Abstract
To investigate the mechanisms of human T-cell reconstitution following allogeneic hemopoietic stem cell transplantation (alloSCT), we analyzed the clonal composition of human cytomegalovirus (HCMV)-specific or Epstein-Barr virus (EBV)-specific CD8+ T cells in 10 alloSC transplant recipients and their donors. All virus-specific CD8+ T-cell clones isolated from recipients after alloSCT contained DNA of donor origin. In all 6 D+/R+ sibling alloSCTs from seropositive donors into seropositive recipients, donor virus-specific clones transferred in the allograft underwent early expansion and were maintained long term in the recipient. In contrast, in 2 of 3 HCMV D+/R- alloSC transplant recipients in whom there was no detectable HCMV infection, donor HCMV-specific clones were undetectable, whereas donor EBV-specific clones were maintained in the same EBV-seropositive recipients, suggesting that transferred clones require antigen for their maintenance. Following D-/R+ transplantation from 3 seronegative donors into seropositive recipients, a delayed primary virus-specific CD8+ T-cell response was observed, in which the T cells contained donor DNA, suggesting that new antigen-specific T cells arose in the recipient from donor-derived progenitors. In 2 of 4 HCMV D+/R+ sibling allograft recipients the clonal composition underwent diversification as compared with their donors, with delayed persistent expansion of HCMV-specific clones that were undetectable in the donor or in the recipient during the early months after transplantation; this diversification may represent expansion of new clones generated from donor-derived progenitors. We conclude that, following alloSCT, late diversification of the HCMV-specific CD8+ T-cell clonal repertoire can occur in response to persistent viral antigen.
In healthy individuals, primary infection with human cytomegalo-virus (HCMV) is followed by life-long persistence with viral latency in cells of the myeloid lineage. HCMV-specific CD8+ T cells play an important role in controlling HCMV reactivation and preventing overt disease. Healthy HCMV carriers have large circulating populations of HCMV-specific CD8+ T cells, many of which recognize defined peptides derived from the virus tegument protein pp651 or the major immediate early protein (IE72).2 In a given virus carrier, the CD8+ T-cell response against a given HCMV peptide is often dominated by relatively few individual clones that are greatly expanded and maintained in peripheral blood mononuclear cells (PBMCs) for long periods.3 In immunosuppressed patients such as recipients of allogeneic stem cell (alloSC) transplants, uncontrolled HCMV reactivation frequently leads to HCMV disease and is a major cause of procedure-related morbidity and mortality.4 In alloSC transplant recipients, reconstitution of HCMV-specific CD8+ T-cell responses is associated with reduced incidence of HCMV disease.5,6
The mechanisms of CD8+ T-cell reconstitution following alloSCT in humans are incompletely understood. Antigen-driven peripheral expansion of mature donor T cells transplanted within the allograft appears to predominate for initial CD8+ T-cell reconstitution.7 In addition, CD8+ thymic emigrants identified by T-cell receptor (TCR) excision circle (TREC) analysis can be observed by 2 months after allogeneic SCT,8 suggesting that there is production of naive T lymphocytes from donor-derived progenitor cells that have undergone TCR gene recombination in the thymus of the recipient. TREC analysis does not directly assess T-cell function, and it is unclear how soon following alloSCT a primary CD8+ T-cell response can develop in the recipient. The T cells that participate in a primary response in the recipient following alloSCT may include preexisting naive T cells transferred from the donor in the allograft, T cells newly generated in the recipient from donor-derived progenitor cells (via thymic or nonthymic pathways), or recipient T cells that survived the preparative chemoradiotherapy regimen.
Some but not all studies have reported an association between donor HCMV-seropositive status and protection against HCMV disease.4,9 Transfer of immune cells within the allograft from HCMV-seropositive donors may provide cellular immunity to the recipient; in addition HCMV-specific CD8+ T cells grown in vitro have been adoptively transferred into alloSC transplant recipients to restore immunity.5 The spontaneous reconstitution of HCMV-specific CD8+ T cells in recipients following alloSCT has been monitored with the use of peptide-major histocompatibility complex (MHC) class I tetramers.6,10,11 In recipients of sibling allografts in which both the recipient and donor were HCMV-seropositive before alloSCT, reconstitution of HCMV-specific CD8+ T cells was rapid; early reconstitution of tetramer+ cells was not observed if either the donor or recipient was seronegative for HCMV. Tetramer staining does not identify whether the CD8+ T cells originate from the donor or from recipient cells, and it does not distinguish the individual T-cell clones that comprise the tetramer+ population. Analysis of the reconstitution of HCMV-specific CD8+ T cells at the clonal level provides an important model to address fundamental mechanisms of human antigen-specific CD8+ T-cell reconstitution following alloSCT. We studied the origin and kinetics of reconstitution of individual virus-specific CD8+ T-cell clones following alloSCT. Individual clones were distinguished by the hypervariable sequence of the T-cell receptor β chain that is generated by the recombination of the V, D, and J gene segments during T-cell development. We derived multiple functional CD8+ T-cell clones specific for defined peptides of HCMV pp65, HCMV IE72, or EBV EBNA3C from healthy allograft donors and from their corresponding recipients before and after alloSCT and sequenced the hypervariable region of the TCR β chain. We used the TCR β chain hypervariable sequence to design a complementary clonotype-specific oligonucleotide to quantify each individual virus-specific T-cell clones in PBMCs by molecular clonotype probing. For each biologic clone obtained from a transplant recipient, we also determined whether the clone was of recipient or donor origin by analysis of DNA for sex chromosome and/or informative single nucleotide polymorphisms (SNPs).
Patients, materials, and methods
Donor-recipient pairs
We studied alloSC transplant recipients and their donors who had either HLA-A02.01, HLA-B07.02, HLA-B08.01, HLA-B35, or HLA-B44.02 alleles, in whom the recipient and/or the donor were seropositive for HCMV and/or EBV (immunoglobulin G [IgG] enzyme-linked immunosorbent assay [ELISA]; Public Health Laboratory Services, Addenbrooke's Hospital, Cambridge, United Kingdom). Ethical approval was obtained from the Addenbrookes NHS Hospital Trust institutional review board for this study. All recipients provided informed consent according to the Declaration of Helsinki (LREC 97/129). Details of donor-recipient pairs are summarized in Table 1. We studied recipients 04 and 06 at 6 years after alloSCT; all other donor-recipient pairs were studied prospectively from the time of transplantation.
Transplantation protocols
Stem cell harvesting, administration of the total body irradiation/cyclophosphamide preparative regimen, and prophylaxis against graft-versus-host-disease (GVHD) were performed as previously described.12 All alloSC transplant recipients received HCMV-negative irradiated blood products. Recipients received prophylaxis with oral or intravenous ganciclovir from day +30 to day +100. HCMV infection was monitored by weekly plasma samples tested for HCMV DNA by polymerase chain reaction (PCR), and HCMV serology (Public Health Laboratory Services, Addenbrooke's Hospital) until cessation of immunosuppression. Recipients who developed HCMV DNAemia or HCMV disease were treated with intravenous immunoglobulin 1 mg/kg on days +1 and +7 and intravenous ganciclovir 5 mg/kg twice daily for at least 2 weeks. To prevent GVHD, sibling allograft recipients received cyclosporin (commencing on day -1) and short-course methotrexate, and HLA-matched unrelated donor allograft recipients received cyclosporin and intravenous alemtuzumab (Campath-1H) monoclonal antibody from day -5 through day +4. Cyclosporin was generally continued for at least 6 months.
