TNF/LTα/LTβ (tumor necrosis factor/lymphotoxin-α/lymphotoxin-β) triple knockout (KO) mice show a significant reduction of dendritic cell (DC) number in the spleen, presumably due to defective recruitment and/or production. To distinguish between these possibilities, DCs were generated from bone marrow (BM) cultures prepared from wild-type (wt) and mutant mice in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4). The yield of CD11c+major histocompatibility complex (MHC) class II+DCs generated from TNF/LTα/LTβ−/− BM culture was significantly reduced compared with wt BM culture. In order to further dissect the individual pathways responsible for defective DC properties observed in TNF/LTα/LTβ−/− mice, the panel of TNF/LT ligand and receptor single KO mice were used. The production of DCs from BM culture was significantly reduced in TNF−/− and TNF receptor (TNFR) p55−/− mice, but normal in LTα−/−, LTβ−/−, LTβR−/−mice. Recombinant TNF (rTNF) exogenously added to TNF/LTα/LTβ−/− BM cultures could reverse this defect, and blocking antibodies showed partial effect on BM cultures of wt mice. Conversely, numbers of mature DCs in spleen were significantly decreased in LTα−/−, LTβ−/−, LTβR−/− mice, but not in TNF−/− and TNFRp55−/− mice. These results reveal 2 distinct contributions of TNF/LT cytokines. First, TNF acting through TNF receptor is involved in the development/maturation of DCs in BM progenitor cultures, but this function appears to be redundant in vivo. Second, the microenvironment in peripheral lymphoid organs associated with LTα/LTβ-LTβR signaling and chemokine production is critical for recruitment efficiency of DCs, and this pathway is indispensable.
Introduction
Dendritic cells (DCs) are known as the most potent antigen- presenting cells (APCs) capable of activating even naive T cells and playing a major role in initiating various T-cell–mediated immune responses.1-3 DCs, which differentiate from bone marrow (BM) progenitors, are positioned in the body to capture antigen, and upon maturation and activation move to T-cell areas of lymphoid organs, such as spleen and lymph node (LN), where they initiate immune response. Many studies have elucidated the role of cytokines required for the differentiation or maturation of DCs in vitro.4-7Although granulocyte-macrophage colony-stimulating factor (GM-CSF) is known to be essential for DC generation from BM progenitor cells,8-10 the mediators responsible for subsequent differentiation and maturation of DCs are not fully understood. As for recruitment to the lymphoid tissues, recent studies proved that chemokines expressed in the lymphoid organs, including secondary lymphoid tissue chemokine (SLC, CCL21) and Epstein Barr virus–induced molecule 1 ligand chemokine (ELC, CCL19), play an important role in mobilization of mature DCs to the spleen and LN.11-15 When peripheral DCs become activated, they down-regulate inflammatory chemokine receptors and up-regulate CCR7, the receptor for SLC and ELC, allowing the traffic of DCs from peripheral tissue into the lymphoid organs.16-18
Tumor necrosis factor (TNF) and lymphotoxins (LTs) are profoundly involved in the organogenesis of secondary lymphoid tissues. LTα−/− and LTβ−/− mice have severe defects in the development of peripheral LN, Peyer patches (PP), and disturbed organization of splenic architecture,19-23whereas TNF−/− and TNFRp55−/− mice show lesser defects in PP formation24-26 and splenic microarchitecture.27 TNF, LTα, and LTβ, are to various degrees required for the expression of SLC, ELC, and B-lymphocyte chemoattractant (BLC) in the spleen and LN.11,28-30 All 3 strains of knockout (KO) mice exhibit defective formation of B-cell follicles, germinal centers, and follicular dendritic cell networks and defective antibody responses to T-cell–dependent antigens.20-22 27
The present study was prompted by the observation of low absolute and relative numbers of DCs in the spleen of the triple TNF/LTα/LTβ−/− mice (these mice completely lack other peripheral lymphoid organs31). Based on published data, we initially hypothesized that both production and recruitment of DCs may be defective in these mice, and we therefore investigated the role of cytokines of the TNF/LT subfamily in DC generation and recruitment. Indeed, we found that mice with combined TNF/LT deficiency in addition to defective recruitment of DCs to peripheral lymphoid organs show lower maturation/production of DCs from BM cells in vitro. By using a panel of mutant mice with single deficiencies in 3 TNF/LT ligands and their receptors, TNFRp55 and LTβR, we elucidated the distinct contributions of specific signaling pathways.
Materials and methods
Mice
LTα−/−, LTβ−/−, LTβR−/−, TNF−/−, and TNFRp55−/− have been described previously.22,23,27,32,33 The generation and phenotypic analysis of mice with a combined TNF/LTα/LTβ deficiency is described in detail elsewhere.31 The mice were backcrossed to the C57BL/6 background, bred in specific pathogen-free conditions, and used for experiments between 6 and 12 weeks of age. Animal care was provided in accordance with the procedures outlined in the “Guide for the Care and Use of Laboratory Animals” (NIH Publication No. 86-23, 1985).
Cytokines and monoclonal antibodies
The following monoclonal antibodies (mAbs) were purchased from PharMingen (San Diego, CA) and used in flow cytometric analysis and cell isolation; anti-CD45R/B220 mAb, anti-Thy1.2 mAb, fluorescein-isothiocyanate (FITC)–conjugated anti–I-AbmAb, FITC-conjugated anti-CD80 mAb, FITC-conjugated anti-CD86 mAb, and phycoerythrin (PE)–conjugated anti-CD11c mAb. FITC-conjugated anti–c-kit mAb, PE-conjugated anti-CD4 mAb, PE-conjugated anti-CD8a mAb, PE-conjugated anti-B220 mAb, PE-conjugated anti-NK1.1 mAb, PE-conjugated anti–Gr-1 mAb, PE-conjugated anti-CD11b mAb, and PE-conjugated anti-CD11c mAb were used in cell sorting, and all of them were kindly provided by Dr O. Shimozato (NCI, Frederick, MD). Recombinant GM-CSF (rGM-CSF), recombinant interleukin (IL)–4 (rIL-4), and rTNF were purchased from Biosource (Camarillo, CA) and from Pepro Tech (Rocky Hill, NJ) under contract by the Biological Resources Branch, Division of Cancer Treatment and Diagnosis, NCI-Frederick.
