Abstract
After an episode of unstable angina or myocardial infarction, a high proportion of patients show biochemical signs of coagulation activation, expressed as persistently elevated thrombin generation, in their blood. It is not known whether this has any influence on long-term outcome. In this prospective multicenter cohort study, we assessed the relation of persistently elevated thrombin generation to outcome in 319 consecutive patients with acute coronary syndromes enrolled in the Global Use of Strategies To Open occluded coronary arteries (GUSTO) IIb trial. Plasma prothrombin fragment 1 + 2 levels, an index of “in vivo” thrombin generation, was measured during the acute phase and after 1, 6, and 12 months, and its relation to outcome was assessed during a median 29-month follow-up period. The primary end point of cardiac death or myocardial (re)infarction occurred in 61 patients (19%). There was a U-shaped relationship between plasma prothrombin fragment 1 + 2 levels and the risk of developing the primary end point; intermediate levels (1.5-1.9 nM) were associated with the lowest risk, whereas both higher (> 1.9 nM) and lower (< 1.5 nM) values were associated with an increased risk (relative risk [RR] 1.56 and 95% confidence interval [CI], 1.25-2.28; RR, 1.35 and 95% CI, 1.11-1.86, respectively). After an episode of acute coronary syndrome, both high and low levels of thrombin generation are predictors of an increased risk of an unfavorable outcome.
Introduction
Intracoronary thrombosis over plaque disruption or fissuring is considered to be the major pathogenetic mechanism of unstable angina and myocardial infarction.1,2 After an episode of an acute coronary syndrome, the occurrence of cardiac complications, such as sudden death, (re)infarction, or recurrent rest angina, has also been attributed to the development of intracoronary thrombosis,3 but no large prospective study concerning the effect of increased hemostatic system activity on long-term outcome has yet been reported.
During the acute phase of unstable angina or myocardial infarction, elevated plasma concentrations of prothrombin fragment 1+2 (F1+2) and fibrinopeptide A, markers of factor Xa–mediated prothrombin activation and thrombin action on fibrinogen, respectively, have been related to an early unfavorable outcome.4,5 After an episode of acute coronary syndrome, a high proportion of patients have persistently elevated levels of prothrombin F1+2, but not fibrinopeptide A.6 This condition indicates increased hemostatic system activity and has been considered indicative of a hypercoagulable state.7 However, it is not known whether this has any effect on the long-term clinical outcome.
The Italian Haematologic Substudy of the Global Use of Strategies To Open occluded coronary arteries (GUSTO) IIb trial was prospectively designed to investigate the relationship between the plasma levels of prothrombin F1+2 measured at different time points after an episode of acute coronary syndrome and the occurrence of adverse cardiac events during long-term follow-up.
Patients and methods
Study population
All of the patients consecutively enrolled in the GUSTO IIb trial at 4 Italian centers participating to the GUSTO IIb Trial (the Division of Cardiology IRCCS Policlinico San Matteo, Pavia; the Division of Cardiology, Ca' Granda Niguarda and Hospital, Milan; Ospedale Giovanni Battista Morgagni, Forlì; and Ospedale Civile, Ravenna) were considered eligible for the Italian Haematologic Substudy. The GUSTO IIb study8 enrolled the whole spectrum of acute coronary syndromes, that is, unstable angina and non–Q-wave and Q-wave myocardial infarction. All of the patients had to have symptoms of cardiac ischemia at rest within the 12 hours preceding enrollment and electrocardiographic signs of acute myocardial ischemia, that is, persistent or transient ST-segment elevation or depression more than 0.5 mm, or definite T-wave inversion more than 1 mm. The exclusion criteria for GUSTO IIb have been previously reported.8 The specific exclusion criteria for the Haematologic Substudy were comorbid conditions known to alter coagulation system activity, concomitant vascular disorders or valvular heart disease, the need for chronic anticoagulant treatment, and severely limited venous access.
Study protocol
The GUSTO IIb study was a randomized, double-blind, multinational, multicenter trial.8 The patients were randomized to receive antithrombotic treatment with either intravenous heparin or hirudin (desirudin; Ciba-Geigy, Summit, NJ) for a minimum of 3 and a maximum of 5 days. The dose of hirudin was a 0.1-mg/kg bolus followed by 0.1 mg/kg/h. The heparin dose was a 5000-U bolus followed by 1000 U/h, with dose adjustments being made using a standard nomogram to maintain an activated partial thromboplastin time between 60 and 85 seconds. All patients received aspirin (165-325 mg), before starting the study drug. The associated treatments were given as decided by the patients' individual physicians and were not dictated by the study protocol.
The patients enrolled in the Italian Haematologic Substudy underwent blood sampling immediately before study drug discontinuation and after 1, 6, and 12 months. Follow-up visits were scheduled 1, 6, 12, and 24 months after the enrollment of each patient and at the end of the study (ie, at the time of the 24-month visit of the last enrolled patient). Long-term treatment was also left to the discretion of the patients' individual physicians, with aspirin and β-blockers being strongly recommended.
The study was approved by the institutional review boards of the IRCCS, Policlinico di Milano, and informed consent was obtained from all of the enrolled patients.
Study end points
The primary end point was the composite of cardiac death and myocardial (re)infarction. Cardiac death was defined as death due to cardiac causes. Myocardial (re)infarction included Q-wave and non–Q-wave myocardial (re)infarction. Q-wave myocardial (re)infarction required ischemic pain lasting more than 30 minutes with recurrent ST-T changes associated with a (re)elevation of creatine kinase or the MB fraction of creatine kinase to more than twice the upper limit of the reference range, and new significant Q waves in at least 2 leads on a standard 12-lead electrocardiogram. The diagnosis of non–Q-wave myocardial (re)infarction required only the first 2 characteristics. The secondary end point was the composite end point of cardiac death, myocardial (re)infarction, and recurrent angina requiring revascularization or rehospitalization. Angina requiring revascularization was severe rest angina lasting at least 20 minutes, associated with ST-T changes and leading to nonscheduled/unplanned revascularization within 24 hours of the index hospitalization or severe rest angina after discharge that resulted in a rehospitalization during which coronary revascularization was performed; angina requiring rehospitalization was defined as angina at rest as described leading to prompt rehospitalization, with a discharge diagnosis of acute ischemic heart disease as the reason for admission. All clinical events were adjudicated independently by an end-point committee of 3 cardiologists, who were unaware of the biologic results and treatment assignment.
Blood sampling and handling
Clean venipunctures were performed atraumatically by specially trained investigators using 19-gauge butterfly infusion sets and a 2-syringe technique. Inadequate blood samples were prospectively excluded. After the first 4 mL blood was discarded, the samples were directly placed into refrigerated vacutainers containing an anticoagulant mixture of a thrombin inhibitor (D-phenylalanyl-prolyl-arginine cloromethyl ketone [PPACK]), EDTA (ethylenediaminotetraacetic acid), and aprotinin (Byk-Sangtec, Dietzenbach, Germany); the ratio of anticoagulant to blood was 1:9 (vol/vol). The samples were immediately centrifuged at 2500g for 25 minutes at 4°C. The plasma was then snap-frozen and stored at –80°C until analyzed.
Biochemical determinations
All of the samples were centrally analyzed by investigators unaware of the clinical data, using a double-antibody radioimmunoassay as previously described.9 This method has an interassay coefficient of variation of about 8%.
To investigate the biologic significance of plasma F1+2 levels further, we measured plasma activated protein C (aPC) levels as previously described10 in the samples drawn after 1 month. The median plasma F1+2 level was 1.36 nM (interquartile range, 0.99-1.86 nM) and the median aPC level was 112 ng/mL (interquartile range, 95-130 ng/mL). There was a close positive correlation between F1+2 and aPC (rho = 0.66; P = .0001).