Viral peptides
Peptides used were HCMV pp65495-503 NLVPMVATV restricted by HLAA02.01, HCMV pp65417-426 TPRVTGGGAM restricted by HLA-B07.02, HCMV pp65397-411 restricted by HLA-B35,1 EBV EBNA3C281-290 EENLLDFVRF restricted by B44.02, and EBV EBNA3C880-891 PQPRAPIRPIPT restricted by HLA B07.0213 (> 95% pure by high-performance liquid chromatography [HPLC]; Affiniti Research Products, Exeter, United Kingdom). The HCMV IE72198-207 peptide DELRRKMMYM restricted by HLA-B08.012 was a kind gift from Dr Florian Kern (Charité, Humboldt University, Berlin, Germany). Peptides were dissolved in RPMI at a final concentration of 40 μg/mL.
Generation of biologic T-cell clones and determination of TCR β chain hypervariable sequence
Blood samples were taken from donors and from recipients before and after transplantation. PBMCs were prepared by gradient centrifugation (Lymphoprep; Nyegaard, Oslo, Norway). Prior to limiting dilution (LDA) culture, CD16+ natural killer (NK) cells were depleted by using anti-CD16 monoclonal IgM antibody (Leu-11b; Becton Dickinson, Oxford, United Kingdom) and complement. For healthy donors, CD4+ cells were also depleted with anti-CD4-conjugated magnetically activated cell sorter (MACS) microbeads according to the manufacturers' instructions (Miltenyl Biotech, Bisley, United Kingdom).
CD16-depleted responder cells were stimulated with viral peptide in LDA culture by using peptide-labeled irradiated autologous PBMCs in RPMI supplemented with 10% human AB serum and human recombinant interleukin 2 (IL-2; final concentration 5 IU/mL) as previously described.14 The cultures were incubated at 37°C with 5% CO2, were re-fed every 5 days, and on day 14 were assayed for peptide-specific cytotoxicity in split-well analysis against 51chromium-radiolabeled autologous or MHC-mismatched lymphoblastoid B-cell lines pulsed with peptide or unpulsed, as previously described.1 LDA wells that showed high levels of MHC-restricted peptide-specific lysis were used to generate independent CD8+ T-cell clones by formal single-cell cloning by subculture at 0.5 cells/well, followed by restimulation with mitogen phytohemagglutinin (PHA) and irradiated allogeneic PBMCs from unrelated healthy subjects, as previously described.3 After 2 to 3 weeks, clones were retested for peptide-specific lysis and selected on the basis of strong killing (> 30% specific lysis) against the peptide-pulsed target and minimal killing against the unpulsed target (< 10% specific lysis). After a minimum of 10 days from last addition of feeder cells, RNA from each clone was extracted, reverse transcribed, and PCR amplified by using a panel of 36 TCR Vβ family-specific primers together with the corresponding C-region specific primer (synthesized by Genosys Biotechnologies, Cambridge, United Kingdom) as previously described.3 The amplified PCR product was purified (Qiagen, West Sussex, United Kingdom) and sequenced by automated DNA sequencing (Department of Biochemistry, University of Cambridge, Cambridge, United Kingdom).
Quantitation of TCR clonotypes
We designed 12mer to 21mer oligonucleotide probes complementary to the hypervariable nucleotide sequence of TCR β chains of individual CD8+ T-cell clones (Table 2); such probes are highly specific for individual CD8+ T-cell clonotypes.15 Cellular mRNA from donor PBMCs, donor allograft, or recipient PBMCs was reverse transcribed into cDNA and amplified by using the appropriate TCR Vβ region and Cβ region PCR primers. A positive control sample from the original defined CD8+ T-cell clone and a negative control sample from pooled PBMCs of 4 HCMV-seronegative donors were amplified simultaneously by using the same primers. Each PCR product was blotted onto a nylon filter and incubated overnight with γ32P end-labeled clonotypic probe in hybridization buffer. After washing, the amount of probe that had bound to each sample on the filter was quantitated by using an Instant Imager (Beckman, Hialeah, FL). The filter was stripped, washed, and then rehybridized with a radiolabeled TCR Cβ constant region probe that detects all amplified TCR sequences. In each sample studied, we calculated the relative abundance of the clonotype sequence as a proportion of all TCR sequences of the same Vβ family as follows: percentage of relative abundance of clonotype sequence = (cpm clonotypic probe/cpm TCR constant probe) test sample/(cpm clonotypic probe/cpm TCR constant probe) positive control clone × 100.
We used TCR Vβ-specific monoclonal antibodies to quantify by flow cytometry the proportion of CD8+ T cells in PBMCs that expressed the corresponding TCR Vβ chain. The clone size per 106 CD8+ T cells could then be estimated, as previously described.16
In selected samples from donor-recipient pair 01, we also used seminested PCR to increased sensitivity of detection of clonotype sequences. Initial PCR amplification used the Vβand Cβ-specific primers, followed by further PCR amplification using the relevant internal TCR Jβ-specific primer. Clonotype probing was then performed as described in “Quantitation of TCR clonotypes,” and rehybridization was performed using the relevant TCR Jβ chain region-specific probe as a control to detect all Vβ-Jβ amplified sequences. The relative abundance of the clonotype sequence as a proportion of all TCR sequences within the same Vβ-Jβ family was calculated.
Determination of the origin (donor versus recipient) of peptide-specific CD8+ T-cell clones
DNA from donor PBMCs and recipient pretransplantation PBMCs (or buccal scrape) was analyzed to identify an informative DNA polymorphism; peptide-specific CD8+ T-cell clones obtained from the recipient after alloSCT were then analyzed for the appropriate polymorphism. In cases of donor-recipient sex mismatch, PCR amplification using primers at the amelogenin locus was performed by Molecular Genetics Laboratory, Addenbrooke's Hospital as previously described.17 Otherwise, analysis of SNPs was undertaken. Analysis of methyl-tetra-hydrofolate-reductase variant (MTHFRC677T), tumor necrosis factor-α, and the apolipoprotein B (XbaI, EcoRI, and MspI) SNPs were performed by the Department of Haematology, Addenbrooke's Hospital, according to previously published methods.18 HPA genotyping for human platelet antigen (HPA) 1, 2, 3, and 5 was performed using the PCR with sequence-specific priming (SSP) method described by Cavanagh et al19 by the National Blood Service East Anglia Centre, Addenbrooke's Hospital.
Flow cytometry
Monoclonal antibodies conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE), TriColor (TC), or allo phycocyanin were specific for CD3, CD4, CD8, CD16, CD27, CD28, CD45RO, CD45RA, CD57, HLA-DR (TCS Biologicals, Burlingdale, CA) or Vβ2, Vβ14 (Immunotech, Marseilles, France), and Vβ 13.1 (Serotech, Oxford, United Kingdom). IgM-unconjugated monoclonal antibody against antichemokine receptor CCR7 (Pharmingen, San Diego, CA) was followed by biotin-conjugated anti-IgM (Pharmingen), and then stained with streptavidin Red 670 (Gibco BRL, Grand Island, NY). We used peptide-MHC class I tetramers that incorporated HCMV pp65495-503 or pp65417-426 (kind gift of Dr J. Lipolis, NIH Core Tetramer Facility, Atlanta, GA) or HCMV IE72198-207 (kind gift of Florian Kern, Charitie, Humbolt University). Flow cytometry was performed on a fluorescence activated cell sorting (FACS) Calibur (Becton Dickinson) cytometer, and results were analyzed using WinMDI 2.7 software.
Results
All virus-specific CD8+ T-cell clones identified in the recipient after alloSCT were of donor origin
For each virus-specific CD8+ T-cell clone obtained from a recipient (Table 2), we determined whether the clone was of recipient or donor origin by analysis of DNA polymorphisms. All HCMV-specific and EBV-specific CD8+ T-cell clones were obtained from a contained DNA of donor origin (Table 3). An HCMV pp65495-503-specific CD8+ T-cell clone (clone 5.1, Table 4) obtained from the recipient prior to alloSCT could not be detected by clonotype probing following transplantation at 6 sequential time points (range, 3-18 months), indicating that this recipient clone did not survive the preparative regimen.
Following D+/R+ sibling alloSCT, virus-specific CD8+ T-cell clones transferred from the donor expanded and persisted in the seropositive recipient
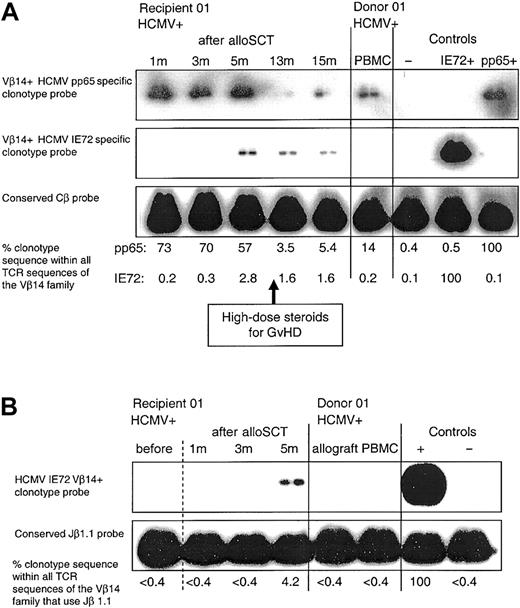
Using peptide stimulation, we generated multiple independent HCMV pp65-specific CD8+ T-cell clones from 4 donor-recipient pairs 01 to 04 after alloSCT (Table 3). Clones from recipients 01 to 03 had identical TCR β-chain nucleotide sequences to the peptide-specific CD8+ T-cell clones obtained from the corresponding donor (clones 1.1, 2.1, 3.1a, and 3.1b, respectively). As we previously reported3 in a given subject many of the independently derived clones specific for a defined peptide had an identical TCR β-chain sequence, for example, in donor 02, 3 of 3 independent clones had the same TCR β-chain sequence (clone 2.1). In recipient 03, 6 of 8 independent clones had an identical sequence (clone 3.2a), which was the same as that of 3 of 4 clones in donor 03 (clone 3.1a); similarly in the same recipient 2 of 8 clones (clone 3.2b) had an individual sequences to 1 of 4 clones in donor 03 (clone 3.1b). We designed clonotype probes specific to the hypervariable region of the TCR β chain of donor clones (1.1, 1.2, 2.1, 3.1a, 4.1). It was not possible to use a probe for clone 3.1b because the probe underwent self-annealing with formation of a secondary structure. For clonotype probing, results are expressed as the percentage of clonotype sequence within all the TCR sequences of the same Vβ family. In each D+/R+ pair, we detected donor clonotype within donor PBMCs (and in allograft harvest material in donor 01), and in the recipient PBMCs at all time points tested from 1 month to 6 years following alloSCT. Clonotype was undetectable in any PBMC samples obtained from recipients 02 and 03 prior to transplantation (Figure 1A; Table 4). Following alloSCT, corticosteroid therapy for GVHD was associated with a relative decline in HCMV-specific clonotype after alloSCT (Table 4, recipient 01). In recipients 01 to 04 blood tests to detect HCMV DNAemia were performed weekly until cessation of immunosuppression and were consistently negative.
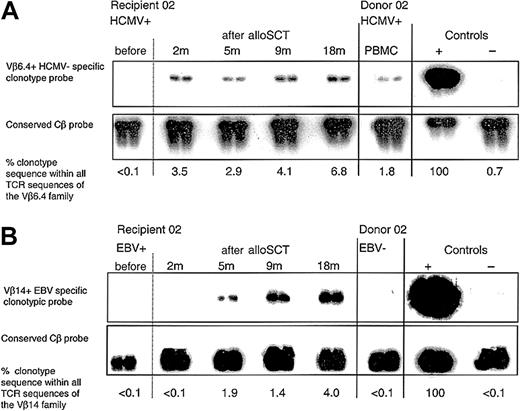
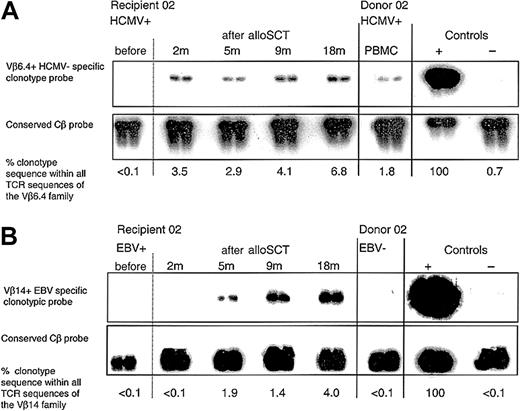
Following HCMV D+/R+, EBV D-/R+ alloSCT, an immunodominant HCMV-specific donor CD8+ T-cell clone expanded and persisted in recipient 02, and a primary EBV-specific CD8+ T-cell response developed within 5 months. (A) Clonotypic probing was used to quantify the donor CD8+ T-cell clone specific for HCMV pp65417-426 (clone 2.1) in sequential peripheral blood samples taken from recipient 02 before and after alloSCT. The clone was not detected in recipient 02 prior to transplantation but was detectable at all time points tested following alloSCT. All blood tests for HCMV DNAemia (performed weekly until 5 months after alloSCT) were negative. Because of renal dysfunction cyclosporin was discontinued at 3 months. (B) In the same EBV-seropositive recipient 02, an EBV EBNA3C880-891 CD8+ T-cell clone (clone 2.3) was not present in the donor but following alloSCT was detected in the recipient from 5 months onward.
Following HCMV D+/R+, EBV D-/R+ alloSCT, an immunodominant HCMV-specific donor CD8+ T-cell clone expanded and persisted in recipient 02, and a primary EBV-specific CD8+ T-cell response developed within 5 months. (A) Clonotypic probing was used to quantify the donor CD8+ T-cell clone specific for HCMV pp65417-426 (clone 2.1) in sequential peripheral blood samples taken from recipient 02 before and after alloSCT. The clone was not detected in recipient 02 prior to transplantation but was detectable at all time points tested following alloSCT. All blood tests for HCMV DNAemia (performed weekly until 5 months after alloSCT) were negative. Because of renal dysfunction cyclosporin was discontinued at 3 months. (B) In the same EBV-seropositive recipient 02, an EBV EBNA3C880-891 CD8+ T-cell clone (clone 2.3) was not present in the donor but following alloSCT was detected in the recipient from 5 months onward.
Recipient 10 underwent HCMV D+/R+ HLA-matched unrelated donor alloSCT, and this recipient received in vivo alemtuzumab monoclonal antibody to deplete T cells. This recipient also received cyclosporin for 4 months after alloSCT, then cyclosporin was discontinued in an attempt to induce a graft-versus-leukemia response to combat leukemia relapse. Despite ongoing ganciclovir prophylaxis, this recipient became HCMV PCR positive on day +46. HCMV viremia resolved after 2 weeks of ganciclovir therapy, then ganciclovir prophylaxis was restarted. HCMV viremia recurred on day +97 and again responded to antiviral therapy. Recipient 10 died of leukemia relapse at 6 months. In donor 10, we generated 2 different peptide-specific CD8+ T-cell clones (pp65495-503-specific clone 10.1 and pp65397-411-specific clone 10.2). By clonotype probing, both clones were undetectable in recipient 10 prior to T-cell-depleted alloSCT and at time points before and after viremia.
We also analyzed 2 EBV D+/R+ pairs. An EBV EBNA3C880-891-specific CD8+ T-cell clone from donor 07 (clone 7.2) was detected in recipient 07 at 4 time points from 2 to 30 months after alloSCT (Figure 2). In recipient 09, at 1 year after alloSCT 2 independently derived EBV EBNA3C880-891-specific CD8+ T-cell clones had identical TCR β-chain sequences (clone 9.1). We detected this clonotype in donor 09 PBMCs, in donor allograft material, and in the recipient at all time points tested from 4 to 30 months after alloSCT (Table 4).
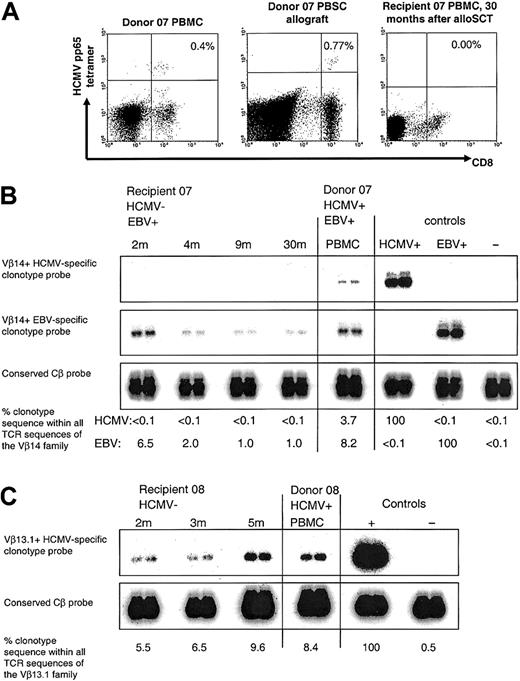
Following D+/R- alloSCT, HCMV pp65-specific CD8+ T cells were undetectable in HCMV-seronegative recipient of alloSC transplant 07, but an immunodominant HCMV-specific donor CD8+ T-cell clone was detectable in recipient 08 who developed severe GVHD. (A) Gated on CD3+ T cells. MHC class I HCMV pp65417-426 tetramer+ CD8+ T cells were present in donor 07 PBMCs and allograft but were undetectable in recipient 07 at 30 months after alloSCT. (B) With the use of clonotypic probing, the Vβ14+ donor CD8+ T-cell clone specific for HCMV pp65417-426 (clone 7.1) was not detectable in any of the sequential peripheral blood samples taken from recipient 07 following alloSCT. Weekly blood tests for HCMV DNA were consistently negative. In the same donor-recipient pair, the Vβ14+ donor CD8+ T-cell clone specific for EBV EBNA3C880-891 (clone 7.2) was detected in recipient PBMCs at all time points tested following alloSCT. In the controls, the HCMV+and EBV+-labeled lanes refer to the positive control cDNA used from clones 7.1 and 7.2, respectively. (C) The Vβ13.1+ donor CD8+ T-cell clone specific for HCMV pp65495-503 (clone 8.1) was detectable in sequential peripheral blood samples taken from recipient 08 after alloSCT. Recipient 08 had severe GVHD and died at 6 months after alloSCT. Weekly blood tests for HCMV DNA were negative.
Following D+/R- alloSCT, HCMV pp65-specific CD8+ T cells were undetectable in HCMV-seronegative recipient of alloSC transplant 07, but an immunodominant HCMV-specific donor CD8+ T-cell clone was detectable in recipient 08 who developed severe GVHD. (A) Gated on CD3+ T cells. MHC class I HCMV pp65417-426 tetramer+ CD8+ T cells were present in donor 07 PBMCs and allograft but were undetectable in recipient 07 at 30 months after alloSCT. (B) With the use of clonotypic probing, the Vβ14+ donor CD8+ T-cell clone specific for HCMV pp65417-426 (clone 7.1) was not detectable in any of the sequential peripheral blood samples taken from recipient 07 following alloSCT. Weekly blood tests for HCMV DNA were consistently negative. In the same donor-recipient pair, the Vβ14+ donor CD8+ T-cell clone specific for EBV EBNA3C880-891 (clone 7.2) was detected in recipient PBMCs at all time points tested following alloSCT. In the controls, the HCMV+and EBV+-labeled lanes refer to the positive control cDNA used from clones 7.1 and 7.2, respectively. (C) The Vβ13.1+ donor CD8+ T-cell clone specific for HCMV pp65495-503 (clone 8.1) was detectable in sequential peripheral blood samples taken from recipient 08 after alloSCT. Recipient 08 had severe GVHD and died at 6 months after alloSCT. Weekly blood tests for HCMV DNA were negative.
At late time points after cessation of immunosuppressive therapy, the functional peptide-specific cytotoxic T lymphocyte precursor (CTLp) frequencies determined by LDA in recipients 01 to 04 were similar to those observed in their respective donors (Table 5). In donor-recipient pair 01, in which the responses against 2 different HCMV peptides were studied, the higher relative frequency of pp65-specific CTLp compared with IE72-specific CTLp in the donor was also maintained in the recipient. The clone sizes per 106 CD8+ T cells in PBMCs in donor 01 were 24 000 cells for the pp65-specific clone 1.1 and 12 000 cells for the IE72-specific clone 1.2; in recipient 01 at 5 months following alloSCT the clone sizes were 19 000 cells for the pp65-specific clone 1.1 and 4000 cells for the IE72-specific clone 1.2 and 1000 cells for the IE72 clone 1.4. To address the in vitro cloning efficiency in donor-recipient pairs 01 to 04, the frequency of pp65 tetramer+ CD8+ T cells in PBMCs was determined by flow cytometry, and the ratio of the frequency of pp65 peptide-specific CTLp in LDA to the frequency of pp65 tetramer+ CD8+ T cells was calculated (Table 5). Within each D+/R+ donor-recipient pair, the frequencies of pp65 tetramer+ CD8+ T cells were similar, and the ratio of the CTLp frequency to the frequency of tetramer+ cells was also generally consistent. In both donors and recipients, the frequency of pp65-specific CTLp was generally only 3-fold lower than the frequency of pp65 tetramer+ CD8+ T cells. The in vitro cloning efficiency was also assessed in recipient 05 (after alloSCT), and in donors 06 to 08 and 10. Results are shown in Table 5.
Following D+/R- sibling alloSCT, donor HCMV-specific CD8+ T-cell clones were undetectable in 2 of 3 HCMV-seronegative recipients
To determine whether the absence of detectable HCMV in the recipient influenced the reconstitution of HCMV-specific CD8+ T cells, we analyzed 3 D+/R- pairs (06-08). None of these 3 recipients had HCMV DNAemia on weekly blood samples, and none showed evidence of seroconversion following transplantation; in each case, anti-HCMV IgG antibody titers declined with time and were persistently negative after 6 months, reflecting waning donor humoral immunity. In these 3 recipients the kinetics of reconstitution of the total CD4+ T-cell population was not delayed compared with other recipients studied (data not shown).
In donor 06, 2 different HCMV pp65495-503-specific CD8+ T-cell clones were obtained (clones 6.1a and 6.1b). Neither of these clones was detectable in the recipient PBMC sample taken at 6 years after transplantation (Table 4). In donor PBMCs we found HCMV pp65495-503 tetramer+ CD8+ T cells (650 × 104/L tetramer+ CD8+ CD3+ T cells), but we were unable to detect any pp65 tetramer+ cells in the recipient PBMCs following alloSCT (Table 4). Using LDA, we could not detect HCMV pp65495-503-specific CTLp in this recipient. The donor EBV was seronegative, and the recipient was EBV-seropositive; as an internal control we did detect a primary EBV-specific CD8+ T-cell response in the recipient, both as functional EBNA3C281-290-specific CTLp in LDA, and EBNA3C281-290-specific clonotype 6.2 (which contained donor DNA) by clonotype probing was detectable in the recipient but not the donor (Table 4).
An immunodominant HCMV pp65 417-426-specific clone 7.1 from donor 07 could not be detected in any of the recipient 07 PBMC samples taken at 2, 4, 9, and 24 months following alloSCT (Table 4). We detected HCMV pp65417-426 tetramer+ cells in both donor 07 PBMCs (0.4% of CD8+ CD3+ T cells were tetramer+, total 200 × 104/L tetramer+ CD8+ CD3+ T cells) and within donor 07's mobilized peripheral blood stem cells (0.77% of CD8+ CD3+ T cells were tetramer+, total 300 × 104/L tetramer+ CD8+ CD3+ T cells), but not in recipient 07 PBMCs at 30 months after alloSCT (Figure 2A; Table 4). Of interest, this donor-recipient pair was D+/R+ with respect to EBV; the donor EBV EBNA3C880-889-specific clone 8.2 obtained from the EBV-seropositive donor was readily detectable in the EBV-seropositive recipient at all time points after alloSCT (Figure 2B; Table 4).
In the third D+/R- pair, a donor HCMV pp65495-503-specific CD8+ T-cell clone (clone 8.2) was detected in donor 08 PBMCs (8.4%), and in recipient 08 at 2 months (5.5%), 3 months (6.5%), and 5 months (9.6%) after transplantation (positive control, 100%; negative control, 0.5%) (Figure 2C; Table 4). This recipient developed severe acute GVHD, was treated palliatively from 4 months onward, and died of leukemia relapse at 6 months. Weekly blood tests for HCMV DNA were consistently negative. We observed HCMV pp65495-503 tetramer+ CD8+ T cells in donor PBMCs (700 × 104/L) and in a recipient PBMC sample taken at 3 months (890 × 104/L).
Following D-/R+ alloSCT a primary CD8+ T-cell response was detected by 5 months after transplantation, from cells that contained donor DNA
We analyzed EBV-specific CD8+ T-cell responses in 2 D-/R+ pairs, 02 and 06. In recipient 02, an EBNA3C880-891-specific CD8+ T-cell clone was obtained in the female recipient at 5 months following alloSCT from a male donor (clone 2.3), and this clone contained male DNA. We were unable to detect clonotype within donor PBMCs, or within PBMC samples taken from the recipient prior to transplantation or at 2 months following alloSCT. However, clonotype was detected in recipient PBMCs at 5, 9, and 18 months (Figure 1B), indicating that a primary EBV-specific CD8+ T-cell response had occurred between 2 and 5 months after alloSCT, from cells of donor origin. In this recipient, because of renal insufficiency, cyclosporin was stopped at 3 months. In recipient 06, an EBV EBNA3C281-290-specific CD8+ T-cell clone that contained donor DNA (clone 6.2) was obtained in this recipient at 6 years following alloSCT. We were unable to detect this clonotype within donor PBMCs, but clonotype was present in the recipient PBMC sample taken at 6 years following alloSCT (Table 4).
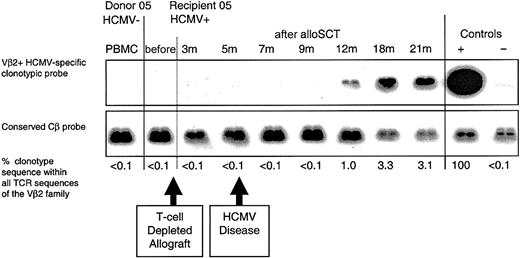
Following D-/R+ alloSCT in which donor T cells were depleted from the graft, the CD8+ T cells participating in the primary CD8+ T-cell response may have been generated de novo in the recipient from donor-derived progenitor cells
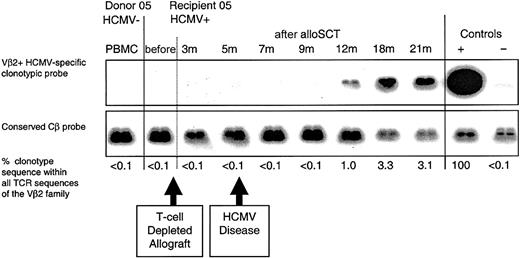
HCMV-seropositive recipient 05 underwent an HLA-matched unrelated donor alloSCT from HCMV-seronegative donor 05. The recipient received in vivo alemtuzumab monoclonal antibody to deplete T cells (days -5 to +4 from the allograft infusion) and also received prolonged immunosuppressive therapy (until 20 months) for chronic GVHD. Recipient 05 had an episode of HCMV retinitis at 5 months following transplantation (having ceased ganciclovir prophylaxis at day +100), accompanied by a strong anti-HCMV IgM response; the retinitis was successfully treated with ganciclovir. Ganciclovir prophylaxis was then re-instituted and continued until 10 months after alloSCT. An HCMV pp65495-503-specific CD8+ T-cell clone obtained from recipient 05 at 21 months following alloSCT (clone 5.2; Table 4), contained donor DNA. We were unable to detect clonotype (or pp65495-503 peptide-specific CD8+ T cells using tetramers) within donor PBMCs or within recipient PBMC samples taken prior to transplantation or at 3, 5, 7, and 9 months following alloSCT. Clonotype was detected at 12, 18, and 21 months, indicating that a primary CD8+ T-cell response had occurred between 9 and 12 months after alloSCT in cells of donor origin, whereas the patient was receiving immunosuppressive therapy (Figure 3).
Following T-cell-depleted D-/R+ alloSCT, a primary HCMV-specific CD8+ T-cell response developed in recipient 05 within 12 months. An HCMV pp65495-503-specific CD8+ T-cell clone (clone 5.2) was obtained from the HCMV-seropositive recipient at 21 months after T-cell-depleted alloSCT. The clone was not detected in the donor or in recipient 05 prior to transplantation. Following an episode of HCMV retinitis at 5 months, the clone was first detected in recipient 05 at 12 months after alloSCT. The retinitis resolved following ganciclovir therapy, and subsequent weekly blood tests for HCMV DNA were negative.
Following T-cell-depleted D-/R+ alloSCT, a primary HCMV-specific CD8+ T-cell response developed in recipient 05 within 12 months. An HCMV pp65495-503-specific CD8+ T-cell clone (clone 5.2) was obtained from the HCMV-seropositive recipient at 21 months after T-cell-depleted alloSCT. The clone was not detected in the donor or in recipient 05 prior to transplantation. Following an episode of HCMV retinitis at 5 months, the clone was first detected in recipient 05 at 12 months after alloSCT. The retinitis resolved following ganciclovir therapy, and subsequent weekly blood tests for HCMV DNA were negative.
Following D+/R+ sibling alloSCT, there is late diversification in the clonal composition of HCMV-specific CD8+ T cells within the HCMV-seropositive recipient
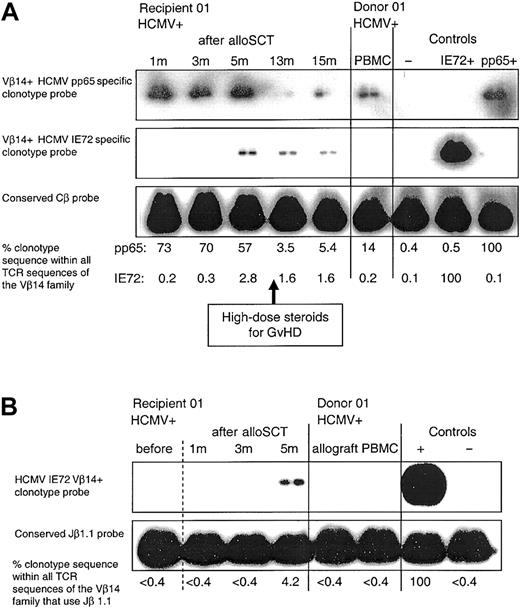
Following (D+/R+) alloSCT in HCMV-seropositive recipients 01 and 04, we identified HCMV peptide-specific CD8+ T-cell clones (clones 1.4 and 4.2, which both contained donor DNA) that were different from the HCMV peptide-specific CD8+ T-cell clones obtained from their respective HCMV-seropositive donors (Table 4). The IE72198-207-specific clonotype (clone 1.4) was first detected in PBMCs from recipient 01 at 5 months (while still receiving cyclosporin), and at all time points tested thereafter (Figure 4A). The delayed emergence of this T-cell clone in the recipient following alloSCT may be due to the generation of a novel CD8+ T-cell clonotype that developed from donor-derived progenitor cells. Alternatively, this CD8+ T-cell clone might represent delayed expansion of a subdominant clone already present in the donor at very low levels, below the levels of detection. To increase the sensitivity of clonotype detection, we used seminested PCR to amplify samples using Vβ14and Cβ-specific primers, and then subsequent amplification using Vβ14- and Jβ1.1-specific primers. Repeated clonotype probing was again positive in the recipient PBMC sample taken at 5 months following alloSCT but was again negative for all other samples (Figure 4B). In recipient 04, a pp65495-503-specific CD8+ T-cell clone (clone 4.2) was obtained 6 years following alloSCT. This clone was not detectable in donor PBMCs (Table 4). The clonotype frequency was similar at 6 years and 7 years, indicating that in fully reconstituted recipients, clones generated by late diversification are stably maintained over time.
Following D+/R+ alloSCT there is delayed emergence of an HCMV-specific CD8+ T-cell clone that was not detectable in the donor. (A) The CD8+ T-cell clone generated in donor 01, which was specific for HCMV pp65495-503 (clone 1.1), was detectable in recipient 01 PBMCs at all time points tested following alloSCT. An HCMV IE72198-207-specific CD8+ T-cell clone (clone 1.4) was obtained from recipient 01 at 13 months after alloSCT; this clone was not detected in the donor or in the recipient at 1 and 3 months after alloSCT but was first detected at 5 months following transplantation. In the controls, the pp65+and IE72+-labeled lanes refer to the positive control cDNA used from clones 1.1 and 1.4, respectively. Weekly blood tests for HCMV DNA performed until cessation of immunosuppression at 15 months were negative. (B) PBMC samples in the same donor-recipient pair were amplified by seminested PCR as described. Clone 1.4 was not detectable in donor PBMCs or allograft, or in recipient samples taken prior to transplantation or at 1 and 3 months after alloSCT but was detected at 5 months.
Following D+/R+ alloSCT there is delayed emergence of an HCMV-specific CD8+ T-cell clone that was not detectable in the donor. (A) The CD8+ T-cell clone generated in donor 01, which was specific for HCMV pp65495-503 (clone 1.1), was detectable in recipient 01 PBMCs at all time points tested following alloSCT. An HCMV IE72198-207-specific CD8+ T-cell clone (clone 1.4) was obtained from recipient 01 at 13 months after alloSCT; this clone was not detected in the donor or in the recipient at 1 and 3 months after alloSCT but was first detected at 5 months following transplantation. In the controls, the pp65+and IE72+-labeled lanes refer to the positive control cDNA used from clones 1.1 and 1.4, respectively. Weekly blood tests for HCMV DNA performed until cessation of immunosuppression at 15 months were negative. (B) PBMC samples in the same donor-recipient pair were amplified by seminested PCR as described. Clone 1.4 was not detectable in donor PBMCs or allograft, or in recipient samples taken prior to transplantation or at 1 and 3 months after alloSCT but was detected at 5 months.
Following alloSCT pp65 peptide-tetramer+ CD8+ cells are abundant within the expanded CD28-CD8+, CD27-CD8+, and CD11b+CD8+ T-cell subpopulations but very rare in the CD28+CD45RAhigh population
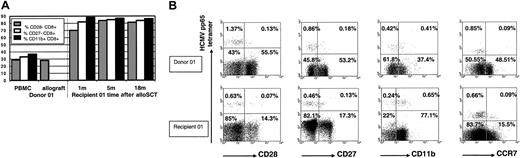
Relative to their donors, in recipients 01 to 04 the CD28-CD8+, CD27-CD8+, and CD11b+ CD8+ T-cell subpopulations were expanded (Figure 5A). This expansion was observed as early as 1 month and persisted long term. In recipients 01 to 04 (> 18 months after alloSCT) MHC class I HCMV pp65495-503 and pp65417-426 tetramer+ CD8+ T cells were predominant within the expanded CD28-CD8+ population (mean, 75% of all tetramer+ cells were CD28-CD8+; range, 59%-88%), within the expanded CD27-CD8+ population (70%; range, 43%-92%), and within the expanded CD11b+ CD8+ population (80%; range, 67%-90%) (Figure 5B). For other CD8+ T-cell subpopulations, the mean proportion of all tetramer+ cells was 19% (range, 9%-29%) in HLA-DR+ CD8+ cells, 79% (range, 70%-88%) in CCR7-CD8+ cells, 45% (range, 8%-72%) in CD57+ CD8+ cells, and 47% (range, 30%-80%) in CD45RAhigh CD8+ cells. Using 4-color flow cytometry in recipients 01 to 04, tetramer-staining CD8+ T cells were present in the CD28-CD45RAhigh (mean, 10% of tetramer+ cells; range, 8%-49%), CD28-CD45RAlo (mean, 46%; range, 25%-72%), and CD28+CD45RAlo (mean, 16%; range, 11%-51%) populations but were very rarely in the CD28+CD45RAhigh population (mean, 1.4%; range, 0%-2.8%).
