Abstract
We explored the feasibility and toxicity of administering escalating doses of anti-CD3/CD28 ex vivo costimulated T cells as a therapeutic adjunct for patients with relapsed, refractory, or chemotherapy-resistant, aggressive non-Hodgkin lymphoma (NHL) following high-dose chemotherapy and CD34+-selected hematopoietic cell transplantation (HCT). Sixteen patients had infusions on day 14 after HCT of autologous T cells that had been stimulated using beads coated with anti-CD3 and anti-CD28 monoclonal antibodies. At baseline, the subjects had severe quantitative and functional T-cell impairments. The culture procedure partially reversed impaired cytokine responsiveness in T cells in vitro and in vivo. Transient dose-dependent infusion toxicities were observed. There was a rapid reconstitution of lymphocytes; however, there were persistent defects in CD4 T cells. Most interestingly, 5 patients had a delayed lymphocytosis between day 30 and day 120 after HCT. Maximal clinical responses included 5 patients with a complete response (CR), 7 patients with a partial response (PR), and 4 patients with stable disease. At a median follow-up of 33 months (range, 26-60 months), 5 patients are alive with stable or relapsed disease and 3 patients remain in CR. In conclusion, this phase 1 trial demonstrates that adoptive transfer of autologous costimulated T cells (1) is feasible in heavily pretreated patients with advanced NHL, (2) is associated with a rapid recovery of lymphocyte counts, (3) reverses cytokine activation deficits in vitro, and (4) is associated with delayed lymphocytosis in a subset of patients.
Introduction
Hematopoietic cell transplantation (HCT) improves survival in selected patients with various hematologic malignancies, including relapsed, chemotherapy-sensitive, aggressive non-Hodgkin lymphoma.1-5 However, despite the survival advantage of HCT, post-HCT relapse rates range between 40% and 70%.6,7 The high relapse rates after HCT have been attributed to the inability of high-dose therapy to eradicate minimal residual disease and to the absence of graft-versus-tumor immune response that occurs after allogeneic HCT.8
There is a substantial and prolonged T-cell depletion following HCT,9 and infectious morbidity in long-term survivors of allogeneic marrow transplantation is associated with low CD4 T-cell counts.10 T-cell immunodeficiency can be profound after autologous HCT, particularly CD34+-selected HCT.11,12 Recent studies have shown that the early absolute lymphocyte count (ALC) after autologous and allogeneic HCT is associated with prolonged survival. Powles et al13 demonstrated a 3-year relapse probability rate of 16% for an ALC of more than 200 cells per microliter versus 42% for an ALC of fewer than 200 cells per microliter on day 29 after allogeneic HCT (P = .004) in patients with acute myelogenous leukemia (AML). Pavletic et al14 analyzed the ALC in patients with various hematologic malignancies after allogeneic HCT and observed that the overall survival at 1 year with an ALC at day 17 after allogeneic bone marrow transplantation (ABMT) of 500 cells per microliter or more was 79% versus 19% for an ALC of fewer than 500 cells per microliter (P = .002). For patients with non-Hodgkin lymphoma (NHL) following an autologous HCT, Porrata and coworkers15 found that the median overall survival (OS) and progression-free survival (PFS) times were significantly longer in patients with an ALC of 500 cells per microliter or more versus those with fewer than 500 cells per microliter. Prolonged survival was associated with early lymphocyte recovery after autologous HCT for patients with AML, Hodgkin disease, and metastatic breast cancer.16-18 Multivariate analysis demonstrated the day 15 ALC to be an independent prognostic indicator for OS and PFS rates.15
Adoptive immunotherapy with mature T cells has the potential to ameliorate or prevent immunosuppression following HCT. In allogeneic HCT, resting donor T cells may serve as a source of T cells but may cause or exacerbate graft verus host disease (GVHD). In contrast, in autologous HCT, several technical limitations have prevented this approach to date. First, in heavily pretreated patients, cytotoxic therapy causes a severe reduction in available T cells to serve as a source for ex vivo expansion. Second, initial approaches involving T-cell culture and expansion including lymphokine-activated killer/interleukin-2 (LAK/IL-2) regimens or antibody-induced signal transduction with anti-CD3 have resulted in the preferential expansion of CD8+ T cells.19 More recently, we and other investigators have reported successful growth and expansion of CD4+ T cells for adoptive transfer in clinical trials.20-22 Our approach has been based on a novel culture system based on an artificial antigen-presenting cell composed of antibodies to the T-cell accessory molecules, CD3 and CD28, immobilized on a magnetic bead. This system provides costimulatory signals to drive the logarithmic growth and expansion of CD3+CD28+ human T cells.23
To determine whether accelerated T-cell immune reconstitution was possible after HCT, we conducted a phase 1 study to determine (1) the feasibility of producing CD3/CD28 ex vivo–expanded costimulatory T cells in heavily pretreated patients with advanced B-cell NHL and (2) to assess the toxicity of administering escalating doses of CD3/CD28 expanded T cells. Subjects with relapsed or refractory NHL with adverse prognostic features were selected for this trial because it was hypothesized that the administration of costimulated T cells as therapeutic adjunct to high-dose chemotherapy (HDC) may potentially accelerate immune reconstitution after dose-intensive chemotherapy. In this trial we determined a maximal feasible dose of autologous costimulated CD3+ T cells in this group of heavily pretreated patients. Dose-dependent infusion toxicities were observed but were transient and reversible. Most interestingly, a subset of patients had a syndrome of delayed lymphocytosis. The results suggest an approach to accelerate immune reconstitution following high-dose chemotherapy that may contribute to enhanced immune recovery and potentially to improved antitumor immunity.
Patients, materials, and methods
Patient eligibility and enrollment
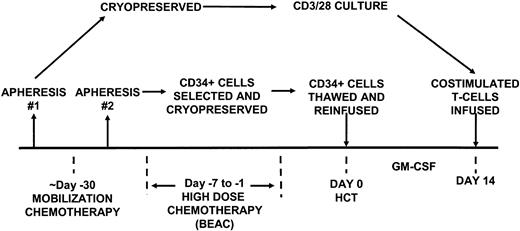
This protocol was approved by the institutional review boards of the University of Chicago and the University of Pennsylvania and was conducted under a Food and Drug Administration (FDA)–approved investigational new drug application. Toxicity grading was performed according to the National Cancer Institute common toxicity criteria. Written informed consent for the protocol (Figure 1) was obtained in accordance with institutional review board guidelines.
Study design. The aphereses were performed as indicated at “steady state” to provide T cells for culture and following mobilization for CD34+ cell selection.
Study design. The aphereses were performed as indicated at “steady state” to provide T cells for culture and following mobilization for CD34+ cell selection.
Eligibility criteria included patients with biopsy-proven NHL (may include low, intermediate, or high grade), which had been refractory to a doxorubicin-based regimen, or relapsed disease from such a regimen. Patients with T-cell NHL were excluded. Relapsed NHL was defined as patients with recurrent disease after complete response (CR) but no more than 5 years after achieving CR, unless tumor could be shown to be identical to the original clone. Refractory disease was defined as patients who achieved less than a CR to first-line therapy. High-risk disease was defined as less than a partial response (PR) to a standard pretransplantation induction regimen. Patients were required to demonstrate an Eastern Cooperative Oncology Group (ECOG) performance status of 0, 1, or 2 with a maximum age of 70 years. Normal renal, hepatic, pulmonary, and cardiac function was required. Patients were excluded with active central nervous system (CNS) disease or active or chronic pulmonary disease.
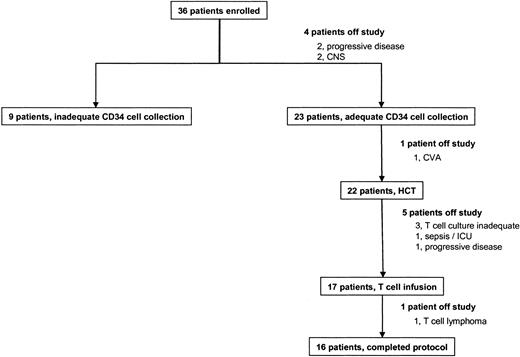
Thirty-six subjects were enrolled on the protocol, and 4 were removed for progressive disease (n = 2) or active CNS involvement (n = 2) (Figure 2). Twenty-three subjects had an adequate CD34 cell collection, and 22 were treated with HCT; 1 was removed for an intercurrent cerebrovascular accident (CVA), stroke. Seventeen of the patients given HCT received T-cell infusions, and 16 were evaluable according to the entry criteria; 6 were taken off study for failure of the T-cell culture process (n = 3), intercurrent sepsis preventing T-cell infusion (n = 1), T-cell lymphoma with aberrant B-cell markers discovered on retrospective pathologic analysis (n = 1), and progressive disease (n = 1).
Patient outcome. Boxes represent treatment pathways for all subjects enrolled onto the protocol. HCT indicates hematopoietic cell transplantation.
Patient outcome. Boxes represent treatment pathways for all subjects enrolled onto the protocol. HCT indicates hematopoietic cell transplantation.
Leukapheresis I
Patients underwent a steady-state single 20 L apheresis procedure using an automated CS3000 blood cell separator (Baxter, Fenwal Division, Round Lake, IL) or the COBE Spectra cell separator (COBE BCT, Lakewood, CO) prior to receiving mobilization chemotherapy. This product served as the source of cells for the CD3+/CD28+ T-cell expansion. See Figure 1 for study design.
Peripheral blood stem cell mobilization/leukapheresis II
Cyclophosphamide 2000 mg/m2 in 500 mL 5% dextrose or normal saline was given over 1 hour every 12 hours for 2 doses followed by etoposide (VP-16) 250 mg/m2 intravenously every 2 hours for 4 doses on the day after the cyclophosphamide was administered. The etoposide was administered in a syringe pump undiluted and infused over 90 minutes. The chemotherapy doses were based on actual body weight. Granulocyte colony-stimulating factor (G-CSF) (Neupogen, Amgen, Thousand Oaks, CA) was given at a dose of 10 μg/kg/d to start 9 days after the cyclophosphamide dose and to continue daily until the last leukapheresis day. Patients were given ciprofloxacin 500 mg orally twice a day and fluconazole 100 mg orally 4 times a day during the period of neutropenia.
Apheresis was performed using the CS3000 blood cell separator (Fenwal). Leukapheresis commenced when (1) circulating CD34+ cells reached more than 5/μL, (2) white blood count (WBC) more than 5000/μL, and (3) platelet count more than 30 000/μL without transfusion support. The end point for successful peripheral blood progenitor cell harvest was more than 5 × 106 CD34+ cells per kilogram. At least 1.0 × 106 CD34+ cells per kilogram were cryopreserved for a “backup” product in case of graft failure. The remaining 4 × 106 CD34+ cells per kilogram underwent positive CD34 selection using the Baxter Isolex 300i Magnetic Cell Separation System (Baxter Oncology, Deerfield, IL) according to the manufacturer's instructions. The positively selected cell fraction was cryopreserved and stored within the vapor phase of liquid nitrogen. Failure to meet the collection end points after 6 daily leukapheresis procedures disqualified the patient from continuing on the protocol.
High-dose chemotherapy
Patients received the BEAC regimen, which consisted of BCNU (N,N-bis[2-chloroethyl]-N-nitrosourea) 300 mg/m2/d on day –6, etoposide 100 mg/m2 on day –5 through day –2 every 12 hours for a total of 8 doses, cytarabine 100 mg/m2 intravenously on days –5 through day –2 every 12 hours for a total of 8 doses, and cyclophosphamide 35 mg/kg/d on days –5 through day –2 intravenously for a total of 4 doses. Granulocyte-macrophage colony-stimulating factor (GM-CSF) (Leukine, Immunex, Seattle, WA) was given at a dose of 5 μg/kg/d beginning on day +1 until the absolute neutrophil count (ANC) was at least 5000 cells per microliter. Supportive care included acyclovir 800 mg orally twice a day and fluconazole 200 mg orally daily from day –6 until ANC was more than 1000/μL. Aerosolized pentamidine 300 mg via inhalation was given once between day +7 and day +21.
Ex vivo costimulation and expansion of T lymphocytes
The premobilization apheresis product was thawed and washed 3 times in phosphate-buffered saline (PBS) with 1% human serum albumin (HSA). The washed peripheral blood mononuclear cell (PBMC) product was enriched for lymphocytes using magnetic bead depletion of monocytes in a closed system. T cells were cultured according to Good Manufacturing Practice regulations as previously described, with the exception that CD8+ T cells and CD20+ B cells were not removed from the starting culture.24 The cells were expanded ex vivo for 14 days in X-VIVO 15 (BioWhittaker, Walkersville, MD) supplemented with 10% Normal Human AB Serum (Valley Biomedical, Winchester, VA), 2 mM l-glutamine (BioWhittaker), and 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) (BioWhittaker) and then processed for reinfusion on day 14. The magnetic beads were removed using a Baxter Fenwal Maxsep magnetic cell separation system. After cell culture, harvest was performed on the day of scheduled cell reinfusion with the Baxter Fenwal cell harvester; the cells were washed and resuspended in Plasmalyte A containing 1% HSA.
In preclinical data, we used a seminested polymerase chain reaction (PCR) assay to identify tumor-specific CDR3 sequences. The sensitivity of the assay was about 1 tumor cell per million cells. In 3 of 6 patients assayed, we demonstrated tumor contamination at the start of the T-cell culture. However, 6 of 6 subjects had no detectable tumor by day 14 of culture. The phenotype of the ex vivo expanded cells was determined at the time of initial seeding, at 7 days of expansion, and then on day 14 of expansion at the time of harvesting. The cells were stained with anti-CD3, anti-CD4, and anti-CD8 and analyzed by flow cytometry to determine the number and relative frequency of total T cells (CD3+) as well as CD4+ and CD8+ T cells. Tumor cells were not detected at the completion of the T-cell culture in any subject.
Dose escalation and reinfusion of expanded T cells
The total dose of reinfused T cells was based on the number of CD3+ cells as determined by the total cell count and flow cytometry with anti-CD3. After the 14 days of expansion, this constituted at least 95% of the total cells in the culture. The expanded T-cell product was resuspended in 100 to 250 mL Plasmalyte A containing 1% HSA. The cells were infused over 30 minutes without a leukocyte filter.
The protocol was a standard dose-escalation study, with the intention to treat 3 patients at each dose level. However, 1 patient at dose level 2 experienced a neurologic syndrome (see “Clinical toxicity”), and therefore 6 patients were intentionally enrolled at dose level 2. In addition, 4 patients received dose level 2 and 1 patient dose level 1 due to feasibility issues, in that insufficient T cells were available for the intended dose level of administration. The ex vivo expanded T cells were infused at the following doses: dose level 1: 2.5 × 109 CD3+ T cells; dose level 2: 5.0 × 109 CD3+ T cells; and dose level 3: 1.0 × 1010 CD3+ T cells.
Intracellular cytokine staining and flow cytometry
Patient cells were obtained by venipuncture or apheresis and cryopreserved as PBMCs. Thawed cells were cultured in X-VIVO 15 (BioWhittaker) supplemented with 5% human AB serum (BioWhittaker), 20 mM HEPES (BioWhittaker), 2 mM l-glutamine (BioWhittaker), and 10 μg/mL DNase I (Sigma, St Louis, MO). Cells were stimulated with 10 ng/mL phorbol 12-myristate 13-acetate (PMA) (Sigma), 2 μg/mL ionomycin (Sigma), and 1 μg/mL 9.3 anti-CD3 mAb in the presence of 1 μg/mL brefeldin A (Sigma). After 6 hours, cells were washed once in Dulbecco phosphate-buffered saline (DPBS) without Ca++ and Mg++ (BioWhittaker) and stained for surface markers with fluorescent conjugated monoclonal antibody (mAb) (BD Pharmingen, San Diego, CA) for 30 minutes at room temperature, fixed, and permeabilized (Caltag, An Der Grub, Austria). The cells were stained for intracellular cytokines using antibody conjugates (BD Pharmingen) for 15 minutes at room temperature. The stained cells were analyzed by flow cytometry using FACSCalibur and CellQuest software (Becton Dickinson, San Jose, CA).
ELISPOT assay for IFN-γ release
PBMCs from NHL study patients with available cryopreserved cells and 6 healthy donors were analyzed for interferon-γ (IFN-γ) release following stimulation for 18 to 24 hours with (a) 20 ng/mL phorbol 12-myristate 13-acetate (PMA) (Sigma) and 2 μg/mL of the calcium ionophore ionomycin (Sigma), (b) 5 μg/mL phytohemagglutinin (PHA) (Sigma) and 100 IU/mL IL-2 (Chiron, Emeryville, CA), or (c) as a measure of allogeneic response, a pool of Epstein-Barr virus (EBV)–negative B-cell lines, including GA-10, ST486, CA46, and Ramos, which were kindly provided by Dr Aaron Rapoport. The B-cell lines were pretreated for 48 hours with 20 μg/mL lipopolysaccharide (Sigma) and irradiated at 70 Gy prior to mixing with patient PBMC cells at a ratio of 2:1 effectors-targets. Cytomegalovirus (CMV) and EBV viral lysate (Advanced Biotechnologies, Columbia, MD) was used at 3 μg/mL.
Enzyme-linked immunospot (ELISPOT) assays were carried out essentially as described.25 The spots were scanned and counted by computer-assisted Elispot image analysis (Hitech Instruments, Edgemont, PA). Digitized images were analyzed for the presence of areas in which color density, spot size, and circularity exceeds background by a factor set on the basis of the comparison of control wells.
MHC class I tetramer analysis
Soluble HLA-A*0201 tetramers were prepared with β2-microglobulin and the immunodominant N495 peptide (NLVPMVATV)26 derived from pp65 of cytomegalovirus (CMV) as described27 and conjugated to phycoerythrin. Negative control tetramer was made with the HLA-A*0201–binding peptide L11 (LLFGYPVYV)28 derived from human T-cell leukemic virus type 1 (HTLV-1) tax. Synthetic peptides (more than 96% pure) were purchased from New England Peptide, Fitchburg, MA. PBMCs from patients were incubated with tetramers and with mAbs CD8–fluorescein isothiocyanate (CD8-FITC) (Immunotech, Marseille, France), CD4–peridinin chlorophyll protein (CD4-PerCP) (Becton Dickinson), and CD14-PerCP (Becton Dickinson) for 30 minutes at room temperature (RT) and analyzed by flow cytometer using a FACSCalibur.
Response criteria and follow-up
Complete response (CR) was defined as no clinical, radiologic, or histopathologic evidence of tumor for 6 or more months' duration. Partial response (PR) was defined as a minimum of 50% reduction in tumor with no evidence of progression at any site and no new sites of disease. Progressive disease (PD) was defined as more than 25% increase in size of an existing lesion or the appearance of any new lesion. Tumor reevaluation with computed tomography (CT) scan and single photon emission computed tomography (SPECT)–gallium scans was performed 30 days following stem cell reinfusion and then every 3 months or as clinically indicated for 18 months following therapy.
Results
Patient characteristics
Thirty-six patients were enrolled between June 1997 and March 2000 as described in “Patients, materials, and methods” (Figure 1). Sixteen patients with a mean age of 49 years (range, 15-70 years) met the study eligibility criteria and received one infusion of CD3+/CD28+ costimulated T cells on day 14 after CD34-selected HCT (Figure 2). Table 1 lists the clinical characteristics of these patients. Most of the patients were male and had diffuse large cell lymphoma and advanced disease (stage III-IV). Most patients had primary refractory lymphoma and had received a median of 3 previous chemotherapy regimens. Fourteen of the 16 evaluable patients had received CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) as first-line therapy, with 14 of 16 patients proceeding to a cisplatin-containing regimen as salvage therapy. All patients had poor-risk prognostic factors, including bone marrow involvement in 2, and short periods of remission.29,30
Autologous HCT and T-cell infusions
The mean number of CD34+ cells present in the HCT grafts of the 16 subjects at the time of autologous infusion was 2.66 × 106 CD34+ cells per kilogram (range = 1.07 × 106-5.78 × 106). All patients showed successful myeloid engraftment with a mean time to achieve an absolute neutrophil count of more than 500/μL in 11.8 days (range, 10-14 days).
On day +14, patients were subsequently infused with T cells as indicated in Table 2. The dose levels of infused costimulated T cells were distributed as follows: 4 at dose level 1, 10 at dose level 2, and 2 at dose level 3. The mean number of T cells infused was 2.9 × 109 CD3+ T cells (range, 0.2 × 109-10.2 × 109) or 34 × 106 CD3+ T cells per kilogram (range, 2.6 × 106-119 × 106). The expanded T cells were infused over 30 minutes without a leukocyte filter. The feasibility of successful T-cell manufacturing was 84%, as there were 3 unsuccessful culture attempts. However, there were improvements in culture technique during the trial; all T-cell culture failures occurred in the first third of the patients enrolled.
Clinical toxicity
Immediate toxicities related to the infusion of the costimulated T cells appeared to be dose dependent and occurred in the 2 patients who were at dose level 3, the highest dose level achieved in this study. Both patients experienced grade 2 fevers and rigors during the infusion that immediately resolved with supportive care only. Pulmonary edema consistent with a grade 3 cardiac toxicity occurred in one of these patients. This patient had a history of congestive heart failure and promptly responded to diuresis. This was attributed to volume overload from the T-cell infusion. A grade 3 neurotoxicity was seen in one patient on dose level 2. This patient presented with right-sided hemiparesis and diplopia on day +86. Magnetic resonance imaging (MRI) of the brain showed thalamic and brainstem hemorrhages with abnormal enhancement of the choroid plexus. A biopsy of the choroid plexus showed a nonclonal population of atypical lymphoid cells. The patient's neurologic symptoms slowly resolved although the etiology of his symptoms or brain MRI findings was never determined.
Lymphocyte recovery
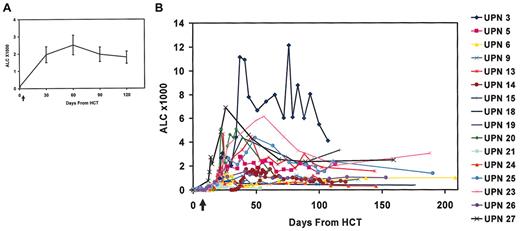
Blood samples on study days 30, 60, and 120 were obtained to assess lymphocyte engraftment (Figure 3). The ALC had a robust and rapid recovery, with mean ALC counts of 1960/μL, 2520/μL, and 1970/μL by days 30, 60, and 120, respectively, after HCT (Figure 3A). However, there was substantial variation in the individual rates and magnitudes of lymphocyte engraftment (Figure 3B). Most interestingly, 5 subjects developed a syndrome of delayed lymphocytosis, with absolute lymphocyte counts more than 4500 cells per microliter, and 10 patients had a relative lymphocytosis. In several cases the lymphocytosis was accompanied by fever and malaise, and extensive investigation failed to reveal a cause for the fever. In the subject with the most marked lymphocytosis (UPN3), tumor regression occurred during the lymphocytosis.
Lymphocyte counts of NHL study patients following HCT and costimulated T-cell infusions given on day 14 after HCT as indicated by arrow. (A) The mean (± SEM) ALC of all study patients receiving T cells (at days 0, 30, 60, 90, and 120; n = 16, 16, 14, 13, and 11, respectively). (B) ALC of individual patients. Day 0 is designated as the day of HCT.
Lymphocyte counts of NHL study patients following HCT and costimulated T-cell infusions given on day 14 after HCT as indicated by arrow. (A) The mean (± SEM) ALC of all study patients receiving T cells (at days 0, 30, 60, 90, and 120; n = 16, 16, 14, 13, and 11, respectively). (B) ALC of individual patients. Day 0 is designated as the day of HCT.
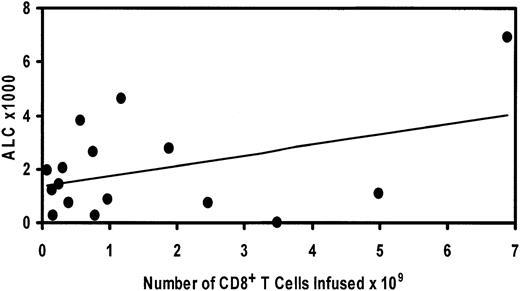
The pace of the robust lymphocyte engraftment compares favorably with patients treated with CD34-selected HCT. In other studies in which CD34+-selected cells have been reinfused in an autologous setting, a delayed lymphocyte repopulation has been observed compared with the patients in this study.11,12,31-33 It is likely that the rapid lymphoid engraftment was derived from the adoptively infused T cells on day 14 after HCT; however, we cannot exclude contribution from residual T cells, progenitors surviving chemotherapy, or lymphocytes matured from CD34+ cells in the graft. There was a positive correlation between the number of CD8+ T cells infused and the ALC on day 30 after HCT that did not reach statistical significance (Figure 4).
Relationship of day 30 ALC to number of CD8+ T cells infused. Regression analysis demonstrates positive correlation that does not reach significance (P = 0.1).
Relationship of day 30 ALC to number of CD8+ T cells infused. Regression analysis demonstrates positive correlation that does not reach significance (P = 0.1).
In vitro T-cell analysis
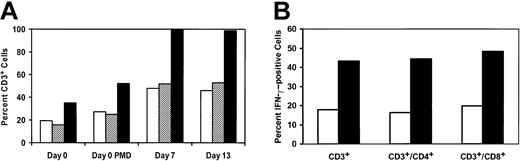
The phenotype of the input culture apheresis product and of the cells following monocyte depletion and after in vitro culture for a representative patient is shown in Figure 5A. In this patient, there was a decrease in CD14+ cells from 48.6% to 22.7% of total cells following the monocyte depletion process. The apheresis product prior to CD3/CD28 stimulation contained 35% CD3+ T cells, and this increased to about 100% following 7 days of in vitro culture. The mean starting percentage of CD3+ T cells of all study patients was 26.9%, which increased to 98.8% after expansion. Contaminating lymphoma cells could be detected at culture initiation in some patients; however, no patients had tumor cells detectable in the final T-cell product. Thus, the culture process was successful at increasing the percentage and absolute numbers of both the CD4+ and CD8+ subpopulations, but preferential expansion of CD4+ T cells occurred in most patients, as there was a greater percentage increase seen in the CD4+ population as compared with the CD8 T cells. Accordingly, with the greater proportion increase in CD4+ T cells, the CD4/CD8 ratios increased from a baseline of 0.9 (range, 0.4-3.6) prior to expansion to 1.80 (range, 0.26-7.7) afterward (Table 2). The mean fold T-cell expansion was 9.0 (range, 0.42-21.5). Together, these data indicate that the growth of NHL patient T cells in vitro was impaired compared with healthy donors23 and, surprisingly, even when compared with HIV-infected donors.21,24 The magnitude of the T-cell proliferative impairment was considerable, as healthy donors undergo a more than 1000-fold expansion of T cells when subjected to the same culture conditions.23 This is most likely a consequence of the multiple prior cytotoxic regimens these patients have undergone and/or the underlying immunosuppressive effects of NHL on T cells.
Effect of CD3/CD28 culture on phenotype and IFN-γ induction in patient T cells. (A) In vitro phenotype of lymphocyte culture: patient mononuclear cells obtained from steady-state pheresis (day 0), after partial depletion of monocytes via adherence to magnetic beads (PMD), and after 7 or 13 days of culture via CD3/CD28 stimulation. Cells were analyzed by flow cytometry for CD3+CD4+ cells (open bars), CD3+CD8+ (hatched bars), and total CD3+ cells (solid bars) as described in “Patients, materials, and methods”. Shown is a representative patient, UPN26. (B) Effect of culture process on IFN-γ expression. Study patient T cells obtained at baseline (open bars) or after 13 days of culture with CD3/CD28 beads (solid bars) were examined by flow cytometry for intracellular cytokine staining. Cells were collected from culture and analyzed as described in “Patients, materials, and methods.” The percent of CD3+, CD3+CD4+, and CD3+CD8+ cells staining for IFN-γ is shown for a representative patient, UPN26.
Effect of CD3/CD28 culture on phenotype and IFN-γ induction in patient T cells. (A) In vitro phenotype of lymphocyte culture: patient mononuclear cells obtained from steady-state pheresis (day 0), after partial depletion of monocytes via adherence to magnetic beads (PMD), and after 7 or 13 days of culture via CD3/CD28 stimulation. Cells were analyzed by flow cytometry for CD3+CD4+ cells (open bars), CD3+CD8+ (hatched bars), and total CD3+ cells (solid bars) as described in “Patients, materials, and methods”. Shown is a representative patient, UPN26. (B) Effect of culture process on IFN-γ expression. Study patient T cells obtained at baseline (open bars) or after 13 days of culture with CD3/CD28 beads (solid bars) were examined by flow cytometry for intracellular cytokine staining. Cells were collected from culture and analyzed as described in “Patients, materials, and methods.” The percent of CD3+, CD3+CD4+, and CD3+CD8+ cells staining for IFN-γ is shown for a representative patient, UPN26.
Previously, we have observed enhanced T-cell secretion of cytokines and chemokines following CD3/CD28 stimulation in immunosuppressed patients with HIV-infected donors.21 To characterize the responsiveness of the expanded T cells compared with T cells obtained directly from NHL study patients at “steady state,” induced intracellular cytokine staining was performed via flow cytometry. Figure 5B illustrates the mean percentage increase of T cells staining for IFN-γ before and after phorbol 12-myristate 13-acetate (PMA) plus calcium ionophore (ionomycin) activation. At baseline, the patient T cells had a significant impairment of IFN-γ induction, as our control studies show that about 70% to 80% of healthy donor T cells have detectable IFN-γ under same conditions.21 The culture process resulted in a significant improvement, as the costimulated T cells had significantly enhanced secretion of IFN-γ at the time of harvest (Figure 5B). This improvement in the originally impaired IFN-γ production was noted in both CD3+CD4+ and CD3+CD8+ T cells.
In vivo immune assessment
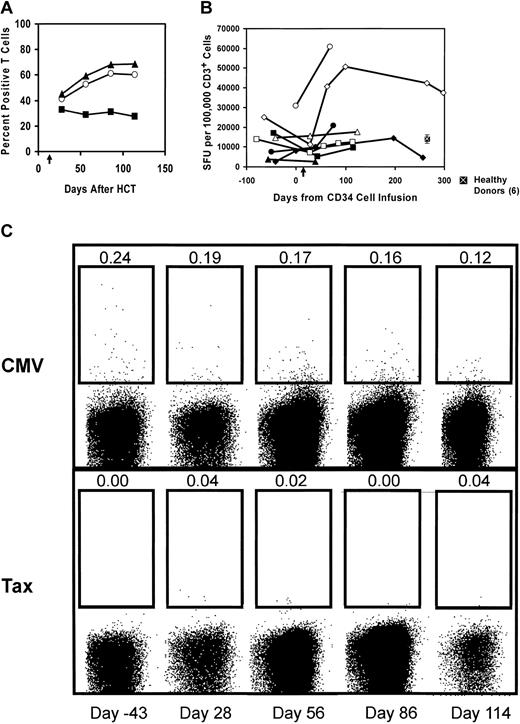
As was noted in “Lymphocyte recovery,” there was a rapid recovery of lymphocyte numbers after HCT and T-cell infusion. Similar to previous studies of immune reconstitution after autologous HCT,11,12,31-34 we found that the T cells were predominantly composed of CD8+ T cells. To assess the recovery of T-cell function after HCT, we obtained peripheral blood after CD3/CD28 costimulated T-cell infusions and examined the IFN-γ production by flow cytometry and ELISPOT analysis following a 6-hour stimulation with phorbol ester and calcium ionophore. The flow cytokine results from a representative patient are shown in Figure 6A. The percent of CD3+ cells producing IFN-γ increased progressively after HCT and reached a plateau at about 60% of the CD3+ T cells. In this patient, all of the increase in the fraction of T cells producing IFN-γ was confined to the CD8+ T-cell subset. In contrast, recovery of IFN-γ production in the CD4+ T-cell subset was not observed.
Kinetics of T-cell functional recovery after HCT and adoptive T-cell infusion. (A) In vivo peripheral blood T cells regain inducible IFN-γ expression. PBMCs obtained following HCT and adoptive T-cell transfer were examined for intracellular IFN-γ expression by flow cytometry following stimulation by PMA and ionomycin as described in “Patients, materials, and methods.” The mean percent of cytokine-positive cells is shown (CD3+ T cells, ○; CD8+ T cells, ▴; and CD4+ T cells, ▪). Results shown are representative of 2 patients (UPN10 and UPN11) examined. (B) Recovery of T-cell IFN-γ secretion after HCT and T-cell infusion: NHL study patient mononuclear cells before and after HCT were stimulated with the phorbor ester and calcium ionophore as described in “Patients, materials, and methods.” Six healthy donors were also tested with the same stimuli, and the mean ± SEM is indicated. Plotted is the average of triplicate wells in spot-forming units per 1 × 105 CD3+ cells. UPN14, ♦; UPN15, ▪; UPN20, ▴; UPN23, □; UPN24, •; UPN25, ⋄; UPN26, ▵; UPN27, ○. Arrow along x-axis denotes day of T-cell infusion. (C) Frequency of CMV-specific CD8+ T cells in vivo via major histocompatibility complex (MHC) class I tetramer analysis. Tetramer analysis using the CMV pp65 HLA-A2–positive tetramer was controlled with the tetramer specific for the immunodominant HTLV-1 tax peptide. Time points shown are days from hematopoietic stem cell transplantation, which was 13 days prior to activated T-cell infusion. The percentage of CD8+ tetramer–positive T cells is shown above the population gated as positive for an HLA-A2–positive subject (UPN23).
Kinetics of T-cell functional recovery after HCT and adoptive T-cell infusion. (A) In vivo peripheral blood T cells regain inducible IFN-γ expression. PBMCs obtained following HCT and adoptive T-cell transfer were examined for intracellular IFN-γ expression by flow cytometry following stimulation by PMA and ionomycin as described in “Patients, materials, and methods.” The mean percent of cytokine-positive cells is shown (CD3+ T cells, ○; CD8+ T cells, ▴; and CD4+ T cells, ▪). Results shown are representative of 2 patients (UPN10 and UPN11) examined. (B) Recovery of T-cell IFN-γ secretion after HCT and T-cell infusion: NHL study patient mononuclear cells before and after HCT were stimulated with the phorbor ester and calcium ionophore as described in “Patients, materials, and methods.” Six healthy donors were also tested with the same stimuli, and the mean ± SEM is indicated. Plotted is the average of triplicate wells in spot-forming units per 1 × 105 CD3+ cells. UPN14, ♦; UPN15, ▪; UPN20, ▴; UPN23, □; UPN24, •; UPN25, ⋄; UPN26, ▵; UPN27, ○. Arrow along x-axis denotes day of T-cell infusion. (C) Frequency of CMV-specific CD8+ T cells in vivo via major histocompatibility complex (MHC) class I tetramer analysis. Tetramer analysis using the CMV pp65 HLA-A2–positive tetramer was controlled with the tetramer specific for the immunodominant HTLV-1 tax peptide. Time points shown are days from hematopoietic stem cell transplantation, which was 13 days prior to activated T-cell infusion. The percentage of CD8+ tetramer–positive T cells is shown above the population gated as positive for an HLA-A2–positive subject (UPN23).
We sought further evidence of immune reconstitution through ELISPOT analysis. Sufficient samples were available to assess the response of 8 patients to pharmacologic stimulation with phorbol ester and calcium ionophore and lectin stimulation with PHA. This assay is more sensitive than intracellular cytokine staining by flow cytometry and can be performed using fewer cells, an advantage in patients where the cells available were limiting. The number of IFN-γ–secreting CD3+ cells in PBMCs after pharmacologic stimulation is shown in Figure 6B. Surprisingly, nearly all patients reconstituted responses to pharmacologic stimulus to levels equivalent or, for UPN25 and UPN27, far above the mean for 6 healthy donors. In contrast, the ELISPOT IFN-γ response of these same patients to stimulation by the lectin PHA and IL-2 was lower than healthy donors, although there was a demonstrable response for 5 of the 8 patients assayed (data not shown).
The recovery of antiviral immunity in 2 subjects was assessed by analysis of samples before transplantation and serially following peripheral blood stem cell transplantation (PBSCT) and T-cell infusion. In the IFN-γ ELISPOT analysis, we found that UPN25 had a rapid and robust recovery of CMV-specific T cells (Table 3). He was borderline positive for EBV at baseline and had an increase above his baseline in the post–T-cell infusion period. The second subject, UPN23, was EBV-positive at baseline and had a rapid recovery of EBV specific T cells following the T-cell infusion. One of the subjects (UPN23) expressed the HLA-A201 allele, so we were able to measure the recovery of CMV-specific CD8 T cells in that patient by flow tetramer staining (Figure 6C). Following the T-cell infusion, a rapid restoration of CMV pp65-specific T cells was observed, to near pretransplantation levels. Finally, the allogeneic response was examined by ELISPOT analysis in 8 patients as described in “Patients, materials, and methods.” Only 1 patient reconstituted to the same level as the healthy donors following HCT and CD3/CD28 costimulated T-cell infusions (data not shown).
Clinical responses
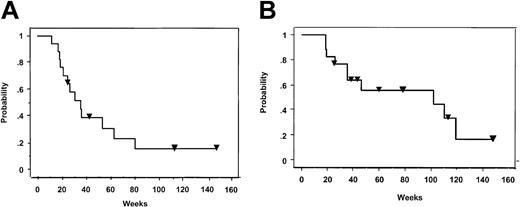
Five patients achieved a complete remission, and 5 patients experienced a partial remission for an overall response rate of 63%. The median progression-free survival and overall survival is 7.2 months and 26.1 months, respectively (Figure 7). With a median follow-up of 33 months (range, 26-60 months), the longest complete remission to date has been 61 months and 5 patients continue to show stable disease. Ten patients died from progressive disease, and one patient died from pulmonary complications due to regimen-related toxicity.
Kaplan-Meier plots of survival. Plots of progression-free survival (A) and overall survival (B) for NHL study patients (n = 16) following HCT and T-cell infusions. Inverted triangles denote patients who are in complete remission (A) or still alive (B).
Kaplan-Meier plots of survival. Plots of progression-free survival (A) and overall survival (B) for NHL study patients (n = 16) following HCT and T-cell infusions. Inverted triangles denote patients who are in complete remission (A) or still alive (B).
Discussion
We have demonstrated the safety and feasibility of infusing CD3/CD28 costimulated T cells in patients with advanced B-cell NHL. Previous reports of T-cell infusions in heavily pretreated patients have been allogeneic donor lymphocyte infusions, where healthy donor lymphocytes are available. Patients with advanced hematologic malignancies have severely depleted T-cell pools, and the remaining T cells are highly prone to apoptosis.31,34,35 Together, these factors have created technical barriers that prevented the routine generation of large numbers of activated autologous T cells from heavily pretreated patients. We used artificial antigen-presenting cells composed of beads coated with anti-CD3/anti-CD28 to facilitate the large-scale expansion and adoptive transfer of costimulated T cells.23,24 The increased efficiency of this culture system has enabled the production of autologous CD4 T cells from HIV-infected patients,21,23 a process that was not possible with previous culture systems.
There is a strong rationale for developing approaches to accelerate T-cell reconstitution after chemoradiotherapy or HCT. First, patients have a pronounced susceptibility to opportunistic infections.10,36 Second, it would be desirable to vaccinate patients following cytotoxic therapy, as there is increasing evidence that patients with earlier stage follicular lymphomas may benefit from therapeutic vaccination.37,38 However, such attempts at vaccination are unlikely to be successful in patients with severe immunodeficiency such as those enrolled in the present study. Third, recent studies show that early lymphocyte recovery after HCT is associated with improved survival.13,15,16
With regard to toxicity attributed to the costimulated T cells, the infusion toxicities in the current study were transient and reversible, and a dose-limiting toxicity was not reached. These observed toxicities appeared to be dose dependent, as the events occurred at the third and highest tested dose level. The fevers and rigors experienced by both patients may possibly be attributed to cytokines and chemokines produced by the activated T cells. One patient succumbed from diffuse alveolar damage, which is a known complication of high-dose chemotherapy, and this was judged to be regimen-related mortality. Patient accrual was terminated at the third dose level due to manufacturing feasibility issues. The feasibility of removing T regulatory cells to improve T-cell expansion and function is being explored in a subsequent phase 1 clinical trial.
CD34-selected HCT is highly immunosuppressive and is associated with prolonged T-cell deficiency.11 A recent review of infections after CD34-selected autologous PBSCT at the Fred Hutchinson Cancer Center concluded that the risk of infections approaches that seen in allogeneic transplantation recipients and that infection surveillance and prevention strategies similar to those used in allogeneic recipients are warranted.39 Our patients had prompt recovery of normal lymphocyte counts and encouraging restoration of immune function following the T-cell infusion. The kinetics of the ALC recovery of our patients (Figure 3) was more rapid than that reported for CD34+-selected autologous HCT (Table 4). Moreover, reconstitution of the peripheral blood T cells equaled or exceeded that of control patients previously reported in the literature who had received T-cell replete, unmanipulated grafts.36,42,43 Further clinical study will be required to determine whether this restoration of lymphocyte counts confers clinical benefit. However, the functional analysis of the T-cell reconstitution showed that a functional and numerical deficit of CD4 cells persisted in the patients for at least 100 days following HCT. This immunodeficiency occurred despite significant numbers of activated CD4 cells that were infused. The delayed reconstitution of CD4 T cells compared with CD8 cells following HCT and T-cell infusion did not appear to be clinically significant, as there were no opportunistic infections in these patients during the year following HCT.
We found restoration of IFN-γ secretion following intracellular stimulation with PMA and ionomycin but incomplete restoration of PHA responsiveness. This may be related to the relative strength of stimulation, as Clerici and coworkers have found a hierarchy of lost responses to APC–antigen-specific T-cell responses first, followed by allogeneic responses and, with late-stage disease, loss of PHA responses in progressive HIV infection and Hodgkin disease.44,45 An alternative explanation for the failure of PHA to activate cells while retaining activation with PMA/ionomycin is a block to upstream signal transduction. For example, nuclear factor–κB (NF-κB) is not activated in response to CD3/CD28 stimulation in either the ZAP-70– or SLP-76–negative Jurkat T cells, whereas PMA/ionomycin activates NF-κB normally.46
Most interestingly, we noted a syndrome of delayed lymphocytosis in a subset of patients. In some patients, this was associated with tumor regression. Recently, Dudley and coworkers at the National Cancer Institute (NCI) noted lymphocytosis and autoimmunity associated with tumor regression in patients with melanoma given autologous infusions of T cells following an immunosuppressive regimen of fludarabine and cyclophosphamide.47 The NCI group gave high-dose IL-2 to the patients following the T-cell transfers. Our results are the first demonstration of lymphocytosis occurring following adoptive immunotherapy in the absence of exogenous cytokines.
The mechanism that results in the lymphocytosis that we observed requires further study. It is not likely to be due simply to homeostatic T-cell proliferation, as this has not been reported in animal models.48,49 Recent data indicate that homeostatic T-cell proliferation can amplify antitumor immune responses in animal models,50 so that the infusion of activated T cells in the HCT setting may take advantage of this phenomenon. While it is likely that homeostatic proliferation contributes to the rapid lymphocyte reconstitution that we observed, it is possible that antigen-induced proliferation contributed. The antigens could be derived from environmental or infectious sources or from endogenous self-antigens. In the later case, this could result in the induction of antitumor responses as well as autoimmune syndromes.47 We did not observe any definite occurrences of autoimmunity; however, a single case of spontaneously resolving encephalitis occurred 86 days after the T-cell infusion. Biopsy of the choroid plexus revealed nonclonal atypical lymphoid cells. The symptoms resolved with supportive care.
T cells collected in the steady state were used as a source of input cells for T-cell culture in our study. In a pilot study, Curti et al used T cells for adoptive transfer that had been collected at steady state, or before or after cyclophosphamide treatment,20 and increased antitumor efficacy was suggested when cells were collected just as patients entered the cyclophosphamide-induced nadir. The mechanisms responsible for this intriguing finding remain unclear, but it is possible that cyclophosphamide selectively depletes regulatory T cells that inhibit antitumor responses.51 Future studies will be required to determine the optimal source of input T cells for adoptive transfer.
In conclusion, our protocol resulted in an encouraging but incomplete restoration of T-cell numbers and functions. We noted a severe immunodeficiency at baseline in the patients enrolled on this study, as only about 15% of patient T cells were capable of producing detectable interferon-γ at baseline. In contrast, we and others find that most T cells from healthy donors secrete interferon-γ. Together, these observations have profound implications for the ability to successfully vaccinate heavily pretreated patients with advanced hematologic malignancies. It is likely that adoptive immunotherapy combined with therapeutic vaccination strategies will be required to induce effective antitumor immunity in patients with advanced hematologic malignancies.
Prepublished online as Blood First Edition Paper, May 22, 2003; DOI 10.1182/blood-2003-01-0095.
Supported by grant 6240 and by a Specialized Center of Research grant from the Leukemia and Lymphoma Society and by the Neil Ruzic Research Foundation.
Two of the authors (B.L.L., C.H.J.) have declared a financial interest in a company whose potential product was studied in the present work.
G.G.L. and B.L.L. contributed equally to this paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Stephanie Williams, Dr Todd Zimmerman, Karen Welborne, Peggy Bennett, Mary O'Connell, Yu Hyon Kim, Dr Sicco Popma, Dr Josephine Cox, and Dr Dennis Van Epps for clinical, technical, and intellectual assistance and thank Dr Aaron Rapoport for cell lines. We especially thank Dr Barbara Vance of the tetramer facility at the Abramson Cancer Center for providing CMV and Tax tetramers.