Statistical analysis
Because the plasma levels of prothrombin F1+2 were nonnormally distributed and had different degrees of dispersion at the various time points, repeated measures were compared by means of the Friedman test and subsequent pairwise comparisons were made using the Wilcoxon signed rank test. The descriptive statistics include mean values and SDs or median values and interquartile ranges as appropriate. Normally distributed variables were compared by means of the unpaired t test; nonnormally distributed variables were compared using the Mann-Whitney U test and correlation calculated with the Spearman rank correlation coefficient.
The relation between plasma prothrombin F1+2 levels and primary or secondary end points was studied using the Cox proportional hazards model with time-varying covariates. This model allows the hazard of the end point at any time t to depend on time and on the most recent prothrombin F1+2 measurement, and controls for potential confounders, that is, age, sex, body mass index, smoking habits, diabetes, hypercholesterolemia, history of hypertension, prior myocardial infarction, clinical diagnosis at presentation, treatment assignment (hirudin or heparin), chronic use of β-blockers, aspirin, or lipid-lowering agents, the direction of ST-segment shift on the qualifying electrocardiogram (ST-segment elevation, depression, or both), or the presence of T-wave changes. The relationships between plasma prothrombin F1+2 levels and the hazard of the end points was further explored using a method based on an analysis of Martingale residuals,11 which represent a suitable form of regression in the presence of censored response data. The method consists of a 3-stage procedure: (1) a Cox regression model is applied to the data assuming a null effect of the explanatory variable of interest (in our case, F1+2 levels); (2) the residuals from the fitting are plotted against the corresponding values of the explanatory variable; and (3) the plot is nonparametrically smoothed, and the presence of substantial deviations from linearity in the smoothing may be interpreted as an indicator of the nonlinear dependence of hazard on the explanatory variable. A 2-tailed P value of less than .05 was considered to indicate statistical significance. The statistical analysis was carried out by means of S-PLUS statistical software.
Results
Three hundred fifty-one consecutive patients enrolled in the GUSTO IIB in the 4 Italian Centers were considered eligible to enter the Italian Haematologic Substudy. Eighteen patients refused to participate in the substudy, 9 patients agreed to participate but did not come to any follow-up visit, and 5 patients were lost to follow-up. The study population therefore consisted of 319 patients, 91% of the eligible patients. The demographic and clinical characteristics of the study population are reported in Table 1.
Demographic, clinical, and electrocardiographic characteristics of the study population according to the occurrence of primary or secondary end points during follow-up
. | . | Primary end point . | . | . | Secondary end point . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics . | All patients, N = 319 . | With events, N = 61 . | Without events, N = 258 . | P . | With events, N = 144 . | Without events, N = 175 . | P . | ||||
| Age, y | 65 ± 7 | 66 ± 10 | 65 ± 9 | NS | 66 ± 10 | 66 ± 7 | NS | ||||
| Male sex (%) | 235 (73) | 46 (77) | 189 (72) | NS | 116 (80) | 119 (68) | NS | ||||
| Height, cm | 168 ± 7 | 168 ± 7 | 168 ± 7 | NS | 169 ± 6 | 167 ± 7 | NS | ||||
| Weight, kg | 73 ± 12 | 73 ± 12 | 73 ± 13 | NS | 73 ± 11 | 72 ± 13 | NS | ||||
| Family history (%) | 106 (33) | 18 (30) | 88 (34) | NS | 45 (31) | 61 (34) | NS | ||||
| Current smokers (%) | 106 (33) | 23 (38) | 83 (32) | NS | 47 (32) | 59 (33) | NS | ||||
| Diabetes mellitus (%) | 60 (18) | 16 (26) | 44 (17) | NS | 36 (25) | 24 (13) | NS | ||||
| History of hypertension (%) | 155 (49) | 28 (45) | 127 (49) | NS | 76 (52) | 79 (45) | NS | ||||
| Hypercholesterolemia (%) | 108 (34) | 12 (17) | 96 (37) | .009 | 45 (31) | 63 (36) | NS | ||||
| Previous myocardial infarction (%) | 112 (35) | 24 (39) | 88 (34) | NS | 60 (41) | 52 (30) | .02 | ||||
| Previous angina (%) | 90 (28) | 21 (34) | 69 (26) | NS | 37 (25) | 53 (30) | NS | ||||
| Chronic treatment, n (%) | |||||||||||
| Aspirin | 312 (98) | 58 (95) | 254 (99) | NS | 142 (98) | 174 (99) | NS | ||||
| Ticlopidine | 4 (1) | 2 (3) | 2 (1) | NS | 2 (4) | 1 (1) | NS | ||||
| β-blockers | 261 (82) | 46 (75) | 215 (83) | NS | 117 (81) | 144 (82) | NS | ||||
. | . | Primary end point . | . | . | Secondary end point . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics . | All patients, N = 319 . | With events, N = 61 . | Without events, N = 258 . | P . | With events, N = 144 . | Without events, N = 175 . | P . | ||||
| Age, y | 65 ± 7 | 66 ± 10 | 65 ± 9 | NS | 66 ± 10 | 66 ± 7 | NS | ||||
| Male sex (%) | 235 (73) | 46 (77) | 189 (72) | NS | 116 (80) | 119 (68) | NS | ||||
| Height, cm | 168 ± 7 | 168 ± 7 | 168 ± 7 | NS | 169 ± 6 | 167 ± 7 | NS | ||||
| Weight, kg | 73 ± 12 | 73 ± 12 | 73 ± 13 | NS | 73 ± 11 | 72 ± 13 | NS | ||||
| Family history (%) | 106 (33) | 18 (30) | 88 (34) | NS | 45 (31) | 61 (34) | NS | ||||
| Current smokers (%) | 106 (33) | 23 (38) | 83 (32) | NS | 47 (32) | 59 (33) | NS | ||||
| Diabetes mellitus (%) | 60 (18) | 16 (26) | 44 (17) | NS | 36 (25) | 24 (13) | NS | ||||
| History of hypertension (%) | 155 (49) | 28 (45) | 127 (49) | NS | 76 (52) | 79 (45) | NS | ||||
| Hypercholesterolemia (%) | 108 (34) | 12 (17) | 96 (37) | .009 | 45 (31) | 63 (36) | NS | ||||
| Previous myocardial infarction (%) | 112 (35) | 24 (39) | 88 (34) | NS | 60 (41) | 52 (30) | .02 | ||||
| Previous angina (%) | 90 (28) | 21 (34) | 69 (26) | NS | 37 (25) | 53 (30) | NS | ||||
| Chronic treatment, n (%) | |||||||||||
| Aspirin | 312 (98) | 58 (95) | 254 (99) | NS | 142 (98) | 174 (99) | NS | ||||
| Ticlopidine | 4 (1) | 2 (3) | 2 (1) | NS | 2 (4) | 1 (1) | NS | ||||
| β-blockers | 261 (82) | 46 (75) | 215 (83) | NS | 117 (81) | 144 (82) | NS | ||||
NS indicates not significant.
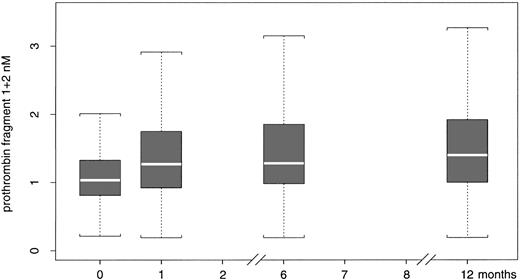
The median and 25th and 75th percentile values of prothrombin F1+2 at the different time points are shown in Figure 1. The median prothrombin F1+2 levels measured in the plasma sample drawn immediately before study drug discontinuation were lower (1.04 nM; interquartile range, 0.83-1.32 nM) than those measured in the plasma samples drawn after 1 month (1.36 nM; interquartile range, 0.99-1.86 nM; P < .001), 6 months (1.33 nM; interquartile range, 1.01-1.88 nM; P < .001), and 1 year (1.46 nM; interquartile range, 1.04-2.12 nM; P < .001). There was no statistical difference between the prothrombin F1+2 plasma levels measured after 1, 6, and 12 months.
Prothrombin F1+2 values distribution. Distribution of prothrombin F1+2 values at the beginning of the study (0), and after 1, 6, and 12 months. The data are median values, 25th and 75th percentiles, and ranges.
Prothrombin F1+2 values distribution. Distribution of prothrombin F1+2 values at the beginning of the study (0), and after 1, 6, and 12 months. The data are median values, 25th and 75th percentiles, and ranges.
The F1+2 levels in the first sample drawn immediately before study drug discontinuation were significantly different between the patients treated with hirudin (median 0.98 nM; interquartile range, 0.71-1.19 nM) and those treated with heparin (1.11 nM; interquartile range, 0.9-1.39 nM), whereas those in the samples drawn after 1, 6, and 12 months were not significantly different between the 2 treatment groups.
The total number of person-months of observation was 6015, with a median follow-up of 29 months (range, 0.1-42 months). During follow-up, 61 patients (19%) experienced a primary end point and 144 (45%) a secondary end point; 24 (8%) died of cardiac death, 37 (12%) had a myocardial (re)infarction, 39 (12%) had recurrent angina requiring revascularization, and 44 (14%) had recurrent angina requiring rehospitalization. The demographic and clinical characteristics of the patients developing a primary or secondary end point compared with those who did not are shown in Table 1. The number of events occurring at each time interval is reported in Table 2.
Primary and secondary end points at each time interval during follow-up
. | Up to 1 mo . | 1-6 mo . | 6-12 mo . | 12 mo . | Total (%) . |
|---|---|---|---|---|---|
| Cardiac death | 6 | 7 | 3 | 8 | 24 (8) |
| Myocardial infarction | 12 | 17 | 3 | 5 | 37 (12) |
| Primary end point | 18 | 24 | 6 | 13 | 61 (19) |
| Angina requiring revascularization | 23 | 14 | 1 | 1 | 39 (12) |
| Angina requiring rehospitalization | 5 | 25 | 8 | 6 | 44 (14) |
| Secondary end point | 46 | 63 | 15 | 20 | 144 (45) |
. | Up to 1 mo . | 1-6 mo . | 6-12 mo . | 12 mo . | Total (%) . |
|---|---|---|---|---|---|
| Cardiac death | 6 | 7 | 3 | 8 | 24 (8) |
| Myocardial infarction | 12 | 17 | 3 | 5 | 37 (12) |
| Primary end point | 18 | 24 | 6 | 13 | 61 (19) |
| Angina requiring revascularization | 23 | 14 | 1 | 1 | 39 (12) |
| Angina requiring rehospitalization | 5 | 25 | 8 | 6 | 44 (14) |
| Secondary end point | 46 | 63 | 15 | 20 | 144 (45) |
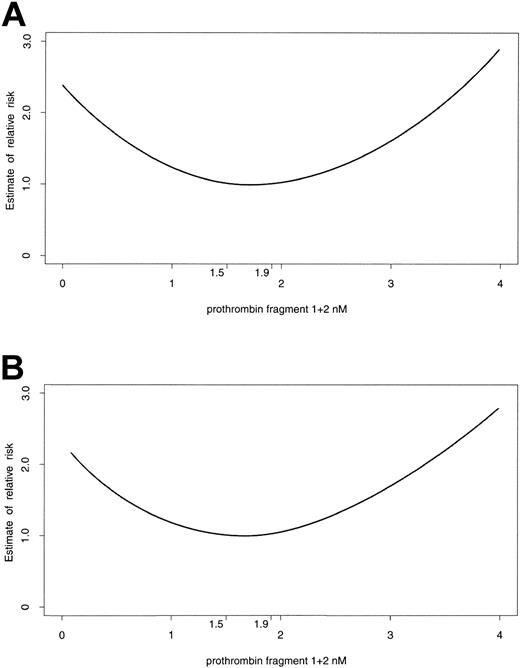
The Cox proportional hazard model with time-varying covariates was used to analyze the relation between plasma prothrombin F1+2 levels and the risk of developing events during follow-up and showed strong evidence of a departure from linearity for both the primary and secondary end points (P < .001 and P = .002, respectively); rather, U-shaped curves emerged (Figure 2). The lowest risk for both the primary and secondary end points was when prothrombin F1+2 levels were in the range of 1.5 to 1.9 nM. Higher values (> 1.9 nM) were associated with an increased risk of developing the primary (relative risk [RR], 1.56; 95% confidence interval [CI], 1.25-2.28) or secondary end points (RR, 1.41; 95% CI, 1.31-1.85), and lower values (< 1.5 nM) were associated with an increased risk of developing the primary end point (RR, 1.35; 95% CI, 1.11-1.85) and a trend toward an increased risk of developing a secondary end point (RR 1.2; 95% CI, 0.93-1.5). Given that plasma prothrombin F1+2 levels increase over time, 2 separate analyses of the relation between this parameter and the risk of developing primary events were performed; one included the events occurring in the first 6 months, the other the events occurring between 6 months and the end of follow-up. Both analyses yielded a U-shaped relationship, with shifted nadirs; the curve describing the relation between prothrombin F1+2 and the events occurring in the early phase had the lowest risk in conjunction with levels of prothrombin F1+2 of 1 to 1.7 nM, whereas the nadir of the curve describing the risk for late events shifted toward the right (1.9-2.5 nM; (Figure 3). In all of our model-based analyses, we controlled for the effect of potential confounders by including them as explanatory terms. Both high and low levels of plasma prothrombin F1+2 were independent predictors of a primary end point (P = .0017 and P = .007, respectively). The presence of ST-segment depression on the qualifying electrocardiogram was the only other variable found to provide an independent contribution to the prediction of risk of primary outcome events (P = .0055).
Relationship between prothrombin F1+2 levels and end points. Relation between prothrombin F1+2 plasma levels and the risk of developing primary (A) and secondary (B) end points during follow-up.
Relationship between prothrombin F1+2 levels and end points. Relation between prothrombin F1+2 plasma levels and the risk of developing primary (A) and secondary (B) end points during follow-up.
Risk for developing primary events. Relation between prothrombin F1+2 plasma levels and the risk of developing primary end points during the first 6 months (thin line) and from 6 months to the end of follow-up (thick line). The 2 curves have similar shapes, but the lowest risk for developing primary events was observed in conjunction with prothrombin F1+2 plasma levels of between 1.0 and 1.7 nM in the first 6 months, and between 1.9 and 2.5 nM from 6 months to the end of follow-up.
Risk for developing primary events. Relation between prothrombin F1+2 plasma levels and the risk of developing primary end points during the first 6 months (thin line) and from 6 months to the end of follow-up (thick line). The 2 curves have similar shapes, but the lowest risk for developing primary events was observed in conjunction with prothrombin F1+2 plasma levels of between 1.0 and 1.7 nM in the first 6 months, and between 1.9 and 2.5 nM from 6 months to the end of follow-up.
Discussion
The new finding of this prospective cohort study is that intermediate levels of thrombin generation were associated with the lowest risk of suffering death or myocardial (re)infarction after an episode of acute coronary syndrome. Both higher or lower levels of prothrombin F1+2 were associated with an increased risk of death or (re)infarction. There was also a U-shaped relationship between this parameter and the development of the secondary end point, which included death, (re)infarction, and rest angina requiring revascularization or rehospitalization.
The U-shaped relationship between thrombin generation and the risk of developing adverse events after an episode of acute coronary syndrome was unexpected. Maintaining normal hemostasis requires keeping a systemic and local balance between procoagulant and anticoagulant pathways. Thrombin is a serine protease, which may be either procoagulant or anticoagulant, depending on its concentration, and therefore plays a critical role in the hemostatic balance. Free thrombin resulting from the action of factor Xa on prothrombin activates platelets, clots fibrinogen, and converts inactive forms of coagulation factors V, VIII, and XIII to their active forms; however, when thrombin is bound to thrombomodulin, a cell-surface vascular endothelium receptor, its procoagulant properties are largely neutralized and its ability to activate protein C is greatly enhanced. Activated protein C is a potent anticoagulant that inactivates factors Va and VIIIa and also has anti-inflammatory actions. At low concentrations, thrombin generates markedly increased levels of endogenous circulating protein C and exerts an anticoagulant effect12-14 ; the relationship between the thrombotic potential of blood and thrombin concentration “ex vivo” is therefore described by a U-shaped curve.
Although this so-called “thrombin paradox” has been well characterized “in vitro,”15 our data suggest that it may be operative in the “in vivo” regulation of hemostatic balance following an initial episode of an acute coronary ischemic syndrome. The lowest relative risk for the primary and secondary end points was actually observed for intermediate concentrations of thrombin generation, thereby suggesting that intermediate levels of thrombin generation are required for the optimal function of the protein C anticoagulant pathway “in vivo.” High levels of thrombin generation may overwhelm the endogenous anticoagulant mechanisms and be prothrombotic. This may be even more critical in the setting of an unstable atherosclerotic plaque, which is associated with a loss of the antithrombotic properties of laminar flow and a normally functioning endothelium. The measurement of plasma levels of aPC16 or the protein C activation peptide17 might provide support for this hypothesis. In our population, the levels of aPC closely correlated (rho = 0.66) with F1+2 levels, thus strengthening the biologic plausibility of our findings. However, other explanations can be suggested such as an important role of thrombin in cardiac neoangiogenesis following an initial coronary ischemic event, that is, low thrombin concentrations might be insufficient for new blood vessel formation, whereas intermediate concentrations might prove salutary for this.18
Previous studies have suggested that prothrombin F1+2 levels change over time in patients with acute coronary syndromes.6,19 In conformity with current practice for other cardiovascular risk factors, such as blood pressure and serum cholesterol, this prospective study was designed to ensure that repeated measurements of plasma prothrombin F1+2 were made at different time points during follow-up. Our results show that there is a significant increase in the levels of thrombin generation between the acute and chronic phase. One possible explanation may be related to the fact that all of the patients were receiving antithrombotic treatment during the acute phase and this may have decreased thrombin generation; previous data have shown that thrombin generation increases 1 month after the discontinuation of heparin or hirudin.19
Our observation that both high and low thrombin generation levels correlate with recurrent myocardial infarction conflicts with the finding of the Second Northwick Park Heart Study that prothrombin F1+2 has no predictive value for a first ischemic event in healthy middle-aged men.20 However, the Second Northwick Park Heart Study refers to healthy individuals, whereas our study refers to patients who suffered an acute coronary event. It can be also surmised that hemostatic activity is altered by an ischemic or infarcted myocardium, which may influence endothelial cell anticoagulant function21 and thereby increase susceptibility to subsequent coronary arterial thrombotic events.22
In conclusion, our findings support the existence of a correlation between prothrombin F1+2 levels and the risk of developing adverse cardiac events during the long-term follow-up of patients with acute coronary syndromes. This relationship is not linear, but U-shaped; there is an “optimal” level of thrombin generation associated with the lowest risk, and both higher and lower values are associated with an increased risk of an unfavorable outcome. Further studies will be required to determine the mechanistic basis of this finding, which may be relevant in the optimization of antithrombotic therapy.
Prepublished online as Blood First Edition Paper, July 3, 2003; DOI 10.1182/blood-2002-03-0954.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal