process.
Clear indications and contraindications for HCT for boys with cerebral X-ALD exist. It is now recognized that boys with advanced cerebral X-ALD and the associated neurologic deficits and neuropsychological dysfunction (especially PIQ < 80) are poor candidates for HCT. However, this only became evident after extensive efforts to both minimize HCT-related complications and preserve neurologic and neuropsychological function failed. We do not recommend HCT for patients with AMN who do not have cerebral involvement, because, at this time, it is not known whether HCT benefits them. This uncertainty, combined with the slow rate of natural progression of this form of X-ALD and the higher risk of GVHD in adults, have made the benefit-risk ratio unfavorable. Long-term follow-up of engrafted young patients with cerebral disease and no evidence of AMN at time of HCT will provide important information, as an increasing number of these patients reach adulthood. Nearly all untreated men with X-ALD who are older than 25 years of age show evidence of AMN on the basis of history, neurologic examination, or neurophysiological studies. If patients who received transplants do not develop AMN, this would be strong evidence that HCT may also prevent the onset of this form of the disease. The converse would be concluded if they develop AMN to the same degree as age-matched patients who did not receive transplants. Follow-up of patients who received transplants for another 5 years will provide important preliminary data.
Clinical judgment and frank discussions with parents are needed to ascertain the utility of HCT for boys diagnosed with X-ALD on the basis of signs and symptoms of cerebral demyelination. The ability to anticipate the timing and rate of disease progression is limited. Boys with advanced cerebral X-ALD are poor candidates for HCT because of the virtual certainty of disease progression leading to severe disability or death, the risk of HCT, and its association with an accelerated rate of disease progression. Alternative therapies are needed and could include neuroprotective agents and neuronal or other stem cells administered systemically or locally.
In the absence of a centralized HCT- and disease-specific database, collection of data for this report was both labor intensive and time consuming. From 1982 to 1999, a total of 126 patients with X-ALD received transplants at 43 international centers; 32 transplantation centers submitted complete, analyzable data on 94 patients with cerebral X-ALD; however, only 11 centers had significant clinical experience transplanting 3 or more cases. Guidelines are urgently needed so that international HCT activity and follow-up for cerebral X-ALD can be both complete and consistent. Data collection for HCT-specific events was relatively simple; longitudinal studies to collect cerebral X-ALD disease information presented far greater challenges. This report of both HCT- and disease-specific outcomes allowed a more complete analysis of the effect of HCT on cerebral X-ALD.
The international experience for cerebral X-ALD confirmed the value of HCT in selected patients, particularly those with limited demyelination and good functional status. More detailed and longer longitudinal studies, including assessments of quality of life, are needed to gauge the effect of HCT in all patients with cerebral X-ALD. To accomplish this goal, a comprehensive, international database for X-ALD and other rare diseases is needed.
Prepublished online as Blood First Edition Paper, April 8, 2004; DOI 10.1182/blood-2003-10-3402.
Supported in part by grants from the National Institutes of Health (NS 29099 to W.K.), University of Minnesota Pediatric Hematology/Oncology, Blood and Marrow Transplantation program (C.P.), the Children's Cancer Research Fund (C.P.), and an anonymous private foundation (C.P.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Colin Steward for his critical review of the manuscript; Janet Hegland, Gerald C. Korblick, Marianne Hesslington, Jill Varner, and Sarah Brumberg for the numerous contributions and support; and the dedicated and skilled nursing and supportive care provided to these patients at transplantation centers around the world. Finally, this report would not have been possible without the contributions of the following clinicians and their coworkers from 43 international transplantation centers.
An asterisk indicates the 32 centers from which complete data were available on the 94 patients reported in this manuscript. All Children's Hospital, St Petersburg, FL, Martin Klemperer, MD; Birmingham Children's Hospital, NHS Trust, Birmingham, United Kingdom, P. J. Darbyshire, MD, and Anne Green, MD; *Bristol Royal Hospital for Sick Children, Bristol, United Kingdom, Colin G. Steward, MD; *British Columbia's Children's Hospital, Vancouver, BC, Canada, Jeffrey H. Davis, MD, and Kirk R. Schultz, MD; *Cardinal Glennon Children's Hospital, St Louis University, St Louis, MO, Donna Wall, MD; Charite-Virchow Klinikum, Berlin, Germany, Wolfram Ebell, MD; *Children's Hospital Medical Center; Cincinnati, OH, Richard E. Harris, MD; *Children's Hospital of Philadelphia, Philadelphia, PA, Paige Kaplan, MD; *Children's Medical Center/Nagoya First Hospital; Nagoya, Japan, Koji Kato, MD; *Cook Children's Medical Center/Ft Worth Children's Hospital, Ft Worth, TX, David M. Friedman, MD; Dana-Farber Cancer Institute; Boston, MA, Clare J. Twist, MD; *Duke Children's Hospital and Health Center, Duke University, Durham, NC, Paul Martin, MD, and Joanne Kurtzberg, MD; *Emory University, Atlanta, GA, Raymond D. Cheng, MD, and Michael Boyer, MD; Great Ormond Street Hospital, London, United Kingdom, D. M. Lewis, MD, and Ashok Vellodi, MD; *Hadassah Medical Organization/University Hospital, Jerusalem, Israel, Shimon Slavin, MD, and Arnon Nagler, MD, MSc; Hannover Medical University, Hannover, Germany; *Health Sciences Centre, Winnipeg, Manitoba, Canada, Marlis Schroeder, MD, and Cheryl R. Greenberg, MD; *Heinrich Heine Universitat Dusseldorf, Dusseldorf, Germany, Stefan Burdach, MD, PhD; *Hôpital Maisonneuve-Rosemont, Montreal, Quebec, Canada, Bernard Lemieux, MD, and Dennis-Claude Roy, MD; *Hôpital Saint-Vincent de Paul, INSERM, Paris, France, Patrick Aubourg, MD; *Hôpital Ste-Justine, Montreal, Quebec, Canada, Grant A. Mitchell, MD; *Hospital for Sick Children, Toronto, Ontario, Canada, Fred Saunders, MD, and J. T. R. Clarke, MD, PhD; *Johns Hopkins Hospital, Baltimore, MD, Allen R. Chen, MD; *Karolinska Institute/Huddinge University Hospital, Stockholm, Sweden, Olle Ringdén, MD, PhD; Mt. Sinai Hospital, New York, NY, Adrianna Vlachos, MD; *National Taiwan University, Taiwan, Kai-Hsin Lin, MD; New Children's Hospital/Royal Alexandra Hospital for Children; Sidney, NSW, Australia, Peter Shaw, MD; Royal Children's Hospital and District Health Service, Melbourne, Australia, Jim McGill, MD; *Sidney Children's Hospital, Randwick, NSW, Australia, Cecilia Oswald, MD; St Anna Kinderspital, Vienna, Austria, Christina Peters, MD; *St Louis Children's Hospital, Washington University School of Medicine, St Louis, MO, Robert J. Hayashi, MD; *Texas Children's Hospital/Baylor College of Medicine, Houston, TX, Angela K. Ogden, MD; *Tokai University School of Medicine, Tokai, Japan, Shunichi Kato, MD, PhD; *Universitaetsklinik fuer Kinderheilkunde u Jugendmedizin, Tuebingen, Germany, Thomas Klingebiel, MD; *University of California San Francisco, San Francisco, Morton Cowan, MD; *University of Chicago (La Rabida) Children's Hospital, Chicago, IL, Charles M. Rubin, MD; *University of Iowa Hospital and Clinics, Iowa City, Fred Goldman, MD; *University of Minnesota, Minneapolis, Charles Peters, MD, and William Krivit, MD, PhD; *University of Padova, Padova, Italy, Alberto Burlina, MD; *University of the Witwatersrand, Johannesburg, South Africa, Richard J. Cohn, MD; University Children's Hospital, Utrecht, The Netherlands, Nico Wulffraat, MD, PhD; *Wolfson Children's Hospital/Nemours Children's Clinic; Jacksonville, FL, Michael J. Joyce, MD, PhD; *Yale University, New Haven, CT, Joel Rappaport, MD.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal