Abstract
Stromal cell-derived factor 1 (SDF1/CXCL12) and its cognate receptor, CXCR4, play key regulatory roles in CD34+ cell trafficking. We investigated whether AMD3100, a selective CXCR4 antagonist, could mobilize hematopoietic progenitor cells from marrow to peripheral blood in healthy human volunteers. Initially, 10 persons each received a single dose of AMD3100 (80 μg/kg subcutaneously), which induced rapid, generalized leukocytosis associated with an increase in peripheral blood CD34+ cells, representing pluripotent hematopoietic progenitors by in vitro colony-forming unit assays, from 3.8 ± 0.5/μL to 20.7 ± 3.5/μL at 6 hours. Subsequent dose-response studies showed a maximum increase in circulating CD34+ cells from 2.6 ± 0.3/μL to 40.4 ± 3.4/μL at 9 hours after 240 μg/kg AMD3100. Serial administration of AMD3100 (80 μg/kg/d for 3 days) resulted in consistent, reversible increases in peripheral blood CD34+ cells. AMD3100 was well tolerated and caused only mild, transient toxicity. These findings suggest potential clinical application of AMD3100 for CD34+ cell mobilization and collection for hematopoietic stem cell transplantation.
Introduction
Stromal cell-derived factor-1 (SDF-1; CXCL12), originally described as a stimulatory factor for pre-B cells but now recognized as a multifunctional cytokine, is a member of the CXC chemokine family and is constitutively expressed on bone marrow stromal cells.1-4 The cognate receptor for SDF-1 is CXCR4, a 7-transmembrane G-protein–coupled receptor expressed on many cell types, including CD34+ hematopoietic progenitor cells, where it plays a critical role in cell survival, proliferation, directed migration, and engraftment.4-13
Because CXCR4 serves as a coreceptor for the entry of HIV into host cells, a number of CXCR4 ligands have been developed as potential therapeutic agents for HIV-infected persons.14-19 AMD3100 (AmorMED, Langley, BC, Canada), a bicyclam derivative, is a specific antagonist of CXCR4,16-19 although a recent report suggests that AMD3100 may exhibit weak, partial agonism at high concentrations.20 AMD3100 was noted to cause modest leukocytosis when administered intravenously to HIV-1–infected persons in a previously reported phase 1 trial.18 Based on this observation, we hypothesized that AMD3100 might mobilize CD34+ and hematopoietic progenitor cells from marrow to peripheral blood.
Study design
Twenty-six healthy human volunteers (aged 24-33 years; 13 men, 13 women) participated in these studies under protocols approved by the Human Subjects Committee/Institutional Review Boards of the University of Washington (Seattle), Indiana University School of Medicine (Indianapolis), and the Scientific Advisory Committee for the Clinical Research Center of the University of Washington Medical Center. Informed consent was obtained from all volunteers before enrollment in study protocols. All subjects had normal blood cell counts, normal liver and kidney function test results, and normal findings on physical examination, and they were taking no regular medications. Because premature ventricular contractions were observed in 2 of 40 HIV-infected persons in a previous trial,18 normal electrocardiogram findings were also required for the enrollment of subjects receiving the highest dose of AMD3100 (240 μg/kg).
Each subject was admitted to the Clinical Research Center for AMD3100 injection, blood sampling, and clinical observation for adverse events. Three groups of subjects were enrolled: Group 1 (n = 10) received AMD3100 at a dose of 80 μg/kg in a single subcutaneous injection; group 2 (n = 13) was enrolled for dose-response (40-240 μg/kg, subcutaneously) studies to extend these initial observations; and group 3 (n = 3) received AMD3100 (80 μg/kg/d, subcutaneously) for 3 consecutive days to test the consistency of response and to test whether previous injection influenced subsequent response to AMD3100.
Before AMD3100 injection and at 1, 3, 6, 9, and 24 hours after injection, EDTA (ethylenediaminetetraacetic acid)–anticoagulated venous blood samples were obtained for blood cell counts, including CD34+ cell count fluorescence-activated cell sorter (FACS) analysis. CD34+ cells were enumerated according to a protocol of the International Society for Hematotherapy and Graft Engineering (ISHAGE) using a single-platform, 2-color assay performed on a Beckman-Coulter XL flow cytometer (Beckman-Coulter, Hialeah, FL).21
Granulocyte-macrophage colony-forming units (CFU-GMs), granulocyte-erythroid-macrophage-megakaryocyte CFUs (CFU-GEMMs), and erythroid burst-forming units (BFU-Es) were enumerated by standardized assays, as previously described.22 The percentage of progenitor cells in S phase of the cell cycle was also measured using a high specific-activity [3H]-thymidine kill assay.23
Statistical analysis of differences between groups was performed using the paired Student t test.
Results and discussion
Single-dose administration of AMD3100 (40-240 μg/kg) caused generalized leukocytosis in healthy human volunteers, with peak increases in neutrophils, lymphocytes, monocytes, eosinophils, and basophils observed at 6 to 9 hours after drug administration (data not shown). AMD3100 induced a modest shift to band forms (maximum, 1.71 × 109/L at 9 hours after 240 μg/kg AMD3100) but did not increase metamyelocytes or myelocytes. No statistically significant effects on circulating levels of erythrocytes or platelets were observed at any of the AMD3100 doses studied in this trial (data not shown).
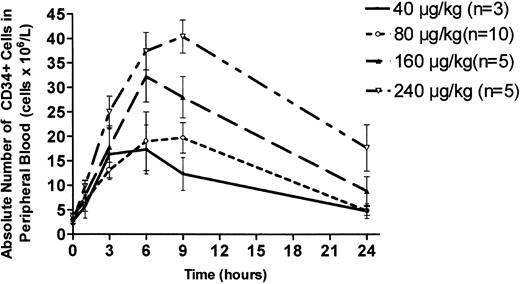
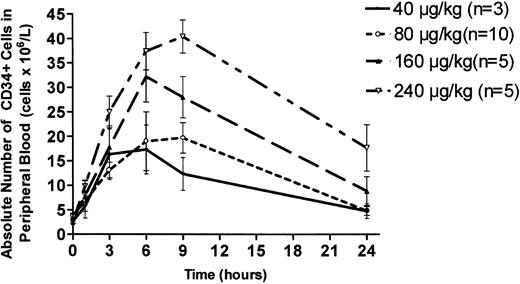
Single-dose administration of AMD3100 at 80 μg/kg caused a 4-fold increase in circulating CD34+ cells, with a peak value at 6 hours (20.7 ± 3.5/μL; mean ± SEM) that declined to baseline by 24 hours (Figure 1). Dose-response studies showed a clear dose-dependent effect, with a peak 10-fold increase in peripheral blood CD34+ cells at 9 hours (40.4 ± 3.4/μL) after subcutaneous injection of AMD3100 at 240 μg/kg (Figure 1). All enrolled subjects showed similar responses. The CD34+ cell response to 240 μg/kg, the highest dose administered in this study, was significantly greater at 9 hours, but not at 6 hours, compared with 160 μg/kg (P < .05). The CD34+ cell response induced by 240 μg/kg was significantly greater than the response to 80 μg/kg at 3, 6, and 9 hours (P < .05). When AMD3100 was administered on 3 consecutive days at 80 μg/kg/d, baseline circulating values of CD34+ cells were similar on each day. On days 1 and 3 of consecutive daily administration, the magnitude of the AMD3100-induced increase in circulating CD34+ cells was similar, suggesting that the CD34+ cell response was not substantially affected by serial injections of AMD3100 (data not shown).
Dose-response analysis of AMD3100-induced mobilization of CD34+ cells into peripheral blood. Healthy human volunteers received a single subcutaneous injection of AMD3100 at the following doses: 40 μg/kg (n = 3; solid line); 80 μg/kg (n = 10; ○); 160 μg/kg (n = 5; ▴); and 240 μg/kg (n = 5; ▿). Peripheral venous blood was withdrawn at time intervals after drug administration, and FACS analysis was used to determine the concentration of CD34+ cells. Each value represents the mean ± SEM.
