Abstract
Postulating favorable antileukemic effect with improved safety, we used intravenous busulfan and fludarabine as conditioning therapy for allogeneic hematopoietic stem cell transplantation (HSCT) for acute myelogenous leukemia (AML) and myelodysplastic syndrome (MDS). Fludarabine 40 mg/m2 and intravenous busulfan 130 mg/m2 were given once daily for 4 days, with tacrolimus-methotrexate as graft-versus-host disease (GVHD) prophylaxis. We treated 74 patients with AML and 22 patients with MDS; patients had a median age of 45 years (range, 19-66 years). Only 20% of the patients were in first complete remission (CR) at transplantation. Donors were HLA-compatible related (n = 60) or matched unrelated (n = 36). The CR rate for 54 patients with active disease was 85%. At a median follow-up of 12 months, 1-year regimen-related and treatment-related mortalities were 1% and 3%, respectively. Two patients had reversible hepatic veno-occlusive disease. Actuarial 1-year overall survival (OS) and event-free survival (EFS) were 65% and 52% for all patients, and 81% and 75% for patients receiving transplants in CR. Recipient age and donor type did not influence OS or EFS. Median busulfan clearance was 109 mL/min/m2 and median daily area-under-the-plasma-concentration-versus-time-curve was 4871 μmol-min, with negligible interdose variability in pharmacokinetic parameters. The results suggest that intravenous busulfan-fludarabine is an efficacious, reduced-toxicity, myeloablative-conditioning regimen for patients with AML or MDS undergoing HSCT.
Introduction
Busulfan-based chemotherapy is a popular conditioning treatment for hematopoietic stem cell transplantation (HSCT). Most commonly, oral busulfan (Bu) is given every 6 hours for 3 to 4 days followed by cyclophosphamide (Cy), given in 2 (BuCy2) to 4 daily doses (BuCy4).1-3 This combination is generally well tolerated, but unpredictable intestinal absorption and erratic bioavailability of oral busulfan has been associated with serious, often lethal, hepatic veno-occlusive disease (VOD).4 Because intravenous delivery of solubilized busulfan yields 100% bio-availability,5 a parenteral busulfan formulation was developed.6 We postulated that an intravenous BuCy2 regimen would yield less toxicity and overall improved safety compared to BuCy2 with oral busulfan.7,8 Although our studies suggested a lower incidence of VOD than expected after oral BuCy2,8 we still experienced some VOD. The risk of this implication is of particular concern when busulfan is combined with another agent, for example, cyclophosphamide, which is known to cause VOD.9,10 Furthermore, regimen-related toxicity, engraftment, and acute graft-versus-host disease (GVHD) may be altered by variable systemic busulfan exposure.11-16
Fludarabine is being increasingly used for HSCT conditioning and is not known to enhance VOD. Furthermore, it has been shown to be as immunosuppressive as cyclophosphamide when combined with total body irradiation (TBI).17 Further, Gandhi and Plunkett18 demonstrated that fludarabine potentiates alkylator-induced cell killing through inhibition of DNA damage repair. The nucleoside analog has previously been used in preparative regimens for allogeneic HSCT combined with oral busulfan in total doses of 8 to 16 mg/kg, and with melphalan at 140 to 180 mg/m2.19-21 Fludarabine has a long plasma half-life, allowing once-daily administration. We proposed to follow each dose with 1 busulfan dose to achieve synergistic cytotoxicity.18 Recently, safety and efficacy data were obtained with a once-daily intravenous busulfan and fludarabine conditioning regimen for allogeneic HSCT in patients with hematologic malignancies.22 That report demonstrated the combination to be efficacious, with impressively low treatment-related mortality (TRM). Accompanying preliminary pharmacokinetic (PK) data indicated highly reproducible intrapatient and interpatient busulfan exposure.22
Allogeneic transplantation for acute myelogenous leukemia (AML) and myelodysplastic syndrome (MDS) is commonly associated with 100-day TRM rates of 10% to 40%, with higher rates seen with alternative donors and in patients with advanced disease or older age.20,23,24 Nonlethal toxicity is also a problem, preventing intensified conditioning and posttransplantation interventions to prevent relapse.
Based on these considerations, we hypothesized that once-daily intravenous busulfan combined with fludarabine would yield improved safety and acceptable antileukemic activity. Here we report the results of using this combination as conditioning for HSCT in high-risk AML and MDS patients, including PK information gained using intravenous busulfan in these patients.
Patients and methods
Patient eligibility
AML patients should have failed initial induction chemotherapy or have high-risk disease in first complete remission (CR1), characterized by cytogenetics other than t(8;21), inv(16), or t(15;17), or by the need for more than one cycle of chemotherapy to achieve CR.25 Patients in CR beyond CR1 were also eligible. Subjects with MDS were eligible if they had a high International Prognostic Score System (IPSS) score (≥ 2)26 or if they progressed after chemotherapy.
The eligibility criteria included acceptable renal and hepatic function with alanine aminotransferase (ALT) less than or equal to 3 times the upper normal limit; a ZUBROD performance status less than or equal to 2; negative serology for hepatitis B or C and HIV; left ventricular ejection fraction (LVEF) 45% or more; forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), and diffusing capacity of lung for CO2 (Dlco) equal to or more than 50% of predicted; absence of uncontrolled infection; and no chemotherapy within 30 days prior to entry. An HLA-compatible related (fully matched or one antigen mismatched) or matched unrelated donor (MUD) was required. All patients signed informed consent according to institutional guidelines. One patient was treated off protocol with approval from M.D. Anderson Cancer Center's Institutional Review Board, under a “compassionate plea” mechanism due to chronic renal failure developed after a previous nonmyeloablative transplantation.
Conditioning regimen
The treatment was modified from Russell et al22 and consisted of fludarabine (Berlex Laboratories, Wayne, NJ), 40 mg/m2 given over 60 minutes daily for 4 days, each dose immediately followed by intravenous busulfan6 (ESP Pharma, Edison, NJ), 130 mg/m2 over 3 hours daily (days -6 to -3). The drug was infused via a controlled-rate infusion pump through a central venous catheter (CVC). Dosing was based on patients' actual weight up to 120% of ideal body weight, above which it was based on adjusted ideal body weight (ideal weight plus 50% of the difference between ideal and actual weight). Patients with a one-antigen mismatched related donor or a MUD received equine antithymocyte globulin (Pharmacia & Upjohn, Kalamazoo, MI) 20 mg/kg daily (days -3to -1). Day 0 was the day of transplantation.
Supportive care
Phenytoin 300 mg orally was used during and 1 day after intravenous busulfan therapy, starting the evening before or in the morning of the first dose. All supportive care measures were used according to extant institutional protocols. All patients received filgrastim (Amgen, Thousand Oaks, CA) 5 μg/kg subcutaneously daily from day +7 until achieving an absolute neutrophil count (ANC) equal to or more than 1.5 × 109/L for 3 days.
GVHD prophylaxis consisted of tacrolimus (Fujisawa Healthcare, Deerfield, IL) adjusted to maintain whole blood trough levels of 5 to 15 ng/mL and mini-dose methotrexate 5 mg/m2 on days 1, 3, 6, and 11 following transplantation.27 Tacrolimus was to be continued for 6 to 8 months. Pentostatin (Supergen, Dublin, CA) was added in 10 cases receiving MUD grafts (n = 8) or one-antigen mismatched related donor grafts (n = 2), under an investigational protocol. The dose of pentostatin was 0.5 mg/m2 (n = 2), 1 mg/m2 (n = 4), and 1.5 mg/m2 (n = 4) given on days 8, 15, 22, and 30 following HSCT.
Hematopoietic stem cell grafts
Procurement of donor peripheral blood progenitor cells (PBPCs) has been described.28 Donors were treated with recombinant human granulocyte colony-stimulating factor (rhG-CSF) 10 to 12 μg/kg every 12 hours over 3 days and in the morning of day 4 prior to PBPC collection. Three times the donor's total blood volume was processed by an apheresis procedure. In case a second procedure was performed, rhG-CSF treatment was continued through prior to the second apheresis procedure. The PBPC dose was targeted to approximately 5 × 106 CD34+ cells/kg patient body weight, in keeping with our data indicating a correlation between higher cell doses and incidence of GVHD.29 Bone marrow or PBPCs from unrelated donors were obtained through the National Marrow Donor Program.
HLA typing
HLA typing for class I antigens was performed using standard serologic techniques. Class II alleles (HLA-DRB1) were resolved with low-resolution molecular typing using sequence-specific oligonucleotide primers for hybridization of amplified DNA, followed by high-resolution typing in all patients and donors. Donor-recipient pairs were considered fully matched by compatibility for HLA-A, -B, and -DRB1.
Analysis of chimerism
Peripheral blood or bone marrow donor-recipient chimerism was evaluated by analysis of DNA microsatellite polymorphisms by polymerase chain reaction (PCR) with D6S264, D3S1282, D18S62, and D3S1300 fluorescence-labeled primers, then analyzed using GeneScan software (Applied Biosystems, Foster City, CA) in all cases. In addition, we used conventional cytogenetic analysis with G-banding or fluorescent in situ hybridization studies for the Y chromosome in sex-mismatched cases. Mixed chimerism was defined as the presence of any detectable (≥ 1%) recipient DNA or cells in addition to donor-derived DNA or cells.
Clinical outcome variables
Engraftment was defined as the first of 3 consecutive days with ANC equal to or more than 0.5 × 109/L. Failure to engraft by day +30 was considered primary engraftment failure. Secondary graft failure was initial engraftment with documented donor-derived hematopoiesis followed by loss of graft function without recurrent malignancy. Platelet engraftment was defined as the first of 7 consecutive days with a platelet count of 20 × 109/L or more without transfusion support. Criteria for CR prior to transplantation included absence of circulating blasts, less than 5% marrow blasts, lack of chromosomal abnormalities, and platelet count equal to or more than 100 × 109/L. CR after transplantation was defined using the same criteria except for platelet count, with donor cell engraftment.
Cytogenetics were considered prognostically favorable for patients with t(15;17), inv(16), or t(8;21); poor risk for patients with deletions of chromosome 5 or 7 or both, multiple chromosomal abnormalities, and trisomy of chromosome 8; and intermediate risk in all others. Standard morphologic criteria, conventional cytogenetics, or both were used to diagnose recurrent disease. Cytogenetic relapse was documented by the presence of a clonal chromosomal abnormality in more than 2 consecutive tests, taken at least 4 weeks apart. Time to relapse/progressive disease was calculated from transplantation to the day of documented event. Patients who did not achieve a CR after transplantation were scored as failures at the date of documented persistent disease. Toxicity was scored using the modified National Cancer Institute (NCI) Common Toxicity Criteria version 2.0 (NCI, Bethesda, MD).
Overall survival (OS) was calculated from the day of transplantation, with patients alive at the time of last follow-up administratively censored, and TRM was defined as death due to any cause other than relapse. Event-free survival (EFS) time was counted from day 0 to relapse or death.
Adverse events and hematologic parameters were monitored daily, clinical chemistry parameters at least twice weekly during the initial hospitalization, and then at increasing intervals up to day HSCT +100. Subsequently, patients were followed at least quarterly during the first year, then at gradually increasing intervals.
Statistical methods
The 2 major patient outcomes used for safety monitoring were death within the first 30 days and TRM between transplantation days +31 and +100. In this single-arm trial, Bayesian early stopping rules based on the observed rates of these 2 outcomes were implemented separately for patients with a good prognosis (first or subsequent remission or untreated disease prior to HSCT) and those with a poor prognosis (all others). Given the low TRM, treatment was not stopped in either prognostic group.
Unadjusted OS and EFS were estimated by Kaplan-Meier curves.30 Differences in OS or EFS between subgroups were evaluated using the log-rank test.31 The Cox proportional hazards regression model32 was used to assess the ability of patient characteristics (covariates) to predict OS and EFS time, with goodness- of-fit assessed by the Grambsch-Therneau test and Martingale residual plots,33 smoothed using the lowess method of Cleveland.34 Covariates used in the Cox models were transformed as appropriate based on these plots. For each Cox model regression analysis, a final model was obtained by performing a backward elimination with P cutoff at .05, allowing any previously deleted covariate to enter the final model if its P was less than .05. The covariate used to represent the patient's bone marrow blast count was log (BM.blast.cell + 1), where “BM.blast.cell” was the percentage of blasts multiplied by bone marrow cellularity. The log transformation was used so that the covariate effect followed the Cox proportional hazards model assumption. Because the bone marrow blast count, the presence/absence of peripheral blood blasts, and the indicator of whether the patient was in CR at time of transplantation were highly associated, to avoid colinearity and unstable model fits at most one of these covariates was included in any model considered. Chronic GVHD was included in the Cox proportional hazards model as a time-varying covariate, taking on the value 0 up to the time of onset of chronic GVHD, and the value 1 thereafter.35 This resulted in 3 different multivariate Cox models for OS and 3 for EFS. These models were compared using the Bayes Information Criterion (BIC).36 Each covariate was compared between the 2 groups of patients who did or did not survive 100 days without progression, using a Wilcoxon signed rank test for quantitative covariates and Fisher exact test for categorical covariates. All computations were carried out in Splus37 using standard Splus functions and the Splus survival analysis package of Therneau.38
PK studies
Blood samples for busulfan plasma levels were collected before drug administration and at 15, 90, and 175 minutes (5 minutes before the end of infusion; “peak”) after starting the first dose. We also obtained samples at 15 and 30 minutes, and at 1, 2, 4, 6, 8 to 10, and around 21 hours after the end of infusion (before the next dose). This sampling schedule was repeated after the third or fourth dose whenever possible. Blood for PK analyses was collected from a peripheral line to avoid sample contamination caused by the proximity between the different CVC ports. Samples were collected in heparinized tubes and transported to the laboratory in ice. Plasma was separated and cryopreserved at -70°C until analysis with high-pressure chromatography after derivatization with diethyldithiocarbamate.39 There was no detectable drug degradation in samples stored for up to 6 months under these conditions. Busulfan peak concentrations (Cmax) and the corresponding peak time (Tmax) were observed values. The area under the plasma concentration-versus-time curve (AUC) per busulfan dose was calculated by dividing the administered dose by the final plasma clearance estimate, whereas the plasma clearance was determined by modeling all plasma concentration versus time data. Parameters such as volume of distribution of the central compartment, elimination rate constant, and micro constants were estimated, whereas steady-state volume of distribution, half-lives (T1/2), and clearance were calculated from the primary parameters.40 All concentration-time plasma busulfan data were analyzed using an open one-compartment PK model. PK modeling was performed using the ADAPT II software program, version 4.0 (Biomedical Simulation Resource, University of Southern California, Los Angeles, CA).41
Results
Patient, disease, and donor characteristics
Patient and disease characteristics are summarized in Table 1. This was a heavily pretreated group, with only 20% of the patients in CR1, and 56% having active disease at HSCT. Median time from diagnosis to transplantation was 12 months (range, 2-88 months), and median CR1 duration was 4 months (range, 0-73 months). Nineteen patients were in CR1; 4 of them required more than one cycle of chemotherapy to achieve CR, and 15 subjects had intermediate or poor prognosis cytogenetics, or secondary AML. The median CR duration of subjects receiving transplants in CR1 was 4 months (range, 0.7-12 months), and median duration of first CR for patients receiving transplants in second or third CR was 19 months (range, 1-73 months). Only 2 of the 23 patients in later CR at HSCT had “favorable” karyotype, whereas 19 had “intermediate” and 2 had “poor risk.” All subjects with favorable cytogenetics underwent transplantation with active disease (induction failure or relapse) or in second/third CR. Secondary AML/MDS was diagnosed in 13 subjects, and 14 cases of AML had evolved from MDS.
Thirteen patients with MDS had refractory anemia with excess blasts, 5 had refractory anemia with excess blasts in transformation, 2 had chronic myelomonocytic leukemia, and 2 had refractory anemia. The latter 4 patients had intermediate-2 IPSS scores.
Donors other than HLA-identical siblings were used in 40 cases. Bone marrow was used in 47 cases (49%), and 49 patients received PBPCs.
Engraftment and chimerism
The median time to neutrophil engraftment was 12 days (range, 9-25 days), and to platelet engraftment 13 days (range, 0-125 days), respectively (Table 2). A platelet count more than 100 × 109/L was observed in 64 cases (67%), at a median of 22 days (range, 12-89 days).
Sixty-two (70%) of 89 evaluable patients were complete chimeras by HSCT day +30. This proportion was 54% among recipients of HLA-identical sibling transplants and 85% among unrelated and one-antigen mismatched donor HSCT recipients. In the subgroup of patients who were in CR but mixed chimeras around day +30, conversion to complete chimerism was seen in most at the re-evaluation around day +100; at this time, the entire group had 78 patients in CR and 1 patient had autologous hematopoiesis after primary graft rejection. Chimerism studies revealed that 66 (84%) of 79 were complete chimeras (100% donor), whereas 12 were stable mixed chimeras with a median of 98% donor cells (range, 83%-99%). Median T-lymphocyte and myeloid cell donor chimerism on day 100 and at 6 months was 100% (Table 2).
Disease response
The CR rate was 85% (n = 47 responders) among 54 patients undergoing transplantation with active disease. Two of the nonresponders had untreated MDS, 3 had failed primary induction therapy, and 2 had AML in a chemotherapy-refractory relapse.
Treatment-related toxicity
There was one regimen-related death in this group of predominantly advanced-stage disease patients. This occurred in a patient with previously treated fungal pneumonia and congestive heart failure who developed engraftment-related cytokine syndrome complicated by pulmonary hemorrhage in the setting of persistent AML.
Transient elevation of liver function tests was a common finding, with ALT peaking during the first week and bilirubin during the second week after HSCT. Reversible NCI grades III and IV elevation of transaminases and bilirubin occurred in 18% and 9% of the cases, respectively. The typical pattern of bilirubin elevation included a transient increase to 2 to 3 times baseline values within 2 days of administering methotrexate for GVHD prophylaxis, returning to normal within another 2 to 3 days. Only in 2 cases could a clinical diagnosis of mild-to-moderate VOD be ascertained using Jones' criteria.9 One case was a 63-year-old man with secondary AML who previously received cyclophosphamide-TBI and autologous HSCT for lymphoma. The second case was a 62-year-old MDS patient who on his own account ingested large doses of acetaminophen for several days immediately preceding busulfan administration.
Grade III mucositis, diarrhea, and abdominal pain occurred in 13% of the patients, and hemorrhagic cystitis in 3%. Another 4% developed “hand-foot syndrome” with neuropathic pain, similar to what they previously experienced after ara-C (cytarabine) exposure.
Significant central nervous system (CNS) toxicity was not observed. One patient developed seizures related to high tacrolimus levels 6 weeks after HSCT. A patient with MDS had a long history of petit mal seizures after previous brain surgery for a malignant glioma, but she neither developed grand mal attacks nor had any increased frequency of petit mal seizures during or after the conditioning regimen. Table 3 details toxicity.
GVHD
The rates of grades II to IV and III/IV acute GVHD were 25% and 5%, respectively (Table 4). This included 4 patients who developed acute GVHD on withdrawal of immunosuppression at leukemia recurrence. Grades II to IV and III/IV acute GVHD among MUD recipients were 43% and 11%, respectively. Forty-eight patients (55%) developed chronic GVHD (63% after MUD transplantation and 53% after related donor grafts). It was extensive in 26 and limited in 22 cases.
Relapse
Thirty-three patients (34%) have had a relapse at a median time of 4 months after transplantation (range, 1.5-12 months), and 7 patients were refractory to treatment. Twenty-five (76%) of the relapses occurred during the first 6 months after treatment. Among subjects receiving transplants in CR1, 5 (26%) have had relapse at 2.5, 3, 4, 4.7, and 5.5 months after transplantation. These patients had poor prognostic characteristics in common, such as high-risk cytogenetics (n = 3) and secondary AML with diploid cytogenetics (n = 2). Six of the 23 CR2 patients have had relapse at 2, 4, 4.5, 4.5, 6, and 6.6 months after transplantation (2 had poor prognosis and 4 had intermediate-risk cytogenetics). Overall, 11 (26%) of 43 patients receiving transplants in CR, and 23 (43%) of 53 patients with active disease at HSCT have had relapses.
OS and EFS
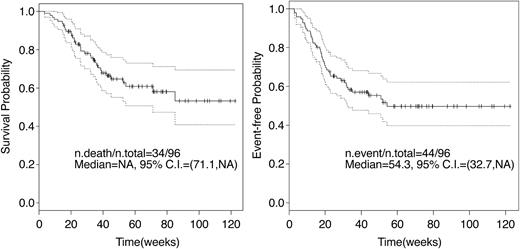
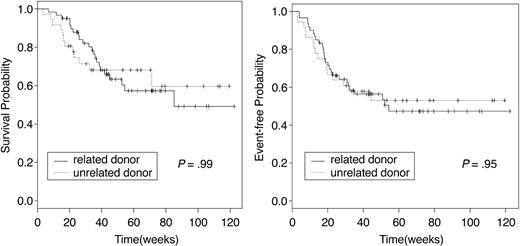
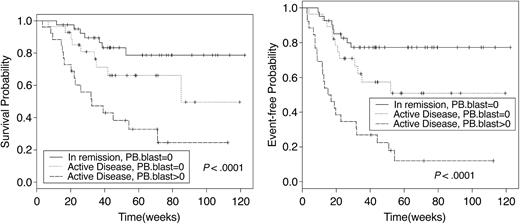
Sixty-two patients are alive at a median follow-up of 12 months. Causes of death were chronic GVHD (n = 2), disease relapse or progression (n = 31), and regimen-related toxicity and refractory disease (n = 1). The median OS of the 34 patients who died was 6 months (95% CI, 4.8-8.1 months). Overall, 100-day posttransplantation mortality was 5% (n = 5). All deaths before day 100 were associated with persistent or relapsing disease. One-year actuarial OS and EFS for the entire group were 65% and 52%, respectively (Figure 1). However, among those receiving transplants in CR, the 1-year OS and EFS were 81% and 75%, respectively, contrasting with 52% and 34%, respectively, for those receiving transplants with active disease. Related and MUD recipients had similar outcomes (Figure 2). The presence of circulating blasts at HSCT was associated with significantly worse OS and EFS, both when compared with patients given transplants in CR and with those in relapse without circulating blasts (Figure 3). AML and MDS patients had similar OS and EFS. The 1-year EFS for patients with deletions of chromosome 5 or 7 was 34% (95% CI, 19%-61%). Development of acute GVHD did not influence outcome. Chronic GVHD, when considered as a time-varying covariate, was not a significant predictor of survival (P = .71) or EFS (P = .45; Table 5). OS and EFS of patients with mixed chimerism on transplantation day 30 was similar to that of fully chimeric individuals (1-year EFS of 58% versus 48%, respectively, P = .86, and 1-year OS of 66% versus 62%, respectively, P = .84).
OS and EFS. Kaplan-Meier curves of OS and EFS for all patients. NA indicates not achieved; and n, number. Dotted lines indicate the confidence interval.
OS and EFS. Kaplan-Meier curves of OS and EFS for all patients. NA indicates not achieved; and n, number. Dotted lines indicate the confidence interval.
OS and EFS by donor type. OS and EFS of patients receiving related or unrelated donor transplants were similar.
OS and EFS by donor type. OS and EFS of patients receiving related or unrelated donor transplants were similar.
OS and EFS survival by disease status and peripheral blood blasts. Patients with active disease at HSCT were divided into 2 subgroups according to the presence or absence of circulating blasts. The outcome of patients with refractory disease without circulating blasts was significantly better than that of patients with peripheral blood disease. PB indicates peripheral blood.
OS and EFS survival by disease status and peripheral blood blasts. Patients with active disease at HSCT were divided into 2 subgroups according to the presence or absence of circulating blasts. The outcome of patients with refractory disease without circulating blasts was significantly better than that of patients with peripheral blood disease. PB indicates peripheral blood.
Associations among variables and Cox model analyses
As expected, patients with active disease undergoing transplantation had a shorter OS and EFS. Subjects with circulating blasts at the time of transplantation had a worse outcome than those with disease confined to the bone marrow. These results suggest that the presence of peripheral blood blasts may be a reliable indicator of a heavier disease load, hence predicting worse survival in this group of patients. The 3 fitted Cox models for OS (Table 5) show that a higher level of bone marrow blasts, or presence of peripheral blood blasts, each predicts worse survival. Similar results were obtained for EFS (Table 5).
PK studies
Busulfan PK parameters were calculated from the data obtained from blood samples of 45 consenting patients. The drug was cleared in less than 24 hours without drug accumulation observed over the 4-day dosing interval, and with little (< 20%) interdose variation in PK parameters between day 1 and day 3 or 4. The mean (CV%) population Cmax,Vd,T1/2, and Cl for once-daily dosing were 3.6 μg/mL (13.8%), 22.6 L/m2 (20.2%), 2.7 hours (27.5%), and 109 mL/min/m2 (26%). Overall interdose variability in the day-to-day estimated clearance was less than 20% from day 1 to day 3 or 4. The mean and median daily AUCs were 4891 and 4871 μmol-min, respectively (range, 2931-8271 μmol-min).
Discussion
Searching for an alternative to TBI, Santos and coworkers introduced oral busulfan into pretransplantation conditioning therapy.1-3 However, early TRM remained a major obstacle when the oral formulation was used in myeloablative regimens. Deeg and colleagues42 have shown that outcomes of patients with MDS treated with oral busulfan and cyclophosphamide can be improved by careful adjustment and targeting of busulfan dosing, with multiple individualized adjustments based on plasma drug levels. In the referenced study, nonrelapse mortality at 100 days after transplantation was 12% after related and 13% after MUD transplantations. Nonrandomized comparisons between patients treated with intravenous BuCy2 and those receiving unadjusted oral BuCy or TBI-based therapy appear to favor the intravenous busulfan-based conditioning regimen,7,8,43 although definitive data from randomized studies are lacking. We hypothesized that the more precise dose delivery achieved with intravenous busulfan and a tighter range in systemic drug exposure would lead to less regimen-related toxicity, less graft rejection, and a lower risk of leukemic recurrence.
Furthermore, it became clear from the careful and elegant studies of Vaughan et al44 and McDonald et al10 that hepatic irradiation as well as the use of high-dose cyclophosphamide are associated with an increased risk of VOD. The latter investigators found a strong correlation between blood levels of various cyclophosphamide metabolites and VOD. It is conceivable that the formation of these metabolites deplete the liver of glutathione (GSH), resulting in serious toxicity. This risk would be exacerbated if cyclophosphamide were administered with busulfan, which also effectively reduces hepatic GSH stores. However, other interactive mechanisms of normal organ toxicity are also possible.
Gandhi and Plunkett18 reported that nucleoside analogs combined with alkylating agents exert synergistic cell kill, but sequence and timing to reach maximum synergy between the agents mandated that the target tissues be exposed to fludarabine prior to the alkylator. Fludarabine is not known to deplete GSH content. The described observations formed a compelling argument for replacing cyclophosphamide with fludarabine in a busulfan-based conditioning regimen. The long T1/2 of fludarabine allows it to be administered once daily, so that maximum intracellular busulfan levels occur concomitantly with optimal fludarabine-triphosphate concentrations when busulfan was administered immediately following fludarabine. Single daily busulfan doses produce higher peak concentrations than the same total dose delivered on a 4-times-daily schedule, which should produce better penetration of poorly vascularized “sanctuary sites.” Furthermore, dosing based on body surface area rather than on body weight would provide a closer adjustment for changes in systemic exposure with changing body size.45,46 This has for a long time been common practice with most chemotherapeutic agents.
The current study demonstrated that the intravenous busulfanfludarabine combination was very well tolerated, with only one death due to regimen-related complications. Likewise, failure to engraft was seen in only one patient. Overall, 5 patients died during the first 100 days after transplantation. All had persistent or recurrent disease, but in one the direct cause of death was pulmonary hemorrhage. Beyond the first 100 days, the most common cause of death was recurrent disease, followed by GVHD. Nonrelapse mortality at 12 months was 3%. Our findings contrast with the report of Bornhauser et al,21 who reported a 100-day TRM of 7%, and nonrelapse mortality of 18%, using a regimen of fludarabine and PK-targeted oral busulfan given 4 times daily for 4 days. They treated a group of older patients (median age of 52 versus 45 years in our cohort). However, our study included 30 patients older than 50 years, and age did not influence outcome. We observed that by day 100 and 180 after HSCT, our patients had a median T-lymphocyte and myeloid cell chimerism of 100%, values that are slightly higher than those observed after oral busulfan and fludarabine (median T-cell chimerism of 75% on day 75 and of 98% 1 year after transplantation).21 Further, the early mixed chimerism rate after intravenous busulfanfludarabine was no different from that seen after intravenous BuCy (12%).7 One is led to conclude that more consistent delivery of pretransplantation conditioning therapy is of major importance for the safety of the whole transplantation process.8,12,15,16,22,42-44,47
Although our patients represented a high-risk population with mostly active AML, the incidence of VOD was low (2 cases). However, reversible bilirubin elevations in the absence of GVHD occurred in 46% of the patients. This typically occurred within a few days of administering methotrexate for GVHD prophylaxis, with rapid resolution in most cases. Although both drugs are known to penetrate the CNS and have been connected with serious neurotoxicity, our patients experienced no such problems. The moderately severe side effect spectrum confirmed the observations of Russell and coworkers.22
Our data indicate that daily intravenous busulfan yields reproducible and predictable PK, with considerably less interdose and interpatient variability than anticipated with oral busulfan. PK patterns on day 1 and on days 3 or 4 virtually overlapped, and there was no detectable interdose drug accumulation. The median T1/2 of about 2.7 hours is not significantly shorter than the median of 2.9 hours previously reported using every 6 hours dosing.7,48 The target systemic exposure dose (AUC) of 900 to 1500 μmol-min per dose in intravenous BuCy2 would be equivalent to a daily systemic exposure dose of 3600 to 6000 μmol-min, close to our median level of 4871 μmol-min. This median number is well below the cumulative daily AUC of 6000 μmol-min previously associated with an increased risk of VOD.47 The daily AUC exceeded this exposure in about 10% of our patients without encountering serious hepatic adverse effects.
We did not perform a direct, randomized comparison of intravenous busulfan-fludarabine with either the BuCy or TBI-based regimens. However, EFS in our cohort appears to be comparable to that reported previously with either regimen.49,50 Bearing in mind the controversy of TBI-based versus non-TBI–based preparative regimens for transplantation of AML patients, the low regimen-related mortality observed here may confer a survival advantage by decreasing the high early mortality rates reported in the past, especially among heavily pretreated patients with active disease. The reduced toxicity profile is further illustrated when comparing outcomes of recipients of matched related grafts with the outcomes of alternative donor transplants. There was no difference in OS or EFS between these subgroups, contrasting with previous observations indicating a worse prognosis for MUD transplant recipients in all phases of AML and MDS.24
Development of acute or chronic GVHD did not have an impact on OS or EFS. It is possible that the low incidence of acute GVHD in this group was due at least in part to the low toxicity of the regimen, as previously suggested.16
The well-known impact of advanced disease stage on prognosis was illustrated here. However, patients with active disease at HSCT could be divided in 2 subgroups, depending on the presence or absence of circulating blasts, and patients without circulating blasts had significantly better OS and EFS. Despite significant advances in both therapy and supportive care over the last 3 decades, long-term survival of patients with refractory AML or MDS has remained poor.51,52 Accordingly, disease relapse was the major cause of treatment failure, with a median time to disease progression of only 4 months. This subgroup of patients might benefit from additional early interventions after transplantation, such as antitumor vaccines or targeted T cell–specific therapy, or from complementary pharmacologic strategies. In this context, a myeloablative regimen with low toxicity profile is likely a valuable contribution. The conditioning program can be considered a platform on which to add posttransplantation treatments that will be better tolerated.
The more predictable behavior of intravenous busulfan could make it possible to safely escalate the doses as part of a strategy to intensify therapy based on systemic drug exposure, rather than on body weight. Pharmacologic monitoring and individualized dose adjustments are possible, as shown by the low intrapatient and interpatient variability in PK parameters seen here. Ongoing studies include the implementation of individualized therapy based on PK parameters obtained from a test dose given prior to the first dose of the actual high-dose regimen. Based on the observed adverse effect spectrum, it appears questionable whether PK-directed individualized dosing will improve safety, although more precise dose delivery may be of value to increase EFS of patients at high risk for disease recurrence. A more widespread use of individualized dosing based on PK parameters should await the definition of a therapeutic systemic exposure interval for intravenous busulfan and fludarabine, similar to that recently reported with the intravenous BuCy2 regimen in chronic myelogenous leukemia.16 Hopefully, our promising preliminary results will lead to enrollment of larger numbers of patients and eventually to the conduct of randomized, multicenter studies designed to compare this conditioning regimen against “standard” myeloablative combinations, such as cyclophosphamide-TBI.
Prepublished online as Blood First Edition Paper, April 8, 2004; DOI 10.1182/blood-2004-02-0414.
Supported in part by National Institute of Health grants 2PO1 CA55164 and 2P30CA16672-26.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.