Abstract
It has been proposed that paroxysmal nocturnal hemoglobinuria (PNH) cells may proliferate through their intrinsic resistance to immune attack. To evaluate this hypothesis, we examined the impact of alloimmune pressure on PNH and normal cells in the clinical setting of nonmyeloablative allogeneic hematopoietic cell transplantation (HCT). Five patients with severe PNH underwent HCT from an HLA-matched family donor after conditioning with cyclophosphamide and fludarabine. PNH neutrophils (CD15+/CD66b–/CD16–) were detected in all patients at engraftment, but they subsequently declined to undetectable levels in all cases by 4 months after transplantation. To test for differences in susceptibility to immune pressure, minor histocompatibility antigen (mHa)–specific T-cell lines or clones were targeted against glycosylphosphatidylinositol (GPI)–negative and GPI-positive monocyte and B-cell fractions purified by flow cytometry sorting. Equivalent amounts of interferon-γ (IFN-γ) were secreted following coculture with GPI-negative and GPI-positive targets. Furthermore, mHa-specific T-cell lines and CD8+ T-cell clones showed similar cytotoxicity against both GPI-positive and GPI-negative B cells. Presently, all 5 patients survive without evidence of PNH 5 to 39 months after transplantation. These in vitro and in vivo studies show PNH cells can be immunologically eradicated following nonmyeloablative HCT. Relative to normal cells, no evidence for a decreased sensitivity of PNH cells to T-cell–mediated immunity was observed.
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare clonal disorder of hematopoietic stem cells characterized by a somatic mutation within the phosphatidylinositol glycan–class A (PIG-A) gene on the X chromosome.1,2 As a consequence of this mutation, glycosylphosphatidylinositol (GPI) synthesis is blocked, resulting in the production of blood cells deficient in surface expression of GPI-anchored proteins. Deficiency of GPI-anchored proteins decay-accelerating factor (DAF) (CD55) and membrane inhibitor of reactive lysis (MIRL) (CD59) leads to the production of red blood cells that are susceptible to complement-mediated lysis.3 Clinically, the syndrome of PNH is characterized by recurrent hemolytic episodes, pancytopenia, and thrombotic events.4,5 Infections related to neutropenia, bleeding associated with thrombocytopenia, and thrombosis all contribute to morbidity and mortality. Therapy is mostly supportive and includes oral corticosteroids, blood transfusions, and systemic anticoagulation when clinically indicated. Allogeneic hematopoietic cell transplantation (HCT) can be curative, although the high risk of treatment-related mortality precludes this approach for most patients.6
Although the PNH phenotype is explained at the molecular level, the basis for clonal expansion of mutant cells in vivo remains unclear. PIG-A–mutated blood cells with a PNH phenotype can be identified at a very low frequency in most healthy individuals.7 These abnormal populations do not expand under most circumstances. In vitro studies and a PIG-A knock-out model further demonstrate that a mutation in the PIG-A gene does not confer a proliferative growth advantage.8-10 In contrast to healthy individuals, large populations of PIG-A–mutated cells are frequently detected in patients with immune-mediated severe aplastic anemia (SAA).11,12 These observations have indirectly supported the hypothesis that immune selection may permit the proliferation of PNH clones through resistance to immune attack.13,14 Two potential mechanisms are theorized to mediate this resistance; in one, PNH cells escape autoreactive T cells recognizing antigens derived from GPI-anchored proteins on normal cells. In the second, a mutation in PIG-A confers a generalized resistance to immune effectors, perhaps the consequence of a deficiency of GPI-anchored protein ligands involved in the regulation of immune cells. Indeed, some recent work has shown that PIG-A–mutated cells are resistant to natural killer (NK) cell– and T-cell–mediated cytotoxicity in vitro.15,16
Nonmyeloablative allogeneic hematopoietic cell transplantation (HCT) provides a unique opportunity to investigate the role of the immune system in eradicating PNH. In a single case report, PNH was treated successfully after a reduced-intensity allogeneic HCT.17 In general, chimerism studies indicate that recipient hematopoietic stem cells that survive transplant conditioning are immunologically eradicated or “ablated” by engrafting donor T cells following nonmyeloablative HCT (a phenomenon referred to as graft-versus-host hematopoiesis). We hypothesized that the PNH cells would be susceptible to immune attack from donor T cells recognizing minor histocompatibility antigens (mHa's) expressed on both normal and PNH cells of the recipient.
Beginning in 1999, 5 consecutive patients with PNH underwent a nonmyeloablative HCT using granulocyte colony-stimulating factor (G-CSF)–mobilized peripheral blood mononuclear cells from their HLA-matched sibling donors. Cytotoxic T lymphocytes (CTLs) recognizing mHa's expressed on patient cells were generated from peripheral blood lymphocytes (donor in origin) obtained after the transplantation. These CTLs were used to evaluate in vitro differences in susceptibility of PNH compared with normal cells to cellular immune attack.
Here we present data showing that nonmyeloablative HCT can completely eradicate GPI-negative cell populations and can cure PNH. Our in vitro and in vivo data demonstrate that normal and PNH hematopoietic cells appear to have similar susceptibility to immune killing by mHa-reactive T cells. Relative to normal cells, we found no in vitro or in vivo evidence to demonstrate a decreased sensitivity of PNH cells to this type of T cell–mediated immune attack.