Dose-response analysis of AMD3100-induced mobilization of CD34+ cells into peripheral blood. Healthy human volunteers received a single subcutaneous injection of AMD3100 at the following doses: 40 μg/kg (n = 3; solid line); 80 μg/kg (n = 10; ○); 160 μg/kg (n = 5; ▴); and 240 μg/kg (n = 5; ▿). Peripheral venous blood was withdrawn at time intervals after drug administration, and FACS analysis was used to determine the concentration of CD34+ cells. Each value represents the mean ± SEM.
In vitro CFU assays demonstrated that AMD3100 (80 μg/kg) increased circulating levels of myeloid and erythroid progenitor cells (n = 10; Table 1). Significant increases in circulating levels of all types of colonies assayed were observed within 3 hours of AMD3100 administration. The greatest relative increase occurred in the number of CFU-GMs assayed in either the agar or the methylcellulose system. Specifically, 20-, 10-, and 6-fold peak increases were observed in circulatory CFU-GM, CFU-GEMM, and BFU-E, respectively, at 6 hours after AMD3100 administration (Table 1). In terms of cell cycle status, mobilized CD34+ cells were quiescent, as determined by pulse-treatment of cells with high specific-activity [3H]-thymidine (0%-5% kill at each time point; P > .05; n = 10; data not shown).
All adverse effects observed in healthy human volunteers were mild and transient. The following adverse effects were reported or observed: erythema or stinging at the AMD3100 injection site (18 of 26 volunteers); headache (7 of 26 volunteers); perioral paresthesias (8 of 26 volunteers); nausea (10 of 26 volunteers); and sensation of abdominal distention without diarrhea (5 of 26 volunteers). No arrhythmias were detected. All adverse effects resolved within 24 hours. There was no apparent difference in frequency or severity of adverse effects for any of the doses of AMD3100 studied. No significant changes in serum values of electrolytes, blood urea nitrogen (BUN), creatinine, hepatic aminotransferases, bilirubin, or alkaline phosphatase were observed in any of the 26 healthy human volunteers after AMD3100 administration.
These studies demonstrate that single-dose administration of AMD3100 induces a reversible, dose-dependent increase in CD34+ hematopoietic progenitor cells in peripheral blood. Currently, G-CSF is the most widely used agent for the mobilization of CD34+ cells, with the collection of approximately 5 × 106 CD34+ cells/kg recipient body weight generally regarded as sufficient for engraftment and hematopoietic recovery in most persons.24-26 An increase in circulating CD34+ cells to more than 20/μL generally predicts the collection of sufficient cells from the donor with 1 to 3 leukapheresis procedures.26 The magnitude and rapid time course of increases in circulating CD34+ cells, particularly at the 160 μg/kg and 240 μg/kg doses, are sufficient to suggest that AMD3100 may be clinically useful for the mobilization of progenitor cells for hematopoietic transplantation.
Developing new agents for efficient and safe mobilization of peripheral blood hematopoietic progenitor cells is critically important for the clinical practice of hematopoietic stem cell transplantation.27 Further studies are indicated to determine the role of AMD3100, administered either as a single agent or in combination with granulocyte colony-stimulating factor (G-CSF), in human CD34+ hematopoietic progenitor cell mobilization and collection.
Prepublished online as Blood First Edition Paper, July 10, 2003; DOI 10.1182/blood-2003-02-0663.
Supported by research funding from AnorMED Inc and conducted through the Clinical Research Center facility at the University of Washington. Supported also by National Institutes of Health grants MO1RR 00037, ROI DK53674, ROI HL6738, and ROI HL 56416.
Presented at the 43rd Annual Meeting of the American Society of Hematology, December 7-11, 2001, Orlando, FL, and published in part in abstract form.28
G.J.B., G.W.H., and G.C. are employed by AnorMED Inc, whose product, AMD3100, was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Christine Dehner, Karen Badel, and Jeff Christensen for their assistance with the research, and Theresa Wittenberg, Linda Peyton, and Tanya Webb for their assistance in preparing the manuscript.