Preparation of splenocytes
For analysis of splenic DCs, spleens were cut into small fragments, suspended in complete medium containing 2.4 mg/mL collagenase (Worthington Biochemical, Freehold, NJ), and 1 mg/mL DNAase (Sigma, St Louis, MO) and digested with intermittent agitation for 45 minutes at 37°C. After lysis of red blood cells (RBCs) in ammonium chloride potassium (ACK) lysing buffer (Biosource), cells were resuspended in complete medium.
Preparation of dendritic cells from bone marrow cells
DCs were generated from BM cells, as previously described, with some modifications.8 34 Briefly, BM cells were flushed out from the femurs and tibias. After lysis of RBCs, whole BM cells (1 × 106 cells/mL) were cultured on 24-well tissue-culture plates (Corning, Corning, NY) in 1 mL/well of complete medium containing 20 ng/mL of each murine rGM-CSF and murine rIL-4. On days 3 and 5 of the culture, nonadherent cells were removed by pipetting, followed by aspirating of the medium, and then fresh complete medium containing 10 ng/mL each of rGM-CSF and rIL-4 were added. On day 9 of the culture, nonadherent DCs and loosely adherent DCs were harvested by gentle pipetting.
Flow cytometric analysis
Cells were stained with indicated mAbs and analyzed mainly by flow cytometric analysis using a FACScan with CellQuest software (Becton Dickinson, Mountain View, CA). Nonconjugated anti-Fcγ III/II receptor mAb (PharMingen) was used to block the nonspecific binding. Propidium iodide (Sigma) was used to exclude dead cells from the analysis of the cultured DCs.
Mixed lymphocyte reaction (MLR)
DCs generated from either wt or TNF/LTα/LTβ−/−BM cells were irradiated with 137Cs at a dose of 30 Gy. Indicated numbers of DCs were cocultured with responder cells (1 × 106 cells/mL) in a flat-bottomed 96-well microtiter plate (Corning) for 4 days. The responder cells were prepared from mesenteric LNs of either naive C57BL/6 (syngeneic MLR) or BALB/c (allogeneic MLR) mice. During the last 18 hours of the culture, 37 kBq [3H]thymidine (dThd) was added to each well. The cultured cells were then harvested, and the incorporation of [3H]thymidine was counted with a Beta Plate system (Pharmacia LKB Biotechnology, Uppsala, Sweden). All samples were assayed in quadruplicate, and the values were shown as the means ± SD.
Northern analyses
Ten micrograms of total or poly A+ mRNA were separated on 1.5% denaturing agarose gel and transferred to Supported Nitrocellulose-1 (GibcoBRL, Gaithersburg, MD) membrane. Probes for chemokines ELC and SLC were generated by reverse transcription–polymerase chain reaction (RT-PCR) from total splenic cDNA prepared from C57BL6 mice using the following primers: 5′-aggacatctgagcgattcc-3′ and 5′-ccaataaagctgcttggtac-3′ (ELC); 5′-tacagctctggtctcataca-3′, and 5′-ccttgtccttgcacctatg-3′ (SLC). Hybridization with [32P]-labeled probes was performed in ExpressHyb solution (Clontech, Palo Alto, CA) and washed following the protocol provided by the manufacturer. Radioactivity was detected and quantified using Molecular Dynamics plates and ImageQuant software (Molecular Dynamics, Sunnyvale, CA).
Statistical analyses
The statistical significance of all assays was assessed by using the 2-tailed Student t test.
Results
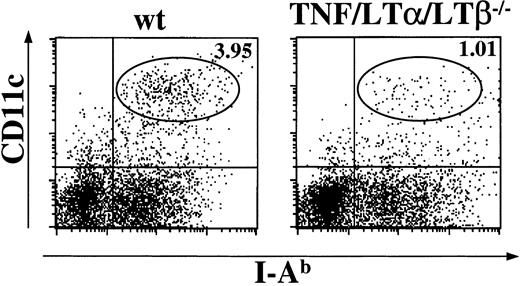
The number of DCs is reduced in the spleen of TNF/LTα/LTβ−/− mice
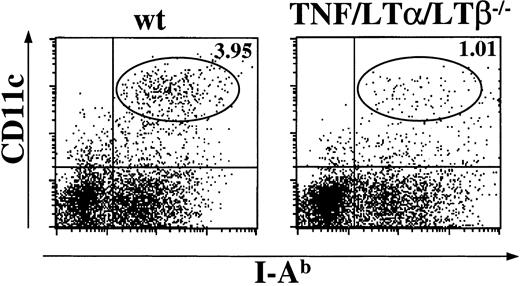
Mice with triple TNF/LTα/LTβ deficiency lack all peripheral lymphoid organs except spleen.31 Fresh splenocytes were prepared from naive wt and TNF/LTα/LTβ−/− mice using collagenase and DNAase. The cells were stained with anti-CD11c mAb, a marker for the DC lineage, and anti–I-Ab mAb, and percentages of CD11c+, I-Ab+ population were determined by flow cytometry. As shown in Figure1, spleen taken from TNF/LTα/LTβ−/− mice contained 3-4 times lower relative numbers of CD11c+, I-Ab+ cells compared with that from wt mice. Because some TNF/LTα/LTβ−/− mice showed splenomegaly and increased white blood cell counts in their spleen, the absolute numbers of CD11c+, I-Ab+ cells were also calculated and were still significantly reduced in TNF/LTα/LTβ−/−(2.28 ± 0.94 × 106 cells/spleen) mice compared with wt mice (4.70 ± 1.21 × 106 cells/spleen).
The number of DCs is reduced in the spleen of TNF/LTα/LTβ−/− mice.
Spleens were taken from naive wt or TNF/LTα/LTβ−/−mice and homogenized after collagenase and DNAase treatment. Cells were stained with FITC-conjugated anti–I-Ab mAb and PE-conjugated anti-CD11c mAb. The percent of events in oval area of each dot plot is indicated. Data from 1 of 4 representative experiments are shown.
The number of DCs is reduced in the spleen of TNF/LTα/LTβ−/− mice.
Spleens were taken from naive wt or TNF/LTα/LTβ−/−mice and homogenized after collagenase and DNAase treatment. Cells were stained with FITC-conjugated anti–I-Ab mAb and PE-conjugated anti-CD11c mAb. The percent of events in oval area of each dot plot is indicated. Data from 1 of 4 representative experiments are shown.
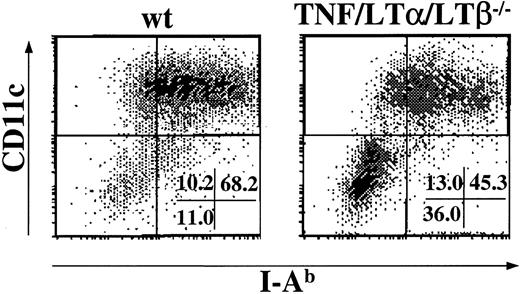
Generation of DCs from BM progenitor cells is impaired in TNF/LTα/LTβ−/− mice
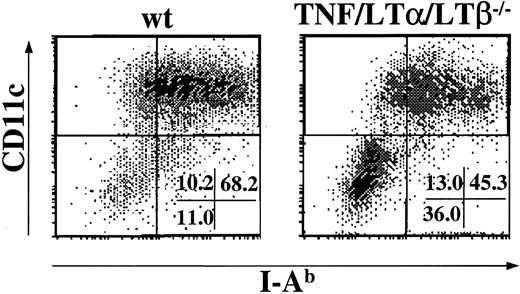
To verify whether the observed deficiency was due to reduced production or maturation of DCs from BM precursors in TNF/LTα/LTβ−/− mice, we generated DCs in vitro from BM cells of naive wt and TNF/LTα/LTβ−/− mice in the presence of GM-CSF and IL-4 as described in “Materials and methods.” After 9 days in culture, the expression of CD11c and of I-Ab was determined by flow cytometry. As shown in Figure2, nearly 70% of cells generated from wt BM culture were CD11c+, I-Ab+ cells (left panel), whereas the percentage of CD11c+, I-Ab+cells was significantly decreased when TNF/LTα/LTβ−/−BM cells were cultured in the same way (Figure 2, right panel). Similar results were obtained when FACS-sorted Lin−, c-kit+ progenitors from either wt or TNF/LTα/LTβ−/− BM cells were cultured in the presence of GM-CSF and IL-4 (data not shown). Many cells generated from wt BM cells under our culture conditions were relatively large and irregularly shaped with various lengths of dendrites that expressed relatively high but various levels of CD80 and CD86 (data not shown), suggesting that they represented DC populations ranging from immature to mature. In contrast, the cells generated from TNF/LTα/LTβ−/− BM culture were more heterogeneous in appearance and contained an increased number of firmly adherent cells. Flow cytometric analysis showed CD11c−/−, I-Ab− cells generated in this culture did not express major costimulatory molecules (data not shown).
The generation of DCs from BM cells is impaired in TNF/LTα/LTβ−/− mice.
BM cells (1 × 106 cells/mL) taken from either wt or TNF/LTα/LTβ−/− mice were cultured with GM-CSF and IL-4 for 9 days as described in “Materials and methods.” Cells were collected on day 9 and stained with FITC-conjugated anti–I-Ab mAb and PE-conjugated anti-CD11c mAb. The percent of events in each quadrant of dot plots is indicated. Dots were plotted based on staining with isotype-matched negative control mAb. Data from 1 of 5 representative experiments are shown.
The generation of DCs from BM cells is impaired in TNF/LTα/LTβ−/− mice.
BM cells (1 × 106 cells/mL) taken from either wt or TNF/LTα/LTβ−/− mice were cultured with GM-CSF and IL-4 for 9 days as described in “Materials and methods.” Cells were collected on day 9 and stained with FITC-conjugated anti–I-Ab mAb and PE-conjugated anti-CD11c mAb. The percent of events in each quadrant of dot plots is indicated. Dots were plotted based on staining with isotype-matched negative control mAb. Data from 1 of 5 representative experiments are shown.
T-cell stimulatory activity is attenuated in the cells generated from TNF/LTα/LTβ−/− BM culture
In order to further characterize functional activity of the cells generated in vitro from TNF/LTα/LTβ−/− mice, we evaluated the T-cell stimulatory activity by MLR. The cells generated from wt BM culture showed some and marked T-cell stimulatory activity in syngeneic (Figure 3A) and allogeneic MLR (Figure 3B), respectively, suggesting that these cells were efficient APCs. On the other hand, the stimulatory activity in both syngeneic and allogeneic MLR of the cells generated from TNF/LTα/LTβ−/− BM culture were severely reduced compared with those from wt BM culture (Figure 3). As the total numbers of cells generated from both wt and TNF/LTα/LTβ−/−cultures were similar (Table 1), it is likely that the impaired stimulatory activity of the cells generated from TNF/LTα/LTβ−/− BM culture was due to the reduced proportion and numbers of mature DCs.
The stimulatory activity of cells generated from TNF/LTα/LTβ−/− BM culture is significantly reduced compared with that from wt BM culture.
BM cells (1 × 106 cells/mL) from either wt (●) or TNF/LTα/LTβ−/− (○) mice were cultured with GM-CSF and IL-4 for 9 days. Cells were collected on day 9 and subjected to 30 Gy irradiation. Indicated number of generated cells were cocultured with syngeneic (A) or allogeneic (B) LN cells (1 × 106 cells/mL) for 4 days. During the last 18 hours of the culture, [3H]dThd was added in each well. The values are the means ± SD of quadruplicate wells. Data from 1 of 3 representative experiments are shown. *P < .01 compared with TNF/LTα/LTβ−/− BM culture. CPM indicates counts per minute.
The stimulatory activity of cells generated from TNF/LTα/LTβ−/− BM culture is significantly reduced compared with that from wt BM culture.
BM cells (1 × 106 cells/mL) from either wt (●) or TNF/LTα/LTβ−/− (○) mice were cultured with GM-CSF and IL-4 for 9 days. Cells were collected on day 9 and subjected to 30 Gy irradiation. Indicated number of generated cells were cocultured with syngeneic (A) or allogeneic (B) LN cells (1 × 106 cells/mL) for 4 days. During the last 18 hours of the culture, [3H]dThd was added in each well. The values are the means ± SD of quadruplicate wells. Data from 1 of 3 representative experiments are shown. *P < .01 compared with TNF/LTα/LTβ−/− BM culture. CPM indicates counts per minute.
DCs from wt and TNF/LTα/LTβ−/− BM culture show comparable stimulatory activity in MLR
To determine whether mature DC subset (defined as CD11c+ and I-Ab+) generated from TNF/LTα/LTβ−/− BM culture possessed the same potency in MLR as DCs from the wt BM culture, we sorted CD11c+, I-Ab+, and CD11c−, I-Ab− cells from TNF/LTα/LTβ−/− BM culture and evaluated the stimulatory activity of these cell subsets. As shown in Figure4, CD11c+, I-Ab low-high cells generated from both wt and TNF/LTα/LTβ−/− BM culture exhibited comparable stimulatory activity in syngeneic (A) and allogeneic (B) MLR. On the other hand, no significant activity was observed when the CD11c−, I-Ab− subset was used as stimulator cells. These results confirm that CD11c+, I-Ab+cells generated from TNF/LTα/LTβ−/− BM culture have no intrinsic functional defect in their role as APCs and are as potent as DCs generated from wt BM culture. The CD11c−, I-Ab− subset generated from TNF/LTα/LTβ−/− BM culture appeared to be functionally distinct from DCs based upon their inability to function as APCs in the MLR.
CD11c+, I-Ab low-high cells generated from TNF/LTα/LTβ−/− BM culture possess stimulatory activity in MLR similar to DCs from wt BM culture.
BM cells (1 × 106 cells/mL) from either wt (●) or TNF/LTα/LTβ−/− (○, ▵, ■) mice were cultured with GM-CSF and IL-4 for 9 days. CD11c+, I-Ab low-high (▵), and CD11c−, I-Ab−(■) cells were sorted from TNF/LTα/LTβ−/− BM culture. After 30 Gy irradiation, indicated number of generated cells were cocultured with syngeneic (A) or allogeneic (B) LN cells (1 × 106 cells/mL) for 4 days. During the last 18 hours of the culture, [3H]dThd was added in each well. The values are the means ± SD of quadruplicate wells. Data from 1 of 2 representative experiments are shown. *P < .01 compared with TNF/LTα/LTβ−/− whole and TNF/LTα/LTβ−/− CD11c− population.
CD11c+, I-Ab low-high cells generated from TNF/LTα/LTβ−/− BM culture possess stimulatory activity in MLR similar to DCs from wt BM culture.
BM cells (1 × 106 cells/mL) from either wt (●) or TNF/LTα/LTβ−/− (○, ▵, ■) mice were cultured with GM-CSF and IL-4 for 9 days. CD11c+, I-Ab low-high (▵), and CD11c−, I-Ab−(■) cells were sorted from TNF/LTα/LTβ−/− BM culture. After 30 Gy irradiation, indicated number of generated cells were cocultured with syngeneic (A) or allogeneic (B) LN cells (1 × 106 cells/mL) for 4 days. During the last 18 hours of the culture, [3H]dThd was added in each well. The values are the means ± SD of quadruplicate wells. Data from 1 of 2 representative experiments are shown. *P < .01 compared with TNF/LTα/LTβ−/− whole and TNF/LTα/LTβ−/− CD11c− population.
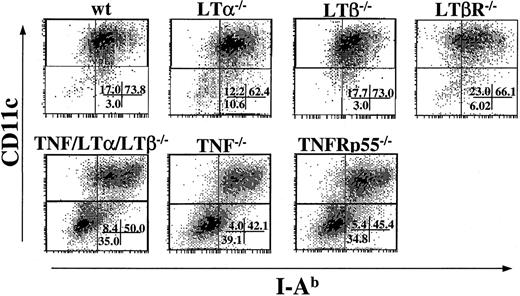
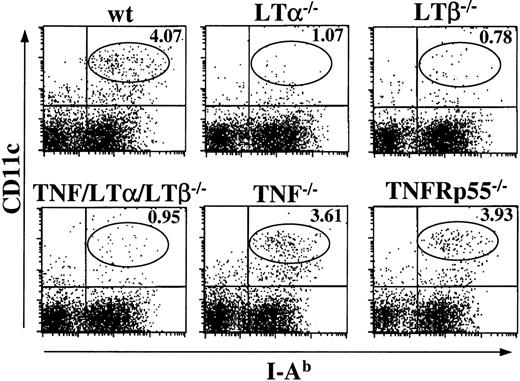
Generation of DCs from BM progenitor cells is impaired in TNF−/− and TNFRp55−/− mice, but not in LTα−/−, LTβ−/−, and LTβR−/− mice
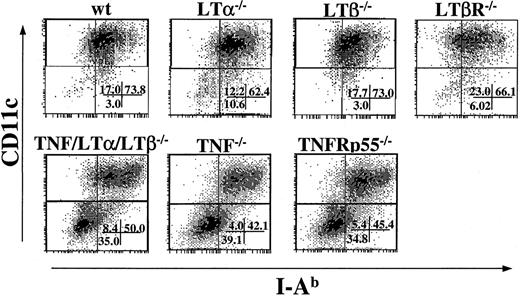
Because TNF/LTα/LTβ−/− mice have deficiencies in several signaling pathways, including TNF-TNFR, LTα-TNFR, and LTβ-LTβR, we next addressed which pathway would be primarily responsible for the generation of mature DCs in vitro. BM cells from several strains of KO mice were cultured in the presence of IL-4 and GM-CSF, and the percentage of CD11c+, I-Ab+cells were determined after 9 days. As shown in Figure5, the percentage of loosely adherent CD11c+, I-Ab+ cells generated from LTα−/−, LTβ−/−, and LTβR−/− BM cultures were almost comparable to that from wt BM culture. This cell population was reduced in TNF−/−and TNFRp55−/− BM cultures, similarly to the yield from TNF/LTα/LTβ−/− BM culture. These results indicate that the TNF-TNFRp55 pathway is critical in the development or maturation of CD11c+, I-Ab low-high DCs from BM progenitor cells in the presence of GM-CSF and IL-4, while the contributions from LTα-TNFR and LTβ-LTβR pathways are not critical or indispensable.
DC generation from BM progenitor cells is impaired in TNF−/− and TNFRp55−/− mice but not in LTα−/−, LTβ−/−, or LTβR−/− mice.
BM cells (1 × 106 cells/mL) from several strains of KO mice were cultured with GM-CSF and IL-4 for 9 days. Cells were collected on day 9 and stained with FITC-conjugated anti–I-Ab mAb and PE-conjugated anti-CD11c mAb. The percent of events in each quadrant of dot plots is indicated. Data from 1 of 5 representative experiments are shown.
DC generation from BM progenitor cells is impaired in TNF−/− and TNFRp55−/− mice but not in LTα−/−, LTβ−/−, or LTβR−/− mice.
BM cells (1 × 106 cells/mL) from several strains of KO mice were cultured with GM-CSF and IL-4 for 9 days. Cells were collected on day 9 and stained with FITC-conjugated anti–I-Ab mAb and PE-conjugated anti-CD11c mAb. The percent of events in each quadrant of dot plots is indicated. Data from 1 of 5 representative experiments are shown.