Patients, materials, and methods
Patients
Between May 1999 and April 2002, 5 patients with PNH underwent a nonmyeloablative HCT from a family donor—matched at 6 of 6 HLAs—at the National Heart, Lung, and Blood Institute (NHLBI). Patients and donors gave written informed consent to participate on protocol 99-H-0050, which had been approved by the institutional review board of the NHLBI. Protocol eligibility included age 18 to 75 years and severe PNH, with at least one of the following: (1) red blood cell (RBC) transfusion dependence; (2) history of thrombotic episodes; and (3) pancytopenia associated with clinical debilitation (Table 1). Nonmyeloablative transplant conditioning consisted of cyclophosphamide 60 mg/kg/d given on days –7 and –6, fludarabine 25 mg/m2/d given on days –5 to –1, and antithymocyte globulin (ATG) 40 mg/kg/d given on days –5 to –2.18 Donors received 10 μg/kg granulocyte colony-stimulating factor (G-CSF) given subcutaneously daily for 5 to 6 days. Mobilized peripheral blood stem cells were collected by leukapheresis on day 5 and, if necessary, on day 6 to obtain a target dose of at least 5 × 106 CD34+ cells per kilogram of recipient weight. A T-cell–replete allograft was infused on day 0. All patients received cyclosporine (CSA) as graft versus host disease (GVHD) prophylaxis given alone (patient no. 1) or in combination with mycophenolate mofetil (MMF) given orally (1 g twice a day; patient nos. 2 to 5).
Characteristics of 5 patients with PNH undergoing nonmyeloablative hematopoietic cell transplantation
Patient no. . | Age, y . | Sex . | Disease duration, mo* . | GPI-negative granulocytes, %† . | GPI-negative RBCs, %‡ . | Pretransplantation hemoglobin level, g/L . | Pretransplantation platelet count, × 109/L . | Indication for transplantation . |
|---|---|---|---|---|---|---|---|---|
| 1 | 30 | M | 15 | 92 | 64 | 92 | 43 | RBC transfusion dependent, portal vein thrombosis |
| 2 | 25 | F | 31 | 97 | 45 | 77 | 154 | RBC transfusion dependent, lower extremity DVT |
| 3 | 35 | M | 23 | 92 | 22 | 75 | 15 | RBC transfusion dependent, pancytopenia |
| 4§ | 26 | F | 15 | 13 | 1.1 | 84 | 28 | RBC and platelet transfusion dependent, pancytopenia |
| 5 | 35 | M | 49 | 92 | 36 | 82 | 36 | Splenic vein thrombosis, pancytopenia |
Patient no. . | Age, y . | Sex . | Disease duration, mo* . | GPI-negative granulocytes, %† . | GPI-negative RBCs, %‡ . | Pretransplantation hemoglobin level, g/L . | Pretransplantation platelet count, × 109/L . | Indication for transplantation . |
|---|---|---|---|---|---|---|---|---|
| 1 | 30 | M | 15 | 92 | 64 | 92 | 43 | RBC transfusion dependent, portal vein thrombosis |
| 2 | 25 | F | 31 | 97 | 45 | 77 | 154 | RBC transfusion dependent, lower extremity DVT |
| 3 | 35 | M | 23 | 92 | 22 | 75 | 15 | RBC transfusion dependent, pancytopenia |
| 4§ | 26 | F | 15 | 13 | 1.1 | 84 | 28 | RBC and platelet transfusion dependent, pancytopenia |
| 5 | 35 | M | 49 | 92 | 36 | 82 | 36 | Splenic vein thrombosis, pancytopenia |
DVT indicates deep vein thrombosis.
The duration from the diagnosis of PNH to transplantation.
The percentage of GPI-anchored protein-negative granulocytes prior to transplantation.
The percentage of GPI-anchored protein-negative red blood cells prior to transplantation.
Patient no. 4 had a diagnosis of aplastic anemia and PNH.
Monoclonal antibodies for analysis of GPI-anchored protein expression
Mixtures of fluorochrome-labeled monoclonal antibodies were used to assess the PNH phenotype in different cell populations. To more accurately quantitate the percentage of normal and PNH cells, each combination contained antibodies directed against 2 distinct GPI-anchored proteins. For granulocytes, CD66b–fluorescein isothiocyanate (FITC) (clone 80H3, mouse immunoglobulin G1 [IgG1]; Beckman Coulter, Marseille, France) and CD16–phycoerythrin–cyanine 5 (PECy5) (clone 3G8, mouse IgG1; Caltag, Burlingame, CA) were used to assess GPI-anchored proteins in combination with CD15-PE (clone 28, mouse IgM; RDI, Flanders, NJ) as a non-GPI–anchored marker to gate on granulocytes. For B lymphocytes, CD19-allophycocyanin (APC) (non-GPI linked, clone HIB19, mouse IgG1; BD Biosciences, Palo Alto, CA) was used to identify B cells in combination with CD59-FITC (clone p282, mouse IgG2a; BD Biosciences) and CD55-PE (clone IA10, mouse IgG2a; BD Biosciences) as labels for GPI-anchored proteins. For monocytes, CD59-PE (clone p282, mouse IgG2a; BD Biosciences) and CD14–peridinin chlorophyll protein (PerCP) (clone MoP9, mouse IgG2b; BD Biosciences) were used to assess GPI-anchored proteins in combination with CD64-FITC (clone 10.1, mouse IgG1; BD Biosciences) as a non-GPI–linked marker to gate on monocytes. For red blood cells (RBCs), CD55-PE (clone 143-30, mouse IgG1; RDI) and CD59-PE (clone MEM 43, mouse IgG2a; RDI) were used to assess GPI-anchored proteins in combination with glycophorin A–FITC (clone D2.10, mouse IgG1; Beckman Coulter) as a non-GPI–linked marker to gate on RBCs. Nonspecific Fc receptor–mediated binding of conjugated antibodies to cells was blocked by preincubating 1 mL blood with 30 μL normal mouse IgG (Caltag).
Antibody staining and flow cytometry analysis
The percentage of GPI-anchored protein–negative (GPI-negative) granulocytes and RBCs was determined by flow cytometry analysis of blood samples obtained before transplantation19 and weekly after transplantation until all GPI-negative populations disappeared (less than 0.03%). For granulocytes, peripheral blood was drawn by venipuncture into tubes containing EDTA (ethylenediaminetetraacetic acid) and stained within 8 hours of collection. A 100 μL aliquot of whole blood was incubated for 1 hour at room temperature with 10 μL CD15-PE, 20 μL CD66b-FITC, and 5 μL CD16-PECy5. In parallel, a separate 100 μL aliquot of whole blood was incubated with 10 μL CD15-PE with appropriate isotype controls. Following incubation, erythrocytes in these samples were lysed (Whole Blood Lysing Reagent; Coulter, Fullerton, CA), washed twice, and then fixed with paraformaldehyde. For monocyte and B-lymphocyte analysis, mononuclear cells (isolated by Ficoll gradient separation of either peripheral blood samples or CD40 ligand–cultured B cells) were incubated for 30 minutes at 4°C with 20 μL of each of the 3 relevant antibodies in a total volume of 100 μL.20,21 All samples were analyzed by using the FACSCalibur cytometer (BD Immunocytometry Systems, San Jose, CA). Excitation light sources were 15 mW from an argon laser tuned to 488 nm and 4 mW from a red diode laser emitting at 635 nm. Fluorescence signals were collected using logarithmic amplifiers while forward and side scatter signals were collected on a linear scale. CellQuest software (BD Immunocytometry Systems) was used for data acquisition and analysis. All data were stored as list-mode files.
Assessment of chimerism
Serial samples of peripheral blood were collected at the time of neutrophil recovery and then weekly or every other week thereafter and were analyzed for the percentage of donor/recipient chimerism in myeloid and T-cell fractions using a polymerase chain reaction (PCR)–based analysis of short tandem repeats (STRs).20,21 Heparinized blood samples underwent Ficoll-Hypaque separation and were then sorted into specific lineages using immunomagnetic beads conjugated to anti-CD3 for T cells and anti-CD14 and anti-CD15 for myeloid cells (Dynal, Oslo, Norway). DNA was extracted from cells, and lineage-specific chimerism was performed by PCR using fluorescent primers flanking a single informative STR (Monoplex; Promega, Madison, WI) that had been previously identified to be polymorphic between the patient and donor. A total of 1 to 5 ng DNA was used as a template with PCR being performed under the manufacturer's recommended conditions. Predetermined amounts of pretransplantation patient and donor DNA were mixed (50%:50% or 95%:5%) and run in conjunction with test samples as quantitative controls (sensitivity for minor populations typically 2% to 3%). The PCR products were analyzed with a 310 ABI PRISM Genetic Analyzer sequencer with the percentage of donor/recipient chimerism determined by measuring area under the curve using Genescan software (PE Applied Biosystems, Foster City, CA).
Generation of minor histocompatibility antigen–specific T-cell lines
Minor histocompatibility antigen–specific T-cell lines were generated using previously described methods.22,23 All peripheral blood mononuclear cells (PBMCs), T-cell lines, and clones were cultured in RPMI 1640 supplemented with HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 2 mM l-glutamine, and 10% pooled heat-inactivated human serum. Donor T cells with reactivity for recipient mHa's were generated in 24-well plates by stimulating 1 × 106 to 2 × 106 PBMCs obtained from the recipient after transplantation with 1 × 106 to 2 × 106 irradiated (25 Gy) PBMCs obtained from the recipient before transplantation. Cell lines were restimulated with irradiated recipient pretransplantation PBMCs at 7 and 14 days after the initial stimulation with interleukin-2–supplemented (20 U/mL) media. The resulting T-cell lines were further expanded by weekly restimulation with irradiated Epstein-Barr virus–transformed B cells (EBV-LCLs) derived from the recipient before transplantation. After 4 to 6 weeks, T-cell cultures were tested for cytolytic activity against donor- and recipient-derived EBV-LCLs.
Isolation of CD8+ or CD4+ minor histocompatibility antigen–specific T-cell clones
Minor histocompatibility antigen (mHa)–specific T-cell clones were generated using methods previously published.22,23 Briefly, T-cell lines exhibiting cytolytic activity specific for recipient EBV-LCLs were cloned by limiting dilution in 96-well round-bottom plates (0.3 to 0.5 cells per well) containing irradiated HLA-mismatched (allogeneic) PBMCs (5 × 104 cells per well), irradiated EBV-LCLs from the patient before transplantation (1 × 104 cells per well), recombinant human IL-2 (rhIL-2) (100 IU/mL), and anti-CD3 monoclonal antibody (MAb) (30 ng/mL). Fourteen days later, wells exhibiting cell growth were tested for target recognition by cytotoxicity or interferon-γ (IFN-γ) secretion. T-cell clones that specifically recognized patient EBV-LCLs but not donor EBV-LCLs were further expanded for analysis of phenotype and function using anti-CD3 MAb, irradiated HLA-mismatched PBMCs, and irradiated patient EBV-LCLs.
Expansion of B cells
B cells were expanded from donor and pretransplantation patient PBMCs using CD40 ligand–transfected NIH3T3 cells (t-CD40L) as previously described.24,25 Briefly, 2 × 105 irradiated (75 Gy) t-CD40L cells (kindly provided by Dr M. Nishimura, University of Chicago) were plated into 6-well plates (Costar, Cambridge, MA) and cultured overnight at 37°C in 5% CO2. The following day, media were removed, and new media containing 4 × 106 to 6 × 106 PBMCs suspended in 3 mL Iscove modified Dulbecco medium (IMDM) (Cellgro; Mediatech, Herndon, VA) supplemented with 10% pooled human serum, IL-4 (200 U/mL; PeproTech USA, Rocky Hill, NJ), and clinical-grade CSA (5.5 × 107 M; Novartis, Basel, Switzerland) were added to each well and cultured at 37°C in 5% CO2. Approximately every 3 to 4 days, expanded B cells were washed and then transferred onto freshly prepared irradiated t-CD40L cells in cytokine-replenished medium.
Isolation of GPI-negative and GPI-positive cell populations
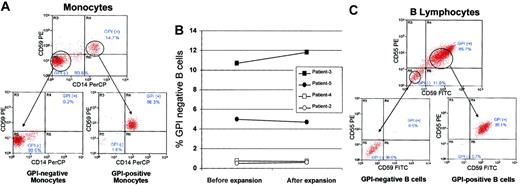
To test in vitro for differences in susceptibility to T-cell–mediated cytotoxicity, monocytes and B cells with a normal and PNH phenotype were isolated from pretransplantation patient samples. Monocytes were sorted by fluorescence-activated cell sorting into PNH (98.5% GPI-negative) and normal (96.3% GPI-positive) populations using CD64 to gate on monocytes and CD14 and CD59 as markers for GPI-anchored proteins (Figure 1A).
Isolation of GPI-anchored protein–negative (PNH) and GPI-anchored protein–positive (normal) B cells and monocytes. Mononuclear cells were collected by apheresis from PNH patients prior to transplantation. (A) Monocytes were sorted by flow cytometry into GPI-negative (CD64+/CD14–/CD59–) and GPI-positive (CD64+/CD14+/CD59+) populations using CD64 (non-GPI–anchored protein) to gate on monocytes and CD14 and CD59 as markers for GPI-anchored proteins. (B) B lymphocytes were expanded from pretransplantation apheresis samples using CD40 ligand–transfected NIH3T3 cells. The percentage of GPI-negative and GPI-positive B cells were similar in expanded versus nonexpanded samples. (C) After expansion, cells were flow sorted into GPI-negative (CD19+/CD55–/CD59–) and GPI-positive (CD19+/CD55+/CD59+) B lymphocytes using CD19 (non-GPI–anchored protein) to identify B cells and CD55 and CD59 as markers for GPI-anchored proteins.
Isolation of GPI-anchored protein–negative (PNH) and GPI-anchored protein–positive (normal) B cells and monocytes. Mononuclear cells were collected by apheresis from PNH patients prior to transplantation. (A) Monocytes were sorted by flow cytometry into GPI-negative (CD64+/CD14–/CD59–) and GPI-positive (CD64+/CD14+/CD59+) populations using CD64 (non-GPI–anchored protein) to gate on monocytes and CD14 and CD59 as markers for GPI-anchored proteins. (B) B lymphocytes were expanded from pretransplantation apheresis samples using CD40 ligand–transfected NIH3T3 cells. The percentage of GPI-negative and GPI-positive B cells were similar in expanded versus nonexpanded samples. (C) After expansion, cells were flow sorted into GPI-negative (CD19+/CD55–/CD59–) and GPI-positive (CD19+/CD55+/CD59+) B lymphocytes using CD19 (non-GPI–anchored protein) to identify B cells and CD55 and CD59 as markers for GPI-anchored proteins.
The minority (0.5% to 10.4%) of B cells from pretransplantation patient peripheral blood lymphocytes (PBLs) had a PNH phenotype. To obtain sufficient numbers of PNH B-cell targets, pretransplantation patient PBMCs were expanded an average of 1000-fold using CD40L-transfected NIH3T3 cells. The percentage of GPI-negative B cells was similar before and after expansion (Figure 1B). Expanded cells were then flow-sorted into GPI-negative (98%) and GPI-positive (99%) populations using CD19 to identify B cells and CD55 and CD59 as markers for GPI-anchored proteins (Figure 1C).
GPI-negative and GPI-positive cells were isolated by cell sorting on a MoFlo flow Cytometer (Dako-Cytomation, Fort Collins, CO). Excitation light sources were 100 mW from an argon laser tuned to 488 nm and 35 mW from a HeNe laser emitting at 633 nm. Fluorescence signals were collected using logarithmic amplifiers while forward and side scatter signals were collected on a linear scale. Sorting was performed using the “single sort 1” sorting mode. Sheath pressure was 60 psi, and the sample differential pressure was 0.4 psi or less. Data acquisition, analysis, and compensation were performed using Summit software (Dako-Cytomation). All data were stored as list-mode files.
Cytotoxicity assays
Cytotoxicity assays were performed using 51Cr release as previously described.26 Briefly, target cells were labeled for 2 hours with 51Cr (100 μCi [3.7 MBq] per 106 cells), washed, and then resuspended at a concentration of 1 × 105/mL; 100 μL of each target (in replicates of 2) was cocultured with varying numbers of effector cells (100 μL) in 96-well round-bottom plates (total volume 200 μL). After incubating at 37°C for 4 hours, 100 μL supernatant was harvested for gamma counting. The percentage of specific lysis was calculated using the following formula: 100 × (cpm released from test sample–cpm spontaneous release)/(cpm maximum release–cpm spontaneous release). All values used represented the average of both replicates.
ELISA for IFN-γ secretion assay and MHC blocking studies
T-cell lines or clones (1 × 105 cells in 100 μL) were added to target cells (1 × 105 cells in 100 μL) in U-bottomed 96-well plates. After 24 hours coculture at 37°C, 50 μL supernatant was collected and assessed for the concentration of IFN-γ by enzyme-linked immunosorbent assay (ELISA; ENDOGEN, Cambridge, MA). All measurements were done in duplicates following the instructions included by the manufacturer. The lower limit of IFN-γ detection was 5 pg/mL. The absorbance was read at 450 nm, and the values were calculated based on the standard curve generated from manufacturer-provided rhIFN-γ standards. To define T-cell restriction for major histocompatiblity complex (MHC) class I or class II molecules, some targets were preincubated (30 minutes at room temperature) with 25 μg/mL W6/32 (antipan class I MHC monoclonal antibody; Dako-Cytomation) or L243 (HLA-DR monoclonal antibody; BD Biosciences) prior to the ELISA.
Results
Nonmyeloablative hematopoietic cell transplantation for PNH
The outcome of the 5 patients with PNH who underwent nonmyeloablative allogeneic HCT is shown (Table 2). Four patients received an allograft from an HLA-identical sibling while one received a transplant from her mother (patient no. 4), who was matched at 6 of 6 HLAs. Transient and self-limited hemolysis associated with fever, hemoglobinuria, and a fall in hemoglobin (10 to 30 g/L [1 to 3 g/dL]) occurred in 3 of 5 patients on day –5, associated with the initiation of ATG conditioning. The regimen was otherwise well tolerated without mucositis, veno-occlusive disease of the liver, or hemorrhagic cystitis. Although the conditioning regimen was nonmyeloablative, it had profound early myelosuppressive effects, as the absolute neutrophil count (ANC) declined to less than 0.01 × 109/L (0.01 × 103/μL) in all patients. The median time to a neutrophil count of at least 0.5 × 109/L (0.5 × 103/μL) was 15 days (range, 10 to 18 days). All patients received intravenous antibiotics for febrile neutropenia. The median time to a platelet count of at least 20 × 109/L was 8 days (range, 0 to 12 days).
Outcome of PNH patients undergoing nonmyeloablative hematopoietic cell transplantation
. | . | No. of cells transfused per kg* . | . | . | . | . | . | . | . | |
|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | Donor HLA match . | CD34, × 106/kg . | CD3, × 106/kg . | ANC nadir, × 103 cells per μL . | Acute GVHD grade/system . | Chronic GVHD . | Day GPI-negative granulocytes disappeared (<0.03%)† . | Status . | Survival after transplantation, days . | |
| 1 | 6 of 6 | 21.1 | 4.3 | 0.007 | No | Yes/limited | 86 | Alive | 1325+ | |
| CR | ||||||||||
| 2 | 6 of 6 | 7.1 | 3.3 | 0.003 | No | Yes/limited | 118 | Alive | 672+ | |
| CR | ||||||||||
| 3 | 6 of 6 | 4.9 | 2.5 | 0.003 | III/GI | Yes/limited | 39 | Alive | 356+ | |
| CR | ||||||||||
| 4 | 6 of 6 | 8.0 | 3.7 | 0.002 | IV/GI | No | 25 | Alive | 315+ | |
| CR | ||||||||||
| 5 | 6 of 6 | 13.4 | 2.6 | 0.002 | No | No | 85 | Alive | 268+ | |
| CR | ||||||||||
. | . | No. of cells transfused per kg* . | . | . | . | . | . | . | . | |
|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | Donor HLA match . | CD34, × 106/kg . | CD3, × 106/kg . | ANC nadir, × 103 cells per μL . | Acute GVHD grade/system . | Chronic GVHD . | Day GPI-negative granulocytes disappeared (<0.03%)† . | Status . | Survival after transplantation, days . | |
| 1 | 6 of 6 | 21.1 | 4.3 | 0.007 | No | Yes/limited | 86 | Alive | 1325+ | |
| CR | ||||||||||
| 2 | 6 of 6 | 7.1 | 3.3 | 0.003 | No | Yes/limited | 118 | Alive | 672+ | |
| CR | ||||||||||
| 3 | 6 of 6 | 4.9 | 2.5 | 0.003 | III/GI | Yes/limited | 39 | Alive | 356+ | |
| CR | ||||||||||
| 4 | 6 of 6 | 8.0 | 3.7 | 0.002 | IV/GI | No | 25 | Alive | 315+ | |
| CR | ||||||||||
| 5 | 6 of 6 | 13.4 | 2.6 | 0.002 | No | No | 85 | Alive | 268+ | |
| CR | ||||||||||
HLA indicates human leukocyte antigen; ANC, absolute neutrophil count; GVHD, graft-versus-host disease; GI, gastrointestinal.
The number of cells per kilogram of recipient body weight that were transfused.
The percent of GPI-anchored protein-negative granulocytes on last assessment following transplantation.
All patients had sustained donor engraftment in both myeloid and T-cell lineages. At the time of neutrophil recovery, T-cell chimerism was at least 95% donor in origin in all 5 patients. In contrast, myeloid chimerism was mixed, showing variable degrees (8% to 55%) of autologous (recipient) hematopoietic recovery. Two patients developed grade II-IV acute GVHD, which resolved completely following treatment with parenteral corticosteroids (patient no. 4) or daclizumab and infliximab (patient no. 3).27,28 Limited chronic GVHD of the skin in 3 patients responded to low-dose, alternate-day CSA (or FK506) and prednisone.
With a median follow-up of 356 days (range, 268 to 1325 days), all 5 patients survive in remission with complete absence of any GPI-anchored protein–negative granulocyte or RBC populations (Table 2). Three patients who previously required coumadin for PNH-related thrombosis discontinued anticoagulation therapy after transplantation once GPI-negative neutrophil populations were no longer detectable.
Decline of GPI-negative granulocytes after nonmyeloablative HCT
All 5 patients had PNH neutrophils detectable by flow cytometry at the time of neutrophil recovery (Figure 2). Thereafter, the percentage of GPI-negative neutrophils declined until undetectable (less than 0.03% by flow cytometry) in all patients by 118 days (range, 25 to 118 days) after transplantation. Representative flow cytometry data from a patient (no. 5) who underwent nonmyeloablative HCT is shown (Figure 3). On day 11 during neutrophil recovery, most neutrophils had a PNH phenotype compatible with recovery of a population of recipient hematopoietic cells; PNH neutrophils subsequently declined rapidly until no longer detectable by posttransplantation day 85. Patient nos. 3 and 4, both of whom had a history of early acute GVHD, had complete disappearance of all PNH cells by day 39. In contrast, the 3 patients who did not develop GVHD had a more gradual decline in PNH neutrophils, with 85 to 118 days elapsing before GPI-negative neutrophils were no longer detectable. The ratio of GPI-negative neutrophils to recipient myeloid chimerism (% GPI-negative neutrophils by flow cytometry/% recipient neutrophil chimerism by STR-PCR) remained relatively constant during the period of graft versus host hematopoiesis (data not shown). Follow-up STR-PCR and flow cytometry studies, after chimerism became 100% donor and GPI-negative populations disappeared, have not shown recurrence of autologous hematopoiesis or PNH cells in any patients.
Decline in the percentage of GPI-anchored protein–negative granulocytes after transplantation. The percentage of GPI-anchored protein–negative granulocytes was measured by flow cytometry (CD15+/CD66b–/CD16–) in 5 patients before and multiple time points after nonmyeloablative hematopoietic cell transplantation. Detection of GPI-negative granulocytes after neutrophil recovery is consistent with the conditioning regimen being nonmyeloablative. The initial decline in GPI-negative populations occurred as a consequence of nonmyeloablative conditioning. The complete erradication of GPI-negative neutrophils in all patients by 25 to 118 days (median, 85 days) after transplantation occurred as a consequence of a graft-versus-host hematopoietic effect.
Decline in the percentage of GPI-anchored protein–negative granulocytes after transplantation. The percentage of GPI-anchored protein–negative granulocytes was measured by flow cytometry (CD15+/CD66b–/CD16–) in 5 patients before and multiple time points after nonmyeloablative hematopoietic cell transplantation. Detection of GPI-negative granulocytes after neutrophil recovery is consistent with the conditioning regimen being nonmyeloablative. The initial decline in GPI-negative populations occurred as a consequence of nonmyeloablative conditioning. The complete erradication of GPI-negative neutrophils in all patients by 25 to 118 days (median, 85 days) after transplantation occurred as a consequence of a graft-versus-host hematopoietic effect.
Gradual decline in GPI-anchored protein–negative granulocytes in a PNH patient after nonmyeloablative HCT. Granulocytes were analyzed in patient no. 5 before and multiple time points after transplantation using CD15 (PE) as a non-GPI–anchored protein and CD66b (FITC) and CD16 (PECy5) as GPI-anchored proteins. Following the white blood cell nadir, a population of GPI-negative granulocytes was detected beginning at neutrophil recovery.
Gradual decline in GPI-anchored protein–negative granulocytes in a PNH patient after nonmyeloablative HCT. Granulocytes were analyzed in patient no. 5 before and multiple time points after transplantation using CD15 (PE) as a non-GPI–anchored protein and CD66b (FITC) and CD16 (PECy5) as GPI-anchored proteins. Following the white blood cell nadir, a population of GPI-negative granulocytes was detected beginning at neutrophil recovery.
In vitro assessment of PNH cell sensitivity to minor histocompatibility antigen–specific CTL lines
To generate minor histocompatibility antigen (mHa)–specific cytotoxic T cells, posttransplantation PBMCs were collected after GPI-negative neutrophils had decreased to 5% or less in the setting of 100% donor T-cell chimerism. Using irradiated patient pretransplantation PBMCs followed by patient EBV-LCLs as stimulators, mHa-specific CTL lines were successfully expanded from patient nos. 3 and 5 who had high degrees of cytolytic activity against recipient- (but not donor-) derived EBV-LCLs.
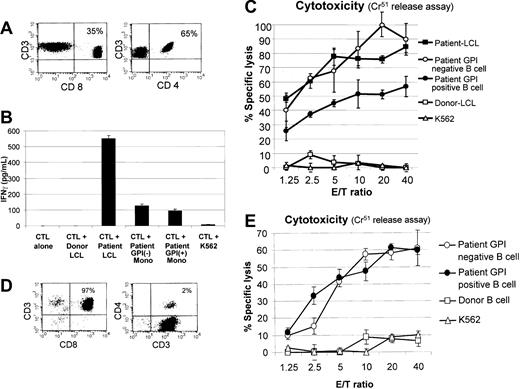
Flow cytometry analysis of the mHa-reactive CTLs expanded from patient no. 3 showed a mixed population of CD3+/CD4+ and CD3+/CD8+ T lymphocytes (Figure 4A). This CTL line (donor in origin) secreted IFN-γ and demonstrated cytotoxicity against patient EBV-LCLs but not to donor EBV-LCLs, consistent with recognition of patient-specific minor histocompatibility antigens (Figure 4B-C). Furthermore, these CTLs showed similar recognition of GPI-negative and GPI-positive monocytes as determined by an ELISA measuring IFN-γ secretion. At multiple different effector-target ratios, a slightly higher percentage of GPI-negative compared with GPI-positive (normal) B cells were killed by these CTLs.
Recognition of GPI-positive (normal) and GPI-negative (PNH) cells by allogeneic mHa-reactive T-cell lines. The mHa-specific CTLs were expanded from posttransplantation PBMCs of patient nos. 3 and 5. (A) The mHa-reactive CTLs expanded from patient no. 3 contained a mixture of CD4+ and CD8+ T cells. (B) These CTLs showed similar recognition of GPI-negative and GPI-positive monocytes (mono) as determined by an ELISA measuring IFN-γ secretion. (C) At multiple effector-target (E/T) ratios, a slightly higher percentage of GPI-negative (CD19+/CD55–/CD59–) compared with GPI-positive (CD19+/CD55+/CD59+) B cells were killed by these CTLs. (D) The mHa-reactive CTLs expanded from patient no. 5 contained predominantly CD8+ T cells. (E) Similar levels of cytotoxicity of GPI-negative and GPI-positive B cells were observed. Error bars represent 1 standard deviation.
Recognition of GPI-positive (normal) and GPI-negative (PNH) cells by allogeneic mHa-reactive T-cell lines. The mHa-specific CTLs were expanded from posttransplantation PBMCs of patient nos. 3 and 5. (A) The mHa-reactive CTLs expanded from patient no. 3 contained a mixture of CD4+ and CD8+ T cells. (B) These CTLs showed similar recognition of GPI-negative and GPI-positive monocytes (mono) as determined by an ELISA measuring IFN-γ secretion. (C) At multiple effector-target (E/T) ratios, a slightly higher percentage of GPI-negative (CD19+/CD55–/CD59–) compared with GPI-positive (CD19+/CD55+/CD59+) B cells were killed by these CTLs. (D) The mHa-reactive CTLs expanded from patient no. 5 contained predominantly CD8+ T cells. (E) Similar levels of cytotoxicity of GPI-negative and GPI-positive B cells were observed. Error bars represent 1 standard deviation.
The mHa-reactive CTLs expanded from patient no. 5 contained predominantly CD3+/CD8+ (97%) T lymphocytes (Figure 4D). These CTL lines demonstrated cytotoxicity against patient EBV-LCLs but not donor EBV-LCLs, consistent with patient-specific mHa recognition. A high level of cytotoxicity against both GPI-negative and GPI-positive patient B cells was induced by these CTLs. At varying effector-target ratios, similar levels of lysis of GPI-negative and GPI-positive B cells were observed (Figure 4E).
In vitro assessment of PNH cell sensitivity to CD8+ or CD4+ T-cell clones
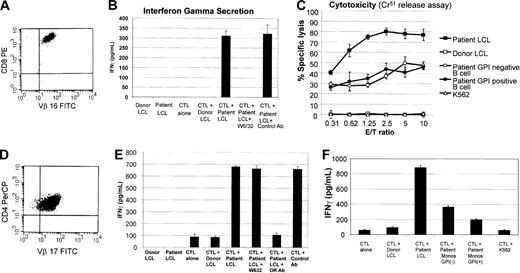
To more specifically assess for resistance to CD8+ or CD4+ T cell–mediated immune pressure, mHa-specific CTL lines were cloned by limiting dilution, and individual clones were then tested for differences in their ability to recognize and/or kill normal versus PNH cells. An mHa-reactive CD8+ T-cell clone that expressed a single T-cell receptor (TCR Vβ-16) was expanded from patient no. 3 (Figure 5A). This clone released IFN-γ against recipient- but not donor-derived EBV-LCLs, consistent with patient-specific mHa recognition. Cytokine secretion was blocked when patient EBV-LCLs were precultured with the monoclonal Ab W6/32 (pan class I MHC Ab), consistent with presentation of the target antigen on MHC class I (Figure 5B). At varying effector-target ratios, this clone induced similar levels of cytotoxicity against GPI-negative and -positive patient B cells (Figure 5C). Similarly, a CD4+ T-cell clone that expressed TCR Vβ-17 was isolated by limiting dilution from the mHa-reactive CTL line of patient no. 3 (Figure 5D). Secretion of IFN-γ against recipient but not donor EBV-LCLs confirmed this T-cell clone to have patient-specific mHa recognition. Diminution of IFN-γ release when patient EBV-LCLs were precultured with an HLA-DR blocking antibody but not with W6/32 was consistent with this T-cell clone having MHC class II–restricted recognition (Figure 5E). This donor-derived CD4+ T-cell clone secreted a slightly higher amount of IFN-γ when cocultured with GPI-negative compared with GPI-positive patient monocytes (Figure 5F).
In vitro assessment of PNH cell sensitivity to mHa-specific CD8+ or CD4+ T-cell clones. mHa-reactive T-cell clones were tested for differences in their ability to recognize and/or kill GPI-anchored protein–positive (normal) versus GPI-anchored protein–negative (PNH) cells. (A) A CD3+/CD8+ T-cell clone expressing a single T-cell receptor (TCR Vβ-16, determined by flow cytometry) was expanded with a cytokine profile (B) compatible with MHC class I–restricted recognition of a patient-specific mHa. (C) This clone was cytotoxic to patient but not donor EBV-LCLs and killed GPI-negative (CD19+/CD55–/CD59–) B cells in a similar fashion as GPI-positive (CD19+/CD55+/CD59+) B cells. (D) A CD4+ T-cell clone that expressed a single T-cell receptor (TCR Vβ-17) was isolated by limiting dilution from the same mHa-reactive CTL line and secreted IFN-γ against recipient but not donor EBV-LCLs, confirming patient-specific mHa recognition. (E) A decrease in IFN-γ when patient EBV-LCLs were precultured with an HLA-DR blocking antibody was consistent with T-cell antigen recognition within the context of MHC class II. (F) This CD4+ T-cell clone secreted a slightly higher amount of IFN-γ when cocultured with GPI-negative (CD64+/CD14–/CD59–) compared with GPI-positive (CD64+/CD14+/CD59+) patient monocytes. Error bars represent 1 standard deviation.
In vitro assessment of PNH cell sensitivity to mHa-specific CD8+ or CD4+ T-cell clones. mHa-reactive T-cell clones were tested for differences in their ability to recognize and/or kill GPI-anchored protein–positive (normal) versus GPI-anchored protein–negative (PNH) cells. (A) A CD3+/CD8+ T-cell clone expressing a single T-cell receptor (TCR Vβ-16, determined by flow cytometry) was expanded with a cytokine profile (B) compatible with MHC class I–restricted recognition of a patient-specific mHa. (C) This clone was cytotoxic to patient but not donor EBV-LCLs and killed GPI-negative (CD19+/CD55–/CD59–) B cells in a similar fashion as GPI-positive (CD19+/CD55+/CD59+) B cells. (D) A CD4+ T-cell clone that expressed a single T-cell receptor (TCR Vβ-17) was isolated by limiting dilution from the same mHa-reactive CTL line and secreted IFN-γ against recipient but not donor EBV-LCLs, confirming patient-specific mHa recognition. (E) A decrease in IFN-γ when patient EBV-LCLs were precultured with an HLA-DR blocking antibody was consistent with T-cell antigen recognition within the context of MHC class II. (F) This CD4+ T-cell clone secreted a slightly higher amount of IFN-γ when cocultured with GPI-negative (CD64+/CD14–/CD59–) compared with GPI-positive (CD64+/CD14+/CD59+) patient monocytes. Error bars represent 1 standard deviation.
Discussion
The severity of symptoms associated with PNH varies considerably among patients. Unfortunately for those who have severe disease, treatment options are limited and consist mostly of supportive-care measures. Eradication of the abnormal PNH clone by the conditioning regimen was thought to be a prerequisite for a successful outcome after allogeneic transplantation. Therefore, transplantation trials in the 1980s incorporated conditioning agents that resulted in myeloablation of host hematopoiesis.29 Although bone marrow transplantation is curative in about 50% of cases, a high treatment-related mortality precludes most patients from pursing this form of therapy.6 Because preliminary data suggest that nonmyeloablative HCT may be a safer alternative to conventional transplantation, we evaluated whether this approach could induce donor immune effects sufficient to eradicate recipient PNH cells. We limited the procedure to those who were significantly incapacitated by their disease, with most having recurrent hemolytic crisis and transfusion dependence. Furthermore, because we anticipated patients would be at a high risk for graft rejection, a profoundly immunosuppressive conditioning regimen was used that incorporated the nucleoside analog fludarabine in combination with cyclophosphamide and ATG.
Here we show that PNH can be cured following nonmyeloablative allogeneic HCT. Our STR-based chimerism analysis showed 8% to 55% of hematopoietic cells were recipient in origin at or shortly following recovery from neutropenia, a level that is substantially higher than the barely detectable amount of host cells measured by fluorescence in situ hybridization (FISH) following fully myeloablative transplantation.21,30 While the conditioning regimen was responsible for the initial decline in GPI-negative cells, the gradual and final eradication of PNH populations likely occurred as a consequence of a donor immune-mediated graft versus host hematopoietic effect. All 5 treated patients survive in complete remission and remain transfusion independent without detectable GPI-negative cell populations. With the exception of transient hemolytic episodes associated with the administration of ATG, the conditioning regimen was safe and well tolerated in this patient population. Of note, the 3 patients with a prior history of thrombosis were able to discontinue systemic anticoagulation after GPI-negative cell populations disappeared and have remained free of recurrent thrombotic episodes. Further, patient no. 2 had full preservation of ovarian function as evidenced by her subsequent pregnancy and uncomplicated delivery at full term.
The mechanisms leading to the expansion of GPI-negative cells to levels sufficient to cause clinical PNH remain enigmatic. The relationship of PNH to aplastic anemia led to the hypothesis that a generalized resistance of GPI-negative targets to immune attack allowed for the emergence of the PNH clone under conditions of autoimmunity.31 However, in vivo and in vitro studies have presented contradictory findings regarding this issue, with evidence that would both affirm and refute that PNH cells are resistant to immune effectors.15,16,32,33 Karadimitris and colleagues33 showed that EBV-transformed GPI-negative and GPI-positive B cells from patients with PNH were equally sensitive to the cytotoxic effects of EBV-specific T-cell lines. In contrast, in vitro and in vivo murine experiments have demonstrated that under some circumstances GPI-negative cells can evade both NK- and T-cell–mediated immune pressure.15,16 Murakami and colleagues16 showed in a murine system that GPI-negative populations had resistance to ovalbumin-specific CD4+ T-cell recognition compared with GPI-positive cells. Furthermore, in a murine transplantation model, this same group showed that GPI-negative hematopoietic cells expanded preferentially in response to alloreactive CD4+ T cells. In other work, Nakakuma and colleagues12 provided evidence PIG-A–mutated K562 (human myeloid leukemia) and JY-5 (human B-cell leukemia) cell lines were less sensitive to NK cell–mediated killing compared with their counterparts with a functional PIG-A gene. These studies showing GPI-negative and GPI-positive cells respond differently to immune pressure seem contradictory to the findings of Karadimitris and collegues33 where no resistance of the PNH clone to immune attack was observed. However, it is important to consider that the experimental methods used in all these studies to draw these conclusions were based on the use of cell lines requiring one or more transfections (including in some cases the PIG-A gene) before antigen specific T-cell recognition could be mediated.
In contrast, our transplantation approach afforded the unique opportunity to test in humans with PNH the effects of cellular immune pressure on normal versus GPI-negative hematopoietic cells. In this setting, donor T cells target mHa's expressed on recipient (but not donor) cells, culminating in their eradication. If PNH populations were indeed resistant to immune attack, one might expect GPI-negative cells to be insensitive to this effect, leading to their prolonged persistence following the procedure. On the contrary, although PNH populations recovered quickly following cytotoxic conditioning, they were unable to escape the effects of allogeneic immune attack. Indeed, by 4 months after transplantation, all recipient cells (both PNH and normal) were completely eradicated.
Although our clinical data were consistent with immunologic ablation of recipient cells, in vitro studies were required to assess for differences in susceptibility of normal and PNH populations to donor immune pressure. In vitro, GPI-negative cell populations were recognized and killed in a similar fashion to normal cells by donor mHa-specific CTL lines. Furthermore, cytotoxicity and cytokine secretion assays evaluating the effects of mHa-specific CD4+ and CD8+ T-cell clones showed virtually identical recognition of PNH monocytes and B cells compared with normal controls. These in vitro findings parallel their in vivo counterparts where selective resistance to alloimmune pressure by PNH cells was not observed.
Given prior evidence showing the insensitivity of PNH populations to attack by NK and alloreactive T cells, we were surprised how rapidly GPI-negative populations were eradicated by the donor immune system following this approach. Our in vivo and in vitro findings do not support the hypothesis that PNH cells have wide-ranging resistance to immune attack. However, they do not exclude the possibility that selective expansion of PNH cells occurs in the setting of autoimmunity to self-antigens absent on GPI-negative cells as a consequence of a mutation in the PIG-A gene. Likewise, relative insensitivity to autoimmune effectors, as might occur in the setting of aplastic anemia, might not have been discernable in our system where exceedingly powerful alloimmune effects against mHa's are generated.
In conclusion, we have shown both in vitro and in vivo that PNH can be immunologically eradicated following nonmyeloablative allogeneic stem cell transplantation. Relative to normal cells, no evidence for a decreased sensitivity of PNH cells to T-cell–mediated cellular immune attack was observed. Although this study is limited by a relatively small patient size, our data suggest that this approach has an acceptable risk-benefit ratio for patients with severe disease. Based on the encouraging results of this pilot trial, further investigation of nonmyeloablative HCT in patients with debilitating PNH should be pursued.
Prepublished online as Blood First Edition Paper, October 2, 2003; DOI 10.1182/blood-2003-04-1281.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank John Barrett and the NHLBI transplantation physicians, nurses, and support staff. We are particularly grateful to Rose Goodwin, Martha Marquesen, Pat Swanson, Othon Mena, Eugenia Oliver, Michael Rosenbaum, Sachiko Kajigaya, and the many important members of the Department of Transfusion Medicine at the Warren Grant Magnusson Clinical Center, National Institutes of Health.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal