Abstract
Plasmacytoid dendritic cells (PDCs) are potent regulators of immune function and the major source of type I interferon (IFN) following viral infection. PDCs are found at sites of inflammation in allergic reactions, autoimmune disorders, and cancer, but the mechanisms leading to the recruitment of PDCs to these sites remain elusive. During inflammation, adenosine is released and functions as a signaling molecule via adenosine receptors. This study analyzes adenosine receptor expression and function in human PDCs. Adenosine was found to be a potent chemotactic stimulus for immature PDCs via an A1 receptor–mediated mechanism. The migratory response toward adenosine was comparable to that seen with CXCL12 (stromal-derived factor-1α [SDF-1α), the most potent chemotactic stimulus identified thus far for immature PDCs. Upon maturation, PDCs down-regulate the A1 receptor, resulting in a loss of migratory function. In contrast, mature PDCs up-regulate the A2a receptor, which is positively coupled to adenylyl cyclase and has been implicated in the down-regulation of DC cytokine-producing capacity. We show that in mature PDCs adenosine reduces interleukin-6 (IL-6), IL-12, and IFN-α production in response to CpG oligodeoxynucleotides (ODN). These findings indicate that adenosine may play a dual role in PDC-mediated immunity by initially recruiting immature PDCs to sites of inflammation and by subsequently limiting the extent of the inflammatory response induced by mature PDCs by inhibiting their cytokine-producing capacity.
Introduction
Dendritic cells (DCs) constitute a highly specialized system of antigen-presenting cells and play important roles in the initiation and regulation of immune responses.1 Two main DC subsets have been identified in human peripheral blood: the CD1c+ “myeloid” DCs and the CD123+ “plasmacytoid” DCs (PDCs).2 PDCs are identical with the type I interferon (IFN)–producing cells3-5 and were previously referred to as plasmacytoid lymphocytes or plasmacytoid monocytes.6,7 PDCs are located in the blood and secondary lymphoid organs and can secrete high amounts of type I IFN (IFN-α, -β, -ω) in response to certain viruses.4,8 Type I IFN production can be mediated by members of the toll-like receptor (TLR) family such as TLR9, a receptor for unmethylated CpG motifs, which are found in the genome of bacteria and viruses at a higher frequency than in vertebrates.9-11 There is increasing evidence that PDCs do not only play a role in innate immune responses by producing high amounts of type I IFN but that PDCs are also powerful regulators of T-cell responses and link innate and adaptive immunity.4,12-17
The localization of PDCs to inflammatory sites in vivo reflects that PDCs probably contribute to various types of immune responses. For instance, increased numbers of PDCs have been observed in inflamed lymph nodes, where they cluster around high endothelial venules.18 PDCs can also be found in inflamed skin lesions, associated with systemic lupus erythematosus,19,20 psoriasis vulgaris and contact dermatitis,20 in epithelioid cell granulomas,21 in nasal mucosa during allergic reactions,22 and in the vicinity of tumors.23,24 However, mechanisms leading to the recruitment of PDCs to inflammatory sites remain unresolved. In vitro, immature PDCs migrate only in response to the chemokine CXCL12 (stromal-derived factor-1α [SDF-1α), the ligand for CXCR4, despite the expression of several other chemokine receptors.25 Migration to CXCL12 may be enhanced by CXCR3 ligands.26 Both CXCR4 and CXCR3 ligands are expressed in high endothelial venules and likely aid PDCs to localize into lymph nodes.25,26 Upon maturation, PDCs acquire the ability to migrate in response to CCL19 (macrophage inflammatory protein-3β [MIP-3β) and CCL21 (6Ckine), ligands of CCR7.25-27 This may allow mature PDCs to home to lymph nodes via lymphatic vessels and to localize in T cell–rich areas.
The nucleoside adenosine is released from activated or stressed cells and dramatically accumulates in tissues during ischemia, inflammation, and tissue damage.28,29 This ubiquitous molecule is used in selective signaling, activating membrane-bound adenosine receptors, denoted A1, A2a, A2b, and A3, which are widely distributed in tissues and mediate diverse biologic effects.30,31 Adenosine has been recognized as an important modulator of immune responses, mediating inflammatory as well as anti-inflammatory effects, such as chemotaxis,32-34 release of allergic mediators,35 reduction of toxic oxygen metabolites,36 neutrophil adherence to endothelium,37 inhibition of T-cell activation,38 and inhibition of cytokine production.39-44 The nonredundant role of adenosine receptors in the regulation of inflammation in vivo has been demonstrated in A2a receptor–deficient mice. In these animals, subthreshold doses of inflammatory stimuli induced extensive tissue damage, increased levels of inflammatory cytokines, and death.45
The first evidence for a potential role of adenosine on the antigen-presenting cell system was gained from studying in vitro generated monocyte-derived DCs. In these cells, adenosine mediated chemotaxis and reduced the capacity to induce T helper (Th)1 type immune responses by inhibition of interleukin-12 (IL-12) production.32,41 No information is available on the function of adenosine on physiological DC types. In this study, we investigated adenosine receptor expression in human PDCs. We propose a dual, maturation-dependent role of adenosine on PDC function: Adenosine may lead to the recruitment of immature PDCs to inflammatory sites via an A1 receptor–mediated mechanism. In contrast, adenosine can also act on mature PDCs expressing A2a receptors by attenuating their cytokine-producing capacity and thereby limiting the detrimental effects of inappropriately sustained cell activation.
Materials and methods
Media and reagents
PDCs were cultured in RPMI 1640 supplemented with 20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 60 mg/L penicillin G, 12.6 mg/L streptomycin, 2 mM l-glutamine, 1% nonessential amino acids, and 10% heat-inactivated fetal calf serum (FCS) (CSL Limited, Melbourne, Australia). Adenosine, CHA (N6-cyclohexyladenosine), DPMA (N6-[2-(3,5-dimethoxyphenyl)-2-(2-methylphenyl)-ethyl]adenosine), IB-MECA (N6-(3-iodobenzyl)adenosine-5′-N-methyluronamide), DPCPX (8-cyclopentyl-1,3-dipropylxanthine), CSC (8-(3-chlorostyryl)caffeine), and MRS 1220 (9-chloro-2-(2-furanyl)-5-((phenylacetyl)amino)-[1,2,4]triazolo [1,5-c]quinazoline) were obtained from Sigma (St Louis, MO). CpG oligodeoxynucleotide (ODN) 2006 (5′-tcgtcgttttgtcgttttgtcgtt-3′) was provided by Coley Pharmaceutical Group (Wellesley, MA) and used at a final concentration of 6 μg/mL. Soluble CD40L trimer (CD40L) was a gift from Immunex, an Amgen company (Seattle, WA). CD40L-transfected BHK cells were a gift from Dr Engelmann (University of Munich, Germany). For activation assays, BHK cells were irradiated with 30 Gy and used as stimulators in a ratio of 1:10 PDCs.
Monoclonal antibodies, cytokines, and ELISA
Fluorochrome-conjugated monoclonal antibodies (mAbs) against CD80, CD86, CD123, HLA-DR, HLA-ABC, CCR7, and CXCR4 were purchased from PharMingen/Becton Dickinson, San Jose, CA; mAbs against BDCA-4 were purchased from Miltenyi Biotec, San Diego, CA. Fluorescence-activated cell sorting (FACS) analysis was performed using a FACScalibur (Becton Dickinson, San Jose, CA). IL-3, CCL21 (6Ckine), and CXCL12 (SDF-1α) were obtained from Peprotech, Rocky Hill, NJ. The human IFN-α enzyme-linked immunosorbent assay (ELISA) (Bender Med Systems, Vienna, Austria), the human IL-12p40, IL-12p70, and IL-6 ELISA (OptEIA; PharMingen/Becton Dickinson) were used according to the manufacturers' protocol.
Isolation of PDCs from the peripheral blood
Peripheral blood mononuclear cells were isolated from buffy coats of healthy volunteers provided by the Red Cross Blood Bank (Melbourne, Australia) by Ficoll-Paque density gradient centrifugation (Amersham Biosciences, Uppsala, Sweden). PDCs were isolated using the BDCA-4 isolation kit (Miltenyi Biotec). Purity of CD123+ HLA-DR+ cells was more than 96%. PDCs were cultured in 96-well plates at 2 × 105 cells per milliliter in RPMI–10% FCS in the presence of 10 ng/mL IL-3 alone or in combination with 1 μg/mL CD40L. CD1c+ blood DCs and skin-derived dermal DCs were isolated as previously described.27
Polymerase chain reactions
Total RNA was isolated from isolated PDCs using the RNeasy Mini Kit (Qiagen, Hilden, Germany) and used to synthesize cDNA as previously described.27 Reverse transcriptase–polymerase chain reaction (RT-PCR) reactions were performed using the following primers: A1 receptor, TCCCCCTCGCCATCCTCATCAACA and GGCCCGCTCCACCGCACTCAG; A2a receptor, CTGGTCCTCACGCAGAGCT and ACTCGCGGATACGGTAGGC; A2b receptor, CGCCCACCAACTACTTC and GCCACCAGGAAGATCT; A3 receptor, GCTTACCGTCAGATACAAG and GCATAGACGATAGGGTTCA. All primers were synthesized by Sigma Genosys (Castle Hill, Australia). A1 receptor PCR products were sequenced by the MicroMon DNA Sequence Facility, Monash University, Melbourne, Australia. A newly identified A1 receptor insert sequence was entered into a BLAST search and identified as part of clone RP11-335013 (AC105940). The sequence and chromosome location of this clone matched the A1 receptor gene. Complementary DNA and protein sequence analysis was carried out using DNASTAR (Lasergene, Madison, WI).
Cell migration assay
For migration studies, 24-well plates were filled with 500 μL RPMI–2% human serum in the absence or presence of CCL21 (100 ng/mL), CXCL12 (30 ng/mL), adenosine, or specific adenosine receptor agonists. Freshly isolated PDCs were rested for 1 to 2 hours (ex vivo) or cultured overnight in the presence of IL-3 before assessing their migratory capacity. To assess migration of mature cells, PDCs were cultured with IL-3 and CD40L for 2 days. From 1.5 × 104 to 2.5 × 104 PDCs in 100 μL RPMI–2% human serum were added into 5-μm Transwell inserts (Costar, Corning, NY), placed into the 24-well plates, and incubated for 2 hours at 37°C. The medium in the lower chambers was concentrated to 50 μL and cells counted with a hemocytometer. All conditions were performed as duplicates.
Calcium signaling studies
Calcium signaling was measured by flow cytometry as described.27 PDCs were allowed to recover from the isolation procedure during overnight culture. In some conditions, PDC maturation was induced by activation with CD40L for 2 days. In short, PDCs were incubated with fluo-3 acetoxymethyl ester (Fluo-3/am) (4 μg/mL) and Fura Red (Fura Red/am) (10 μg/mL) in the presence of 0.02% Pluronic F-127 (all from Molecular Probes, Eugene, OR) for 30 minutes at 37°C. Cells were washed with assay buffer containing 1 mM CaCl2. For analysis, cells were transferred into tubes containing assay buffer at 37°C. Flow rate was adjusted to 100 to 150 events per second, and adenosine receptor agonists were added. Data were analyzed by plotting the LFL1/LFL3 ratios versus time using FloJo software (version 3.4; Tree Star, San Carlos, CA).
Measurement of cytosolic cAMP
PDCs were incubated for 30 minutes in culture medium supplemented with 25 μM rolipram (Sigma), a phosphodiesterase type IV inhibitor. PDCs were stimulated with 100 μM adenosine for 30 minutes, subsequently lysed, and cytosolic cyclic 3′,5′ adenosine monophosphate (cAMP) was measured with an enzyme immunoassay kit from Amersham Pharmacia Biotech (Castle Hill, Australia).
Statistical analysis
Data are expressed as mean values plus SEM. Statistical significance was determined by the Student t test. Differences were considered to be significant for P values less than .05.
Results
Human PDCs express mRNA for adenosine receptors
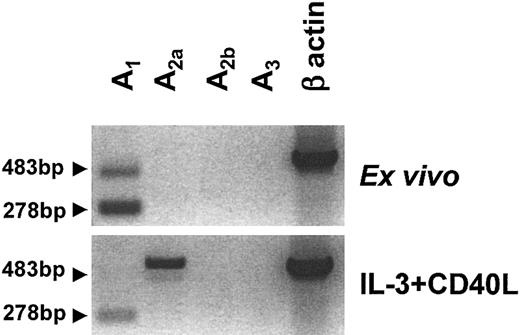
The expression pattern of the 4 adenosine receptor subtypes, denoted A1, A2a, A2b, and A3, in human PDCs was assessed by RT-PCR. Ex vivo, PDCs expressed mRNA for A1 but not for A2b or A3 receptors (Figure 1). A2a receptor mRNA expression was seen at low levels in some donors and was absent in others. Overnight stimulation with IL-3 and CD40L induced down-regulation of A1 and up-regulation of A2a receptor mRNA (Figure 1). As observed with immature PDCs, no A2b or A3 receptor mRNA was detected in mature PDCs. This pattern differed from immature monocyte-derived DCs (MoDCs), which expressed high levels of A1 and A3 receptor mRNA and low levels of both A2a and A2b mRNA (data not shown).
Adenosine receptor expression of human PDCs. Messenger RNA was extracted from highly purified PDCs (purity more than 96%) immediately after isolation or after overnight culture in the presence of IL-3 and CD40L. Profiles of A1, A2a, A2b, and A3 receptor expression were generated by RT-PCR. In addition to the expected 278 bp product for the A1 receptor, a second transcript of 483 bp was amplified (arrows), which contained the expected A1 receptor sequence with a 205 bp insert belonging to the intron between exons 5 and 6. One representative experiment of 6 is shown.
Adenosine receptor expression of human PDCs. Messenger RNA was extracted from highly purified PDCs (purity more than 96%) immediately after isolation or after overnight culture in the presence of IL-3 and CD40L. Profiles of A1, A2a, A2b, and A3 receptor expression were generated by RT-PCR. In addition to the expected 278 bp product for the A1 receptor, a second transcript of 483 bp was amplified (arrows), which contained the expected A1 receptor sequence with a 205 bp insert belonging to the intron between exons 5 and 6. One representative experiment of 6 is shown.
In PDCs, the PCR reaction for the A1 receptor amplified a second transcript, which was larger than the expected 278–base pair (bp) product and which was also down-regulated upon maturation. DNA sequencing revealed that this 483-bp product contained the expected A1 receptor sequence with a 205-bp insert, corresponding to the 3′ region of the intron located between exons 5 and 6 of the A1 gene. These nucleotides were most likely incorporated into the transcript due to an alternative upstream splicing site. However, it is unlikely that the new transcript codes for a functional adenosine receptor, because the frame shift caused by the insert generates a protein that is truncated after helix III, affecting the predicted adenosine-binding site (residues in helices III and VII) as well as the G protein binding site.31
PDCs mobilize intracellular calcium in response to adenosine via A1 receptors
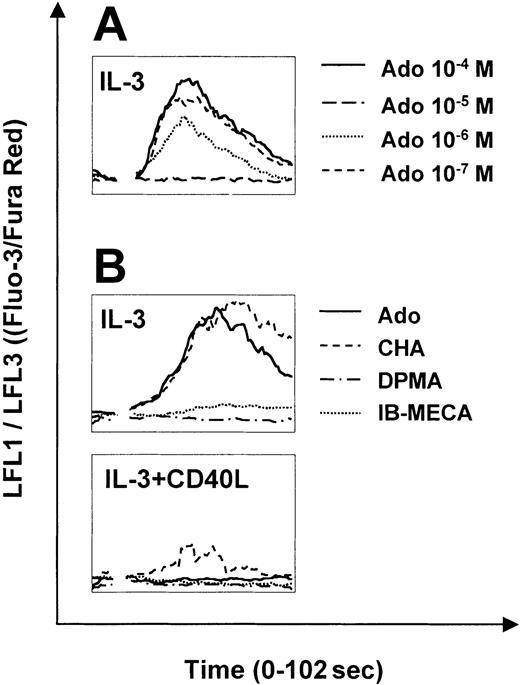
The A1 and A3 receptors signal through phospholipase C and consequently mobilize calcium from intracellular stores. To assess adenosine receptor function in PDCs, we studied calcium signaling in response to adenosine and specific adenosine receptor agonists. Adenosine induced strong calcium transients in immature PDCs (Figure 2A). Responses were observed at concentrations as low as 0.1 to 1.0 μM and were maximal at 10 μM. Receptor subtype-specific agonists revealed that calcium signaling was mediated by the A1 receptor, because calcium transients were only observed in response to the A1 receptor agonist CHA but not to DPMA or IB-MECA, agonists of A2a and A3 receptors, respectively (Figure 2B). Sensitivity of PDCs to adenosine was regulated during maturation. In CD40L-matured PDCs (24 hours), calcium signaling in response to adenosine was significantly decreased, correlating with down-regulation of A1 receptor mRNA expression shown in Figure 1. After 48 hours of activation, calcium transients were no longer induced (Figure 2B). Strong calcium signaling was observed in response to CCL19 (MIP-3β) but not to CXCL12 (SDF-1α) in CD40L-matured PDCs (data not shown).
Calcium signaling of PDCs in response to adenosine and specific adenosine receptor agonists. Calcium transients in PDCs cultured overnight with IL-3 (A-B) or IL-3 plus CD40L for 48 hours (B) in response to adenosine or the specific adenosine receptor agonists, CHA, DPMA, and IB-MECA (each 1 μM), was analyzed by flow cytometry. After establishing a baseline for 10 seconds, reagents were added at the indicated concentrations. Representative data of 4 different experiments are shown.
Calcium signaling of PDCs in response to adenosine and specific adenosine receptor agonists. Calcium transients in PDCs cultured overnight with IL-3 (A-B) or IL-3 plus CD40L for 48 hours (B) in response to adenosine or the specific adenosine receptor agonists, CHA, DPMA, and IB-MECA (each 1 μM), was analyzed by flow cytometry. After establishing a baseline for 10 seconds, reagents were added at the indicated concentrations. Representative data of 4 different experiments are shown.
Adenosine induces chemotaxis of immature PDCs
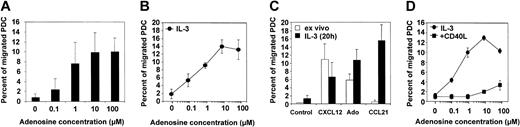
Because the capacity of PDCs to migrate in response to inflammatory chemokines is poor, we were interested in whether adenosine can induce chemotaxis of PDCs, as has been previously reported for other leukocytes.32-34 In a standard Transwell chemotaxis assay, we found that freshly isolated PDCs migrate toward gradients of adenosine in a concentration-dependent manner (Figure 3A). Migration to adenosine was observed at physiologically relevant concentrations of 0.1 to 1.0 μM, with maximal migration between 1.0 and 10 μM (7.5% to 14.5% of total PDCs). The chemotactic response to adenosine increased after an overnight rest period in the presence of IL-3 for some donors, probably reflecting individual differences in the recovery rate from cell stress induced by the isolation procedure (Figure 3B). In contrast, the ability of PDCs to migrate toward CXCL12, which was in magnitude comparable to adenosine, decreased after overnight culture. Furthermore, PDCs cultured with IL-3 showed a strong migratory response to CCL21 (Figure 3C), correlating with down-regulation of CXCR4 and up-regulation of CCR7 expression, as assessed by FACS analysis (data not shown). A distinct migration pattern was observed for PDCs matured with CD40L for 48 hours. CD40L-matured PDCs migrated efficiently in response to CCL21 (data not shown) but no longer to adenosine (Figure 3D).
Migration of PDCs to adenosine or chemokines. (A) Dose-response curve for the migration of freshly isolated PDCs toward adenosine. Mean ± SEM of 7 experiments, each performed in duplicate, are shown. (B) Dose-response curve for the migration of PDCs cultured overnight in the presence of IL-3 toward adenosine. Mean ± SEM of 3 experiments, each performed in duplicate, are shown. (C) Migratory response of PDCs toward adenosine, CXCL12 (SDF-1α), or CCL21 (6Ckine) after isolation (ex vivo) and after 1 day of culture with IL-3. Mean ± SEM of 4 to 6 independent experiments, each performed in duplicate, are shown. (D) PDCs were cultured overnight in the presence of IL-3 or for 48 hours in the presence of IL-3 and CD40L trimer before the assessment of their migratory response to adenosine. Mean ± SEM of duplicates are shown.
Migration of PDCs to adenosine or chemokines. (A) Dose-response curve for the migration of freshly isolated PDCs toward adenosine. Mean ± SEM of 7 experiments, each performed in duplicate, are shown. (B) Dose-response curve for the migration of PDCs cultured overnight in the presence of IL-3 toward adenosine. Mean ± SEM of 3 experiments, each performed in duplicate, are shown. (C) Migratory response of PDCs toward adenosine, CXCL12 (SDF-1α), or CCL21 (6Ckine) after isolation (ex vivo) and after 1 day of culture with IL-3. Mean ± SEM of 4 to 6 independent experiments, each performed in duplicate, are shown. (D) PDCs were cultured overnight in the presence of IL-3 or for 48 hours in the presence of IL-3 and CD40L trimer before the assessment of their migratory response to adenosine. Mean ± SEM of duplicates are shown.
Migration of PDCs toward adenosine is mediated by the A1 receptor
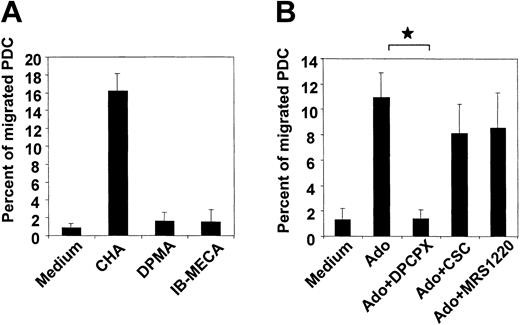
Down-regulation of A1 receptors combined with a loss in migratory function in CD40L-matured PDCs led us to speculate that A1 receptors mediate chemotaxis to adenosine. To test this hypothesis, the influence of selective adenosine receptor agonists and antagonists on PDC migration was studied. As expected, the A1 receptor agonist, CHA (1 μM), strongly induced chemotaxis of PDCs, whereas the A2a and A3 receptor agonists DPMA and IB-MECA were both ineffective (Figure 4A). In this respect, no difference was observed between freshly isolated and IL-3–cultured PDCs (data not shown). Furthermore, the A1 receptor antagonist DPCPX abolished migration of PDCs toward adenosine. CSC and MRS 1220, antagonists of A2a and A3 receptors, were ineffective (Figure 4B).
Chemotaxis of PDCs in response to adenosine is mediated by the A1 receptor. (A) Migration of PDCs cultured overnight in the presence of IL-3 in response to 1 μM of the A1, A2a or A3 receptor agonists CHA, DPMA, or IB-MECA, respectively. (B) Migration of PDCs toward 100 μM adenosine in the absence or presence of 10 μM of the A1,A2a, orA3 receptor antagonists DPCPX, CSC, or MRS 1220, respectively. Data represent mean ± SEM of 3 to 4 different experiments. *P < .05.
Chemotaxis of PDCs in response to adenosine is mediated by the A1 receptor. (A) Migration of PDCs cultured overnight in the presence of IL-3 in response to 1 μM of the A1, A2a or A3 receptor agonists CHA, DPMA, or IB-MECA, respectively. (B) Migration of PDCs toward 100 μM adenosine in the absence or presence of 10 μM of the A1,A2a, orA3 receptor antagonists DPCPX, CSC, or MRS 1220, respectively. Data represent mean ± SEM of 3 to 4 different experiments. *P < .05.
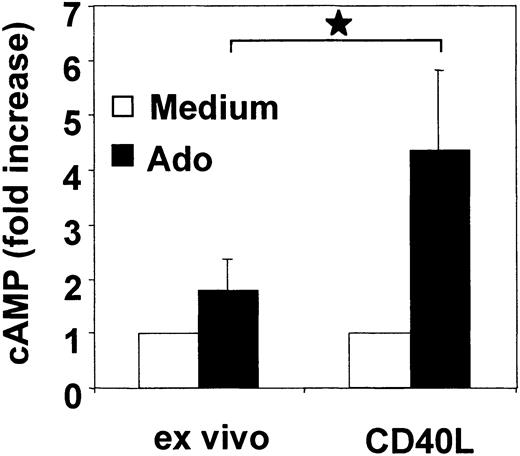
Adenosine enhances cytosolic cAMP and inhibits cytokine production in mature PDCs
A2a receptors are positively coupled to adenylate cyclase and stimulate the formation of the second messenger cAMP. To assess the function of A2a receptors in PDCs, we analyzed cytosolic cAMP levels in response to adenosine in freshly isolated PDCs and following activation with CD40L. To enhance the sensitivity of the cAMP assay on low cell numbers, PDCs were incubated with rolipram, an inhibitor of the cAMP-degrading enzyme phosphodiesterase (PDE) type IV. Compared with immature PDCs, adenosine significantly enhanced cAMP levels in CD40L-activated PDCs (4.4-fold ± 1.4-fold versus 1.8-fold ± 0.5-fold increase, n = 4, P = .01) (Figure 5). These findings are consistent with functional A2a receptor expression in mature PDCs.
Adenosine enhances cytosolic cAMP levels in mature PDCs. Freshly isolated PDCs or CD40L trimer–activated PDCs (24 hours) were incubated with 100 μM adenosine in the presence of rolipram. After 30 minutes, cAMP levels were measured in cell lysates by enzyme immunoassay (EIA). Data are expressed as n-fold increase of cAMP levels and represent mean ± SEM of 4 different donors. *P < .05.
Adenosine enhances cytosolic cAMP levels in mature PDCs. Freshly isolated PDCs or CD40L trimer–activated PDCs (24 hours) were incubated with 100 μM adenosine in the presence of rolipram. After 30 minutes, cAMP levels were measured in cell lysates by enzyme immunoassay (EIA). Data are expressed as n-fold increase of cAMP levels and represent mean ± SEM of 4 different donors. *P < .05.
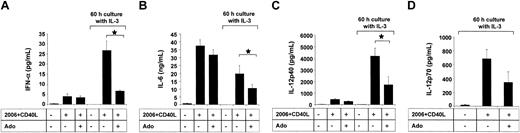
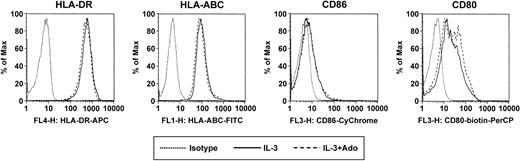
Becaue A2a receptor signaling has previously been linked to anti-inflammatory properties, we investigated whether adenosine has an impact on the cytokine production of PDCs in response to CpG ODN. The stimulation protocol used in our study was previously reported by Krug et al. PDCs were activated with ODN 2006 in combination with CD40L-transfected BKH cells, a stimulation protocol inducing IFN-α, IL-6, as well as IL-12, which is maximal after a 60-hour preculture period with IL-3.13 PDCs stimulated with CpG ODN 2006 and CD40L produced large amounts of IFN-α and IL-6. Adenosine (up to 100 μM) had no significant influence on the production of IFN-α (Figure 6A) or IL-6 (Figure 6B) by immature PDCs. To study the effect of adenosine on the cytokine production by mature cells, PDCs were cultured for 60 hours with IL-3, which resulted in the down-regulation of A1 and up-regulation of A2a receptors (data not shown). In mature PDCs, adenosine significantly inhibited IFN-α and IL-6 production in response to CpG and CD40L (Figure 6A-B). Moreover, culture with IL-3 induced in PDCs the production of high amounts of IL-12 in response to CpG ODN 2006 and CD40L, as previously reported by Krug et al.13 Adenosine also significantly suppressed the production of IL-12p40 and IL-12p70 under these conditions (Figure 6C-D), highlighting the potential role of adenosine in the modulation of cytokine production by mature PDCs. No effect of adenosine on PDC viability was observed for concentrations up to 250 μM, ruling out potential toxic effects of adenosine. Furthermore, adenosine did not induce the maturation of PDCs, as assessed by up-regulation of surface expression of major histocompatibility complex (MHC) class I and MHC class II as well as the costimulatory molecules CD80 and CD86 (Figure 7).
Adenosine regulates cytokine production of mature PDCs. PDCs (2.5 × 105 cells per milliliter) were activated with CpG ODN 2006 and CD40L-transfected BHK cells in the presence or absence of 100 μM adenosine, either directly ex vivo or after culture with IL-3 for 60 hours. Supernatants were harvested after 48 hours, and IFN-α (A), IL-6 (B), IL-12p40 (C), and IL-12p70 (D) concentrations were measured by ELISA. Data represent mean ± SEM of 3 different donors. *P < .05.
Adenosine regulates cytokine production of mature PDCs. PDCs (2.5 × 105 cells per milliliter) were activated with CpG ODN 2006 and CD40L-transfected BHK cells in the presence or absence of 100 μM adenosine, either directly ex vivo or after culture with IL-3 for 60 hours. Supernatants were harvested after 48 hours, and IFN-α (A), IL-6 (B), IL-12p40 (C), and IL-12p70 (D) concentrations were measured by ELISA. Data represent mean ± SEM of 3 different donors. *P < .05.
Surface marker expression of PDCs in response to adenosine. PDCs were cultured with IL-3 for 48 hours in the absence or presence of 100 μM adenosine. Surface expression of HLA-ABC, HLA-DR, CD80, and CD86 was assessed by flow cytometry. A representative experiment of 3 is shown.
Surface marker expression of PDCs in response to adenosine. PDCs were cultured with IL-3 for 48 hours in the absence or presence of 100 μM adenosine. Surface expression of HLA-ABC, HLA-DR, CD80, and CD86 was assessed by flow cytometry. A representative experiment of 3 is shown.
Discussion
Adenosine is constantly formed within cells and can enter the extracellular space via bidirectional transporters. In addition, extracellular adenosine is generated via degradation of released adenine nucleotides by ectonucleotidases.31 In the local inflammatory microenvironment, a combination of enhanced intracellular adenosine production by stressed cells and the release of adenine nucleotides by activated or damaged cells can cause a 150-fold increase of tissue adenosine concentration reaching local levels of up to 30 μM.28,29 There is increasing evidence from in vitro and in vivo studies that adenosine is a modulator of inflammatory responses through activation of adenosine receptors expressed by leukocytes.47 Because PDCs play a role in the regulation inflammatory responses during viral infections, autoimmunity, and possibly cancer, we investigated adenosine receptor expression and function in human PDCs.
We found that immature PDCs express A1 and low levels of A2a receptors. In this respect, PDCs differ from immature MoDCs, which express A1, A2a and A3 receptors and predominantly signal through A3 receptors.32,46 Interestingly, the adenosine receptor expression patterns in PDCs and CD1c+ blood DCs were very similar (data not shown), highlighting differences between physiologic DC types (such as those circulating in peripheral blood) and in vitro–generated MoDCs. Adenosine receptor expression in PDCs was rapidly regulated during in vitro culture. IL-3 and CD40L activation induced down-regulation of A1 and up-regulation of A2a receptor expression in PDCs. A similar regulation pattern has been reported for lipopolysaccharide (LPS)–matured MoDCs.32,46 Furthermore, we found the same adenosine receptor expression pattern in MoDCs activated with proinflammatory cytokines, CD40L or intact Escherichia coli, as well as in mature CD1c+ blood DCs and CD1a+ skin-derived dermal DCs (data not shown). These findings indicate that immature DC types differ in the expression of adenosine receptors. However, because A2a receptor up-regulation was detected in all mature forms of the DC populations examined, A2a receptor expression may represent a useful diagnostic tool to assess the maturational state of different DC populations.
Immature PDCs circulate in the blood and traffic into lymph nodes as well as nonlymphoid tissues during episodes of inflammation. Receptors mediating lymph node homing via high endothelial venules have been identified and include L-selectin (CD62L), CXCR4, and possibly CXCR3.25,26 Up-regulation of CCR7 upon activation can also enable PDCs to reach lymph nodes from peripheral tissues via lymphatic vessels.25,26 However, mechanisms mediating the localization of PDCs to inflammatory sites remain elusive. Because adenosine gradients are formed at these sites of inflammation, we investigated the migration of immature and mature PDCs in response to adenosine. We found that adenosine promotes chemotaxis of immature PDCs at concentrations in the low micromolar range (ie, concentrations that can be found in vivo in inflamed tissues). Adenosine induced the migration of PDCs to a similar degree to that seen with CXCL12, the strongest chemotactic stimulus identified so far.25,26 Migration toward adenosine was mediated by the A1 receptor, the dominant adenosine receptor subtype expressed by immature PDCs, pointing to a role of this receptor in the recruitment of immature PDCs to sites of inflammation. In contrast, mature PDCs were no longer responsive to the chemotactic effect of adenosine but acquired the ability to migrate toward the lymph node–homing chemokine CCL21, confirming previous reports.25,26
Inflammatory cytokines, such as IL-1β, IL-6, tumor necrosis factor-α (TNF-α), IL-12, and IFN-α, play a critical role in the host defense against invading pathogens. In response to viruses or CpG ODN, PDCs are efficient producers of cytokines, such as IFN-α, IL-6, and TNF-α.12,13,48 In addition, Krug et al found that PDCs can be a source of IL-12 in response to stimulation with CpG ODN 2006 and CD40L after a preculture period of several days in the presence of IL-3.13 Adenosine has been shown to inhibit cytokine production in vitro and in vivo via the cAMP-elevating A2a receptor.39-42,45 Because mature PDCs down-regulated A1 and up-regulated A2a receptors, as assessed by signaling studies measuring cytosolic cAMP levels, we investigated the effect of adenosine exposure on the cytokine production of immature and mature PDCs in response to CpG ODN and CD40 ligation using the stimulation protocol published by Krug et al. Adenosine had no influence on the IFN-α and IL-6 production by immature PDCs, which predominantly express A1 receptors. However, in PDCs cultured with IL-3 for 60 hours before activation with CpG and CD40L, which predominantly express A2a receptors (data not shown), adenosine significantly inhibited the production of IFN-α, IL-6, as well as IL-12, pointing to a likely role of adenosine in vivo in the regulation of cytokine production by activated PDCs through A2a receptors.
In conclusion, we propose a dual role of adenosine on PDC function. In immature PDCs, adenosine activates A1 receptors and induces chemotaxis, which may initially lead to the recruitment of PDCs to sites of inflammation. Upon pathogen encounter at these sites, PDCs are activated and produce type I IFN and other cytokines, thereby conditioning the microenvironment and interfacing with both innate and antigen-specific effector cells. Mature PDCs will undergo a switch from A1 to A2a receptor expression, rendering them sensitive to the antiinflammatory effects of adenosine via the cAMP-elevating A2a receptor. This will in turn inhibit the cytokine-producing capacity of mature PDCs and attenuate the potentially detrimental effects of chronic cell activation responsible for tissue damage and disease.
Prepublished online as Blood First Edition Paper, October 9, 20; DOI 10.1182/blood-2003-06-1959.
Supported by the Sylvia and Charles Viertel Foundation, a Program Grant from the Australian National Health and Medical Research Council (NH&MRC), and the Ludwig Institute for Cancer Research. M.S. is supported by the Mildred Scheel Stiftung. I.D.D. was supported in part by an NH&MRC Career Development Award.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We acknowledge Dr Mark Wright from the Austin Research Institute in Melbourne for the protein sequence analysis of the novel A1 receptor transcript.