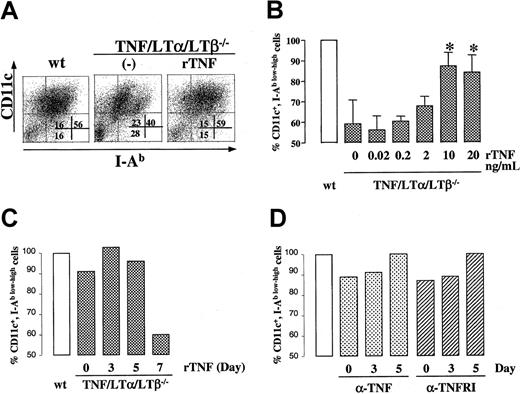
Exogenously added rTNF restores the generation of DCs in TNF/LTα/LTβ−/− BM cultures
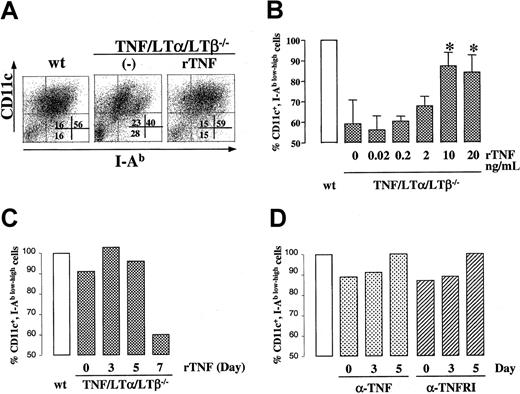
To further address the role of TNF in generating DCs from BM progenitor cells, we added rTNF at various concentrations and at various time points to TNF/LTα/LTβ−/− BM cultures and evaluated the phenotype of generated cells by flow cytometry. As shown in Figure 6A-B), rTNF could restore the percentage of CD11c+ I-Ab low and CD11c+ I-Ab high DC subsets to the level of wt BM culture when added at 10 ng/mL or higher concentrations. Since the number of cells generated at each culture condition was similar at this range of rTNF concentrations, it appears that, under our culture conditions, TNF drives BM progenitor cells to differentiate into mature DCs rather than to push them into the cell death pathway due to proapoptotic signaling via TNFRp55. We also noted that exogenous TNF produced appreciable shift from CD11c+ I-Ab lowcells to CD11c+ I-Ab high (Figure 6A, right panel), consistent with known property of TNF to up-regulate MHC class II expression levels. Additionally, kinetic analysis indicated that the period between days 3 and 5 of culture was critical for the effect of exogenously added TNF in this system, as TNF added on day 7 of culture was unable to restore the generation of CD11c+ I-Ab low-high cells to the wt level (Figure 6C). rTNF added at day 3 and withdrawn on day 5 was sufficient to restore the deficiency (data not shown).
DC population in BM culture is modulated by TNF-TNFR1 signaling.
BM cells (1 × 106 cells/mL) from either wt or TNF/LTα/LTβ−/− mice were cultured with GM-CSF and IL-4 for 9 days. In panels A-C, rTNF was added to some wells of TNF/LTα/LTβ−/− BM culture on day 0 at 10 ng/mL (A) or at various concentrations (B) or at 10 ng/mL on various days (C). In panel D, either anti-TNF (1 μg/mL) or blocking anti-TNFR1 (5 μg/mL) antibodies were added to wild-type BM cultures at the indicated time points. Cells were collected on day 9 and stained with FITC-conjugated anti–I-Ab mAb and PE-conjugated anti-CD11c mAb. Ratios of CD11c and I-Ab–positive cells are presented as a percent of wt (B). In panel B, data from 3 experiments are shown, *P < .05 compared with other concentrations of rTNF. Panels C and D show single representative experiments.
DC population in BM culture is modulated by TNF-TNFR1 signaling.
BM cells (1 × 106 cells/mL) from either wt or TNF/LTα/LTβ−/− mice were cultured with GM-CSF and IL-4 for 9 days. In panels A-C, rTNF was added to some wells of TNF/LTα/LTβ−/− BM culture on day 0 at 10 ng/mL (A) or at various concentrations (B) or at 10 ng/mL on various days (C). In panel D, either anti-TNF (1 μg/mL) or blocking anti-TNFR1 (5 μg/mL) antibodies were added to wild-type BM cultures at the indicated time points. Cells were collected on day 9 and stained with FITC-conjugated anti–I-Ab mAb and PE-conjugated anti-CD11c mAb. Ratios of CD11c and I-Ab–positive cells are presented as a percent of wt (B). In panel B, data from 3 experiments are shown, *P < .05 compared with other concentrations of rTNF. Panels C and D show single representative experiments.
We also attempted to inhibit the production of mature DCs from wt BM cultures by blocking antibodies against either TNF or TNFR55 added to cultures at various time points. As shown in Figure 6D, both types of antibodies had only partial effect on DCs derived from wt cultures. This partial effect could be due to several reasons, including inability to block locally produced TNF, especially, if it is produced in membrane-bound form, and due to inability to block the majority of signaling receptors during such treatment.
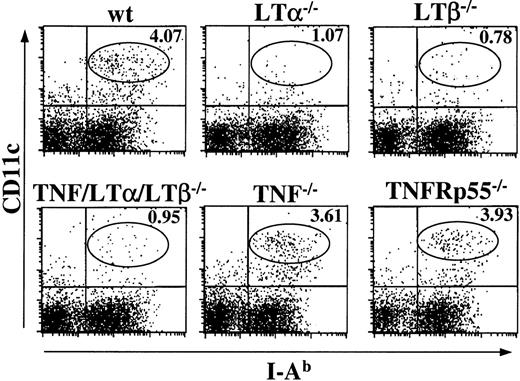
The number of mature splenic DCs is reduced in LTα−/−, LTβ−/− mice, but not in TNF−/− and TNFRp55−/− mice
Finally, to address how our in vitro findings on a panel of KO mice relate to the DC numbers observed in vivo, we analyzed peripheral lymphoid organs in wt and various strains of KO mice. Since only spleen was uniformly present in all KO mice of our panel, we prepared fresh splenocytes from naive mice and determined the numbers and percentages of CD11c+, I-Ab+ population by flow cytometry. As shown in Figure 7, 3.64 ± 0.97% (n = 7) of CD11c+, I-Ab+ cells were detected in wt spleen, whereas a much lower percentage of DCs were observed in LTα−/− (0.98% ± 0.28, n = 3) and LTβ−/− (1.04% ± 0.38, n = 4) mice. Similarly to TNF/LTα/LTβ−/− mice, LTα−/− and LTβ−/− mice frequently showed splenomegaly and increased white blood cells counts in their spleen20,22 31; nevertheless, the absolute numbers of CD11c+, I-Ab+ cells in the spleen were reduced in LTα−/− (2.63 ± 0.74 × 106cells/spleen) and LTβ−/− mice (2.51 ± 0.78 × 106 cells/spleen) as compared with wt mice (4.70 ± 1.21 × 106 cells/spleen). Somewhat unexpectedly, the relative numbers of CD11c+, I-Ab+ cells were normal in TNF−/− (4.40% ± 2.57, n = 3) and TNFRp55−/− (4.66% ± 1.76, n = 3) spleens, suggesting that the role of TNF in differentiation/maturation of DCs from BM may be redundant and can be compensated in vivo by other factors. On the other hand, the role of LT signaling in DCs recruitment to the spleen is indispensable, and this pathway appears to be primarily responsible for the deficiency observed in TNF/LTα/LTβ−/− mice.
The number of splenic DCs is reduced in LTα−/−, LTβ−/−, and TNF/LTα/LTβ−/− mice, but normal in TNF−/− and TNFRp55−/− mice.
Spleens were taken from indicated strains of naive mice and homogenized after collagenase and DNAase treatment. Cells were stained with FITC-conjugated anti–I-Ab mAb and PE-conjugated anti-CD11c mAb. The percent of events in the oval area of each dot plot is indicated. Data from 1 of 4 representative experiments are shown.
The number of splenic DCs is reduced in LTα−/−, LTβ−/−, and TNF/LTα/LTβ−/− mice, but normal in TNF−/− and TNFRp55−/− mice.
Spleens were taken from indicated strains of naive mice and homogenized after collagenase and DNAase treatment. Cells were stained with FITC-conjugated anti–I-Ab mAb and PE-conjugated anti-CD11c mAb. The percent of events in the oval area of each dot plot is indicated. Data from 1 of 4 representative experiments are shown.
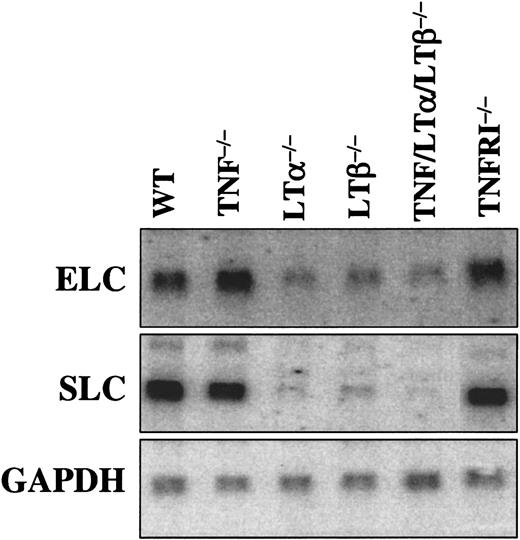
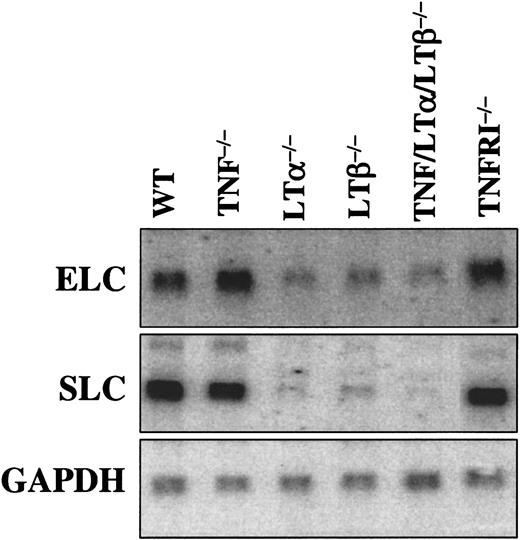
Chemokine expression is defective in spleens of LTα−/−, LTβ−/−, and TNF/LTα/LTβ−/− mice, but normal in TNF−/− and TNFRp55−/− mice
Chemokines of peripheral lymphoid tissues, such as SLC (CCL21) and ELC (CCL19), play a critical role in the recruitment of mature DCs to lymphoid organs. Expression of these chemokines in lymphoid organs, such as spleen, was previously reported to be decreased in LTα−/− mice.29,30 In order to provide a correlate between SLC and ELC expression levels and DC numbers in spleen, Northern analysis of mRNA from spleens of naive mice was employed (Figure 8). Although we observed some quantitative differences with the results earlier reported by Ngo et al,29 ELC and especially SLC were markedly decreased in LTα−/−, LTβ−/−, and TNF/LTα/LTβ−/− mice, but normal in TNF−/− and TNFRp55−/− mice. Interestingly, although the overall splenic microarchitecture was more severely disturbed in TNF/LTα/LTβ−/− mice as compared with LTα−/− and especially LTβ−/− mice (Kuprash et al31), the levels of SLC and ELC did not differ appreciably.
Expression of CCR7 ligands in spleen is associated with LT-LTβR, but not TNF-TNFRp55 signaling.
Northern analysis is described in “Materials and methods.” GAPDH gene was use as a loading control. The LTβR−/− mice produced chemokine mRNA levels similar to LTα−/− and LTβ−/− (data not shown).
Expression of CCR7 ligands in spleen is associated with LT-LTβR, but not TNF-TNFRp55 signaling.
Northern analysis is described in “Materials and methods.” GAPDH gene was use as a loading control. The LTβR−/− mice produced chemokine mRNA levels similar to LTα−/− and LTβ−/− (data not shown).
Discussion
DCs are playing a key role in the immune system. Although many studies have elucidated their ability to initiate immune response or induce tolerance, their ontogeny is still not fully understood. In the mouse model, DCs can be differentiated in vitro from MHC class II negative common myeloid progenitor cells by cultivating them in the presence of GM-CSF.4,8,9,35 Similarly, DCs can be generated from rat BM cultures in the absence of exogenous TNF.36 However, both GM-CSF and TNF seem to be essential for the differentiation and maturation of DCs from human CD34+ progenitor cells.37 38 The different requirement of cytokines for DC generation could be explained by the different level of endogenously produced cytokines among these species. Since even subtle amounts of endogenous cytokines could affect DC generation, KO mouse would be the good model for analysis of DC development.
Homing of DCs to lymphoid organs is another aspect of DC function that could be dissected using KO mice. Chemokines expressed in the lymphoid organs, including SLC (CCL21) and ELC (CCL19), play an important role in mobilization of mature DCs expressing CCR7 to the spleen and LN.11-14,16-18 Previous studies have linked LTβR and TNFRp55 signaling with chemokine expression.11
We recently created mice with a deletion of the entire TNF/LT locus. These triple TNF/LTα/LTβ-deficient mice lack all peripheral lymphoid organs, except spleen, and the microarchitecture of the spleen is severely disrupted.31 By using this model, we analyzed the combined role of TNF/LT cytokines in both generation of DCs from BM progenitor cells and recruitment of DCs to lymphoid organs. We initially found 2 types of defects in these triply deficient mice: decreased in vitro production of mature DCs from BM progenitor cells and inefficient recruitment of DCs into lymphoid organs, such as spleen. Mice with single deficiencies in TNF/LT cytokines or in their receptors were then used to identify distinct contributions of the individual pathways essential to these DC functions.
In this study we detected 2 main subsets, CD11c+ and CD11c−, generated from TNF/LTα/LTβ−/− BM cultures. The CD11c+ subset most likely corresponds to a heterogeneous DC population that is similar to one generated from wt BM culture in terms of the surface marker expression and T-cell stimulatory activity (Figures 3-4, data not shown). Further study will be needed for characterization of CD11c−, I-Ab− subset, which was also observed in TNF−/− and TNFRp55−/− BM cultures (Figure5), and in cultures of Lin−, c-kit+hematopoietic progenitor cells.
We further demonstrated that the relative numbers of DCs, defined as CD11c+, I-Ab+ cells, generated from TNF/LTα/LTβ−/−, TNF−/−, and TNFRp55−/− BM cultures were markedly reduced (Figure 2) and that recombinant TNF, when added to TNF/LTα/LTβ−/− BM cultures, can almost completely correct this deficiency in DC production when added after 3 or even 5 days in culture (Figure 6). Therefore, on top of GM-CSF and IL-4, TNF appears to be critically required for proper DC development and maturation from BM progenitor cells in vitro. The shift from CD11c+, I-Ab low to CD11c+, I-Ab high after addition of endogenous TNF strongly suggests that at least one of the functions of TNF in this culture system is the up-regulation of MHC class II molecules on the surface of immature DCs. Blocking antibodies against TNF or TNFR p55, when added to wt BM cultures, showed only partial blocking effect on the production and maturation of DCs, presumably due to inability to completely block the action of membrane-associated TNF (in the case of α-TNF) or because the action of endogenous TNF is indirect and is mediated through the expression of long-lived mediators.
The fact that numbers of mature DCs are normal in spleens of TNF and TNFRp55-deficient mice (Figure 7), as well as in the peripheral lymph nodes of these mice (data not shown), suggests that the proposed role of TNF can be compensated in vivo by other factors, for example, CD4039,40 or through up-regulation of NFκB,41 which can occur through a variety of mechanisms.
DCs developed normally in LTα−/− and LTβ−/− mice in vitro, suggesting that neither endogenous LTα3 nor LTα1/LTβ2are required in this culture system. However, the numbers of mature splenic DCs in these animals were significantly reduced in vivo (Figure7). This finding is consistent with the report by Wu et al, who demonstrated that signaling via LTβR by membrane LTα/β heterotrimer is required for the homing of DCs into the spleen.42 Several studies have revealed that DCs up-regulate the expression of CCR7 during their maturation stage and chemotactically respond to ELC (CCL19) or SLC (CCL21), which are expressed in the secondary lymphoid organs, allowing DCs to reach the T-cell areas of lymphoid tissues.11,14 17 Splenic mRNA levels of SLC, ELC, and BLC are significantly reduced in mice with single LTα−/−29,30 or combined TNF/LT deficiency (Figure 8). Presumably, lower SLC and ELC expression levels lead to a reduced recruitment of splenic DCs in LTα−/−, LTβ−/−, LTβR−/−, and TNF/LTα/LTβ−/− mice. TNF−/− and TNFRI−/− mice showed normal or almost normal ELC and SLC expression levels in spleen (Figure 8), consistent with normal DC numbers in spleens of these mice. In future studies, it will be interesting to determine how relative DC numbers in spleen (or in LN, where available) of these various mouse strains are affected following a challenge such as immunization or infection through central or local routes.
Overall, our study reveals 2 distinct mechanisms responsible for deficiency in production/maturation and in recruitment of DCs observed in mice with combined TNF/LT. The first mechanism revealed in vitro is associated with TNF signaling through TNFRp55, which leads to DC development and maturation. However, this effect of TNF can be compensated in lymphoid tissues by other factors, and TNF deficiency does not result in defective DC recruitment to lymphoid organs in naive mice in vivo, because the main mechanism of recruitment is associated with the expression of lymphoid tissue chemokines that are essentially normal in TNF or TNFR-deficient mice (Figure 8). Thus, the second component is associated with abnormal expression and/or dislocated positioning of chemotactic signals in lymphoid organs.42This mechanism is controlled primarily by LTα/LTβ-LTβR signaling in a nonredundant manner.
We are indebted to Drs J. J. Oppenheim, W. J. Murphy, J. Keller, and S. Stoll for critically reading the manuscript. We thank Ms L. R. Finch for FACS analysis and Ms K. B. Noer and Dr G. W. Wiegand for cell sorting.
The contents of this publication do not necessarily reflect the view or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Supported in whole or in part with United States federal funds from the National Cancer Institute, National Institutes of Health, under contract no. NO1-CO-12400.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Sergei A. Nedospasov, Basic Research Program, SAIC Frederick, NCI-Frederick, Building 560, Room 31-70, Frederick, MD 21702-1201; e-mail:nedospas@mail.ncifcrf.gov.

![Fig. 3. The stimulatory activity of cells generated from TNF/LTα/LTβ−/− BM culture is significantly reduced compared with that from wt BM culture. / BM cells (1 × 106 cells/mL) from either wt (●) or TNF/LTα/LTβ−/− (○) mice were cultured with GM-CSF and IL-4 for 9 days. Cells were collected on day 9 and subjected to 30 Gy irradiation. Indicated number of generated cells were cocultured with syngeneic (A) or allogeneic (B) LN cells (1 × 106 cells/mL) for 4 days. During the last 18 hours of the culture, [3H]dThd was added in each well. The values are the means ± SD of quadruplicate wells. Data from 1 of 3 representative experiments are shown. *P < .01 compared with TNF/LTα/LTβ−/− BM culture. CPM indicates counts per minute.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/4/10.1182_blood.v101.4.1477/3/m_h80433854003.jpeg?Expires=1765113417&Signature=DOoVT8~u2KlcI1zKzhoWynZyTmg43xSW7qdkyU8QTP7AQZenbjVXgzgXQy-GezYAEKJEKDtEYAZitMA9Pnt4hQLrZh67pwsIx5ZXy00DFDewzzeJTGS~ZKnXp5rM4ZNmK1OcBktv27K6ki2BvBmFlH1YuN9cw0-rSjslhgUOVlut3pYKxcWlOIMi~k3~WI8kPw-QrlKR6HkNe70RUO~zhG5wInz1Gng5ARuPXwdGzq4ROFYYNr8UuVPQ1cHStkkMokbSqtIlc~5nLGlr7JyjVmMnv5zkCCnnZgwU9n0AA51nELGe310UKa20IZu3eL81Dubnoz7YRt7xrgLxU0wKcQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. CD11c+, I-Ab low-high cells generated from TNF/LTα/LTβ−/− BM culture possess stimulatory activity in MLR similar to DCs from wt BM culture. / BM cells (1 × 106 cells/mL) from either wt (●) or TNF/LTα/LTβ−/− (○, ▵, ■) mice were cultured with GM-CSF and IL-4 for 9 days. CD11c+, I-Ab low-high (▵), and CD11c−, I-Ab−(■) cells were sorted from TNF/LTα/LTβ−/− BM culture. After 30 Gy irradiation, indicated number of generated cells were cocultured with syngeneic (A) or allogeneic (B) LN cells (1 × 106 cells/mL) for 4 days. During the last 18 hours of the culture, [3H]dThd was added in each well. The values are the means ± SD of quadruplicate wells. Data from 1 of 2 representative experiments are shown. *P < .01 compared with TNF/LTα/LTβ−/− whole and TNF/LTα/LTβ−/− CD11c− population.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/4/10.1182_blood.v101.4.1477/3/m_h80433854004.jpeg?Expires=1765113417&Signature=djVskqof7n7yo5~7QdD7B1EQm9O4wtITG6U5sUG16Eldan3uJwSmF4p1zpl3Z1NqHmZgSWhQrOw7jM9FEeSaqhLauTPeM9Xrqy-y9TIB0QU3nbyrvHCIXJ6JGdgTouTsYxQqhBas0PhBYnAq0uyGt1na5iW4OQhhfycEo01C8kIkS3UMOPCFfws2SfOWD5SNLL3Gc83lRNZyIhMM0-N6COlI-3JsaCMu3x3lkiKlGrlPVRW23CrmWkhSHrBQhnn7nCyUmoaFEnAqWzB0F8ng3JkPai3Y-VIGXEa5bQVI4~AXtYibSE4vyoZnVCRVeO0-IyrQ9DqbjTvjK3LM-JKCrw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 3. The stimulatory activity of cells generated from TNF/LTα/LTβ−/− BM culture is significantly reduced compared with that from wt BM culture. / BM cells (1 × 106 cells/mL) from either wt (●) or TNF/LTα/LTβ−/− (○) mice were cultured with GM-CSF and IL-4 for 9 days. Cells were collected on day 9 and subjected to 30 Gy irradiation. Indicated number of generated cells were cocultured with syngeneic (A) or allogeneic (B) LN cells (1 × 106 cells/mL) for 4 days. During the last 18 hours of the culture, [3H]dThd was added in each well. The values are the means ± SD of quadruplicate wells. Data from 1 of 3 representative experiments are shown. *P < .01 compared with TNF/LTα/LTβ−/− BM culture. CPM indicates counts per minute.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/4/10.1182_blood.v101.4.1477/3/m_h80433854003.jpeg?Expires=1765610879&Signature=K-87JfOzp-GwKb1Qp0YTgBmN1qqIZ-6-neMdqXifWwRmL~s-kYAfzr8dMEvVc5RgNqEKcVpzHQzHRjM9Jeu~j~HEyLbk0QzwO9TMh-TlG0y-QHdaS317Uniqa9Fpo8cihmB3tKpt844FgbF6XWpwmp5r8rZBpN4xraY5C7tqCtpKDxbm6bHhkuS8bhdNIEbxqJ7qxPPxMv1ZqNzfo6lo3ALFA4Q1FF0TIbKNZDOI1G8VvITTWtv~5hvAdDGu4Wrh0DVVu1S3OY-ZBDB8jvaDblaO5yjocetS35Fl6bpyWAKzNVd5poLAoWd6Px1vzB6vqn1kcd6z0TddRK2t2xKLDw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. CD11c+, I-Ab low-high cells generated from TNF/LTα/LTβ−/− BM culture possess stimulatory activity in MLR similar to DCs from wt BM culture. / BM cells (1 × 106 cells/mL) from either wt (●) or TNF/LTα/LTβ−/− (○, ▵, ■) mice were cultured with GM-CSF and IL-4 for 9 days. CD11c+, I-Ab low-high (▵), and CD11c−, I-Ab−(■) cells were sorted from TNF/LTα/LTβ−/− BM culture. After 30 Gy irradiation, indicated number of generated cells were cocultured with syngeneic (A) or allogeneic (B) LN cells (1 × 106 cells/mL) for 4 days. During the last 18 hours of the culture, [3H]dThd was added in each well. The values are the means ± SD of quadruplicate wells. Data from 1 of 2 representative experiments are shown. *P < .01 compared with TNF/LTα/LTβ−/− whole and TNF/LTα/LTβ−/− CD11c− population.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/4/10.1182_blood.v101.4.1477/3/m_h80433854004.jpeg?Expires=1765610879&Signature=PNXt3khRB3-snDp7OCpkRfFG~dYqH0~rn5WmrEPmALUQK9ljOmwXYYIeM~w~NCgCj11BXMZd9vOC3nhHnwtZxpc1UdzjaaXcBOStJRdZVBWd3h85jq9ytV03Ukhc7xp2XCIxFR6F~Om64OeZNkVpQ8pFKowIVBA-GPTwHQWznDHVhk6QTe~9ngYtcloQSaVTjyyYrAR7JIlr-FXubWHq~D~vdMVcNVPbSK6jeK4azBszKecZdw4HdDO-I6R~89qIwphfRME1BJ6fM6yM0GxR01vMGo55FeIeT-qX-0sQUFsl-krZyHJWyJxWKZwpeJMu-0pFxVmkz14O4Th42N3FIQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)