Following alloSCT, MHC class I HCMV-pp65 peptide-specific tetramer+ CD8+ cells are abundant within the expanded CD28-CD8+, CD27-CD8+, and CD11b+CD8+ T-cell subpopulations. (A) Relative to donor 01 PBMCs, there is an early and persistent expansion in the percentage of CD28-CD8+ CD3+, percentage of CD27-CD8+ CD3+, and percentage of CD11b+ CD8+ CD3+ T cells in the PBMCs of recipient 01 following alloSCT. The proportion of CD28-CD8+ CD3+ T cells was similar in donor PBMCs and donor peripheral blood stem cells (PBSCs); CD27 and CD11b data were not available in donor 01 PBSCs. (B) Upper panels, donor 01; lower panels, recipient 01 at 22 months after alloSCT. Gated on CD8+ T cells. Results are expressed as percentage of CD8+ T cells. In recipient 01 MHC class I HCMV-pp65 peptide-specific tetramer+ CD8+ cells are abundant within the expanded CD28-CD8+, CD27-CD8+, and CD11b+ CD8+ T-cell populations and are predominantly CCR7-CD8+. Results are representative of recipients 01 to 04.
Following alloSCT, MHC class I HCMV-pp65 peptide-specific tetramer+ CD8+ cells are abundant within the expanded CD28-CD8+, CD27-CD8+, and CD11b+CD8+ T-cell subpopulations. (A) Relative to donor 01 PBMCs, there is an early and persistent expansion in the percentage of CD28-CD8+ CD3+, percentage of CD27-CD8+ CD3+, and percentage of CD11b+ CD8+ CD3+ T cells in the PBMCs of recipient 01 following alloSCT. The proportion of CD28-CD8+ CD3+ T cells was similar in donor PBMCs and donor peripheral blood stem cells (PBSCs); CD27 and CD11b data were not available in donor 01 PBSCs. (B) Upper panels, donor 01; lower panels, recipient 01 at 22 months after alloSCT. Gated on CD8+ T cells. Results are expressed as percentage of CD8+ T cells. In recipient 01 MHC class I HCMV-pp65 peptide-specific tetramer+ CD8+ cells are abundant within the expanded CD28-CD8+, CD27-CD8+, and CD11b+ CD8+ T-cell populations and are predominantly CCR7-CD8+. Results are representative of recipients 01 to 04.
Discussion
After alloSCT, T-cell reconstitution could potentially arise from 4 different cell populations: (1) mature postthymic donor T cells present in the graft, (2) donor hemopoietic progenitors in the graft that differentiate in the recipient into naive T cells that undergo TCR gene rearrangement, (3) mature recipient T cells that survive the chemoradiotherapy preparative regimen, and (4) recipient hemopoietic progenitors that survive the preparative regimen. Recipient T cells survive the preparative regimen better than other recipient hemopoietic cells; recipient progenitor cells are destroyed by the preparative regimen.20 Using minisatellite PCR amplification, Roux et al21 found that following receipt of unmanipulated bone marrow, most T cells were of donor origin. In contrast, in recipients of T-cell-depleted (TCD) bone marrow, the T-cell population initially was mixed in origin, containing T cells derived from transferred donor cells and surviving host T cells. In our study, using DNA analysis for sex chromosome and/or informative SNPs, all virus-specific CD8+ T-cell clones identified in the recipient after alloSCT (including clones that arose as primary virus-specific responses in the recipient) contained DNA of donor origin.
Previous studies in humans have shown that antigen-specific T-cell responses can be transferred from donor to recipient by alloSCT, which implies that antigen-experienced donor T cells are transferred in the allograft and persist in the recipient.22,23 We found for the first time at a clonal level (using longitudinal clonotype probing to analyze CD8+ T-cell reconstitution following alloSCT) that in 6 different sibling donor-recipient pairs when both donor and recipient were seropositive for either HCMV or EBV individual antigen-experienced virus-specific CD8+ T-cell clones in donor PBMCs were transferred via the graft into the recipient where they are maintained long term. These clones obtained from the recipient months to years after transplantation were detectable at all early and late time points tested (up to 6 years) and were capable of in vitro proliferation (at least 12 doublings in LDA culture) and antigen-specific cytotoxicity. In the matched-unrelated HCMV D+/R+ alloSC transplant recipient 10 who received alemtuzumab monoclonal antibody, HCMV-specific donor clones failed to reconstitute, and HCMV viremia occurred repeatedly.
In the 4 D+/R+ sibling donor-recipient pairs, following cessation of immunosuppressive therapy 5 to 72 months after alloSCT, the HCMV peptide-specific CTLp frequencies were determined by LDA. In contrast to previous studies that suggested that the human virus-specific CTLp frequency in LDA may be up to 25-fold lower than the frequency of tetramer+ cells,24,25 we found that in both donors and recipients the HCMV peptide-specific CTLp frequency was generally only 3-fold lower than the frequency of tetramer+ cells (possibly because we have developed LDA culture conditions that increase the cloning efficiency of antigen-specific CTLp in vitro). The HCMV peptide-specific CTLp frequencies in the recipients were similar to those obtained in the respective donors. In donor-recipient pair 01, the higher relative frequency of pp65-specific CTLp compared with IE72-specific CTLp in the donor was also maintained in the recipient. In both donor-recipient pairs 01 and 03, the dominant pp65-specific clones obtained from the donors (1.1, 3.1a) were also the dominant clonotypes obtained from their respective recipients following alloSCT (Table 2). It may be that once the antigen-experienced CD8+ T-cell compartment has been fully reconstituted within the long-term surviving recipient, similar homeostatic mechanisms are involved in regulating the size of the pool of peptide-specific CD8+ T cells in both donor and recipient.
Our data suggest that the maintenance of transferred donor HCMV-specific CD8+ T-cell clones following D+/R+ transplantation is related to re-encounter with HCMV antigen in the recipient. The inability to detect HCMV DNAemia by PCR does not preclude subclinical HCMV reactivation at localized tissue sites in these patients. Most active HCMV infections observed in the recipient after alloSCT are the result of reactivation of latent virus in HCMV-seropositive recipients.26 Among HCMV-seronegative recipients who receive hemopoietic allografts from HCMV-seropositive donors and who receive only HCMV-seronegative blood products, the incidence of HCMV infection (with or without seroconversion) is relatively low, only 17%.27 We studied HCMV-seronegative recipients 06 to 08 who received allografts from HCMV-seropositive donors; these recipients had no serologic evidence or evidence of HCMV DNAemia (on weekly monitoring continued until cessation of immunosuppression) after alloSCT. HCMV tetramer+ CD8+ T cells were detected in granulocyte colony-stimulating factor (G-CSF)-mobilized PBSC allografts at a similar frequency to unmanipulated donor PBMCs, indicating that G-CSF mobilization did not result in a selective depletion of donor HCMV-specific CD8+ T cells in the allograft prior to transplantation (Figure 2). In HCMV-seronegative recipients 06 and 07, none of 3 donor HCMV-specific CD8+ T-cell clones (clones 6.1a, 6.1b, and 7.1) were detectable in recipient PBMCs following alloSCT by clonotype probing or by tetramer staining; because internal positive controls in these recipients both of whom were EBV-seropositive, we readily detected the maintenance of donor-derived EBV-specific CD8+ T-cell clones following alloSCT, either a primary EBV-specific response in recipient 06 or transferred in the graft in recipient 07 (Table 4). The maintenance of the EBV-specific clones indicates that there was no global impairment of antigen-presenting cell or CD4+ T-cell function in these recipients. The contrast between the consistent maintenance of donor HCMV peptide-specific clones in 4 HCMV-seropositive recipients and the selective failure to detect transferred donor clones specific for the same HCMV peptides in 2 HCMV-seronegative recipients suggests that the presence of HCMV in the recipient plays an important role in the maintenance of transferred donor HCMV-specific clones.
In HCMV-seronegative recipient 08, the donor HLA-A2-restricted HCMV-specific CD8+ T-cell clone was detectable to 5 months after transplantation, shortly after which the recipient died because of severe GVHD and relapse of the underlying hematologic malignancy. We were, therefore, unable to determine whether this donor clone would have persisted long term in this recipient. A possible explanation for the persistence of this particular clone in recipient 08 in the absence of detectable HCMV infection is that its TCR may have been cross-reactive with another antigen in the recipient. In recipients who possess HLA-A2, donor-derived HCMV-specific T cells can cross-react with HLA-A2-restricted recipient minor histocompatibility antigens.28 Recipient 08 was HLA-A2 positive and experienced severe acute GVHD, raising the possibility that this donor clone might be alloreactive and directly participating in the GVHD and/or that the inflammatory cytokines evoked by severe GVHD contributed to its maintenance. Interestingly, Cwynarski et al10 reported that following HCMV D+/R- alloSCT, pp65 tetramer+ CD8+ T cells were detectable in only 1 of 7 recipients, and this recipient was the only one of the 7 recipients to experience acute GVHD.
The question of whether antigen-experienced T cells require exposure to antigen for their maintenance continues to be debated. Adoptive transfer experiments of antigen-experienced T cells into irradiated naive mice can result in long-term maintenance of antigen-specific T cells in the apparent absence of specific antigen.29 Immune reconstitution following alloSCT in humans is likely to be more complex than in the mouse because adult humans are infected with a number of persistent viruses that undergo intermittent reactivation. Our data and those of Cwynarski et al10 indicate that in human HCMV-seronegative alloSC transplant recipients, any transferred donor HCMV-specific CD8+ T-cell clones may fail to compete successfully with other CD8+ T cells (eg, EBV-specific CD8+ T cells) that do encounter specific antigen in the recipient and proliferate strongly during reconstitution (as we consistently observed in EBV-seropositive recipients).
Human naive T cells can be generated within the first year after alloSCT.8,30 We found that seropositive recipients who received an alloSC transplant from a seronegative donor were able to generate a primary virus-specific CD8+ T-cell response, from cells that contained donor DNA. These primary CD8+ T-cell responses showed delayed kinetics and were not detectable prior to 5 months after alloSCT. Detection rates of both EBV DNA and HCMV DNA in serum are highest between 2 and 3 months after alloSCT,31 and it may be that viral reactivation in vivo is necessary for induction of primary HCMV-specific or EBV-specific CD8+ T-cell responses. The virus-specific CD8+ T-cell clones that participated in the primary CD8+ T-cell response may have been generated from mature naive T cells transferred from the donor within the graft or from newly generated naive T cells produced by de novo TCR gene recombination in the recipient from donor-derived progenitor cells. To address this issue, we analyzed HCMV-specific CD8+ T-cell clones obtained from HCMV-seropositive recipient 05 following alemtuzumab-treated matched-unrelated donor transplantation from an HCMV-seronegative donor. Following an episode of HCMV disease, a primary HCMV-specific CD8+ T-cell response developed in recipient 05 at 12 months after transplantation. In vivo alemtuzumab monoclonal antibody treatment results in a profound and sustained depletion of mature donor T cells transferred in the allograft.32 The delayed emergence of the HCMV-specific T-cell clone in this recipient suggests that this clone was newly produced in the recipient by TCR gene recombination from donor progenitor cells, rather than mature naive donor T cells in the allograft.
In 2 of the 4 sibling pairs in which both donor and recipient were HCMV-seropositive, the CD8+ T-cell clonal composition within the seropositive recipient became diversified in comparison with that of the seropositive donor, by the delayed emergence of additional CD8+ T-cell clones that could not be detected prior to 5 months following transplantation. These additional T-cell clones contained donor DNA but could not be detected in either donor PBMCs or in recipient PBMCs during the first 5 months after transplantation. We have previously established that the probing assay is sufficiently sensitive to detect 1% specific clonotype sequence in a background of 99% different sequences that all have the same Vβ gene segment.15 It is, therefore, likely that the delayed emergence of novel HCMV-specific T-cell clones was due to the generation of new T-cell clones in the recipient from progenitor cells of donor origin, rather than the delayed expansion of a subdominant clone present in donor PBMCs at very low levels.
Relative to the corresponding donor, among the total CD8+ T-cell population in the recipient after alloSCT, the costimulatory molecules CD28 and CD27 are down-regulated, and the CD11b+ (a β2 integrin adhesion molecule) T-cell subpopulation is expanded. The increase in these subpopulations was observed early and persisted long term. In recipients analyzed more than 18 months following alloSCT, we found that most of the pp65 HCMV-specific CD8+ T cells were in the expanded CD28- and CD27- populations and were CD11b+ and CCR7-. Our findings are consistent with the results of Gamadia et al33 who found that in patients receiving renal transplants, circulating HCMV-specific CD8+ T cells were predominantly CD45R0highCD27-CCR7- or CD45RAhighCD27-CCR7-. These antigen-specific cells may use different forms of costimulation other than the CD28 or CD27 pathways, the precise nature of which remains to be determined. The CCR7- and CD11b+ phenotype is consistent with the tissue-homing phenotype proposed by Sallusto et al.34 In agreement with Cwynarski et al10 we found the CD45RA expression of HCMV-specific CD8+ T cells is heterogeneous, but as we found with healthy HCMV carriers,35 the CD45RAhigh population of HCMV pp65-specific CD8+ T cells within HCMV-seropositive recipients after alloSCT are almost entirely within CD28-CD45RAhigh cells but were very rare in the CD28+CD45RAhigh population.
Thus, for both HCMV and EBV, donor virus-specific CD8+ T-cell clones transferred within the graft can expand and persist within the recipient after sibling alloSCT when the recipient is seropositive, and in some but not all seropositive recipients after sibling alloSCT there is late diversification of the HCMV-specific CD8+ T-cell clonal repertoire in response to persistent viral antigen.
Prepublished online as Blood First Edition Paper, July 17, 2003; DOI 10.1182/blood-2002-12-3689.
Supported by the Leukaemia Research Fund (M.K.G.), a Medical Research Council (MRC) program grant (J.G.S. and J.S.) and by a MRC Co-operative Group Grant.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked ”advertisement” in accordance with 18 U.S.C. section 1734.
We thank Karen Brown and Nick Watkins for performing SNP analysis and all the staff of the East Anglian Blood and Bone Marrow Transplant Unit for their assistance.
A.J.C. is a Lister Institute Research Fellow.