LIRs are immunoglobulinlike receptors that have activating and inhibitory functions in leukocytes. Here we report the identification of the first LIR family member, LIR9, expressed as a membrane-bound receptor and as a secreted molecule. We identified 4 different forms of LIR9, 2 of which encode transmembrane molecules and 2 encode secreted molecules. The transmembrane forms of LIR9 contain a short cytoplasmic domain and a charged arginine residue within the transmembrane region that is likely to mediate its association with another coreceptor. LIR9 is mostly expressed in myeloid cells, including monocytes and neutrophils. Cross-linking of LIR9 on the surfaces of monocytes induces calcium flux and secretion of the proinflammatory cytokines interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and IL-6, indicating that LIR9 could play a role in triggering innate immune responses.
Introduction
The LIR family includes 11 members1 that are mostly expressed in monocytes, macrophages, and dendritic cells but that can also be present in T, B, and natural killer (NK) cells.2-4 These receptors are also known as ILTs,5 MIRs,6 or HM and HL clones.7 The LIR family can be subdivided into 3 groups: transmembrane molecules with 2 to 4 immunoreceptor tyrosine-based inhibitory motifs (ITIMs) (LIR1, LIR2, LIR3, LIR5, LIR8); transmembrane molecules with short cytoplasmic domains, a positively charged arginine residue within the transmembrane domain, and no ITIMs (LIR6, LIR7); and a single soluble molecule with no transmembrane domain (LIR4). ITIM-containing LIRs interact with the SH2-domain containing phosphatase SHP-18-10 and send inhibitory signals. LIR6 and LIR7 do not signal by themselves and require association with another cell surface receptor. ILT1/LIR7 associates with the FcR common γ-chain11 to send an activating signal. Most of the LIRs have unknown ligands; the only exceptions are LIR1, LIR2, and LIR6, which bind to major histocompatibility complex (MHC) class 1 molecules.8,10,12 13
LIR4 is the only member of this family that is expressed as a soluble molecule, but its function is still unknown. LIR4 could potentially act as an antagonist of other LIRs by preventing the binding of a common ligand to the membrane-bound LIRs. However, we have not been able to detect any binding of LIR4 to MHC class 1 or any other ligands (M.K. and D. Cosman, unpublished data, 1998-2000), despite the fact that that the extracellular domain of LIR4 is 84% identical to that of LIR1. This has led us to ask whether there is a membrane-bound form of LIR4 or whether other membrane-bound LIRs have soluble versions. To address this question, we performed rapid amplification of cDNA ends (RACE) on leukocyte RNA derived from a large pool of human donors and identified the first LIR family member, LIR9, which is expressed as a membrane-bound and as secreted molecule.
Materials and methods
RACE cloning and cDNA constructs
For the RACE template, we used Marathon-Ready Leukocyte cDNA prepared from a pool of 550 human donors (Clontech, Palo Alto, CA). To clone LIR9, we used the following primers to perform 5′ RACE: 5′-tccaaggtccaggagagcttgtggtctcc-3′ and 5′-gatatgcctgcgagagccatagcatctgagc-3′. The following primers were used to perform 3′ RACE: 5′-gagaccacaagctctcctggaccttggactc-3′ and 5′-gtggatgctcagatgctatggctctcgcagg-3′.
Calcium mobilization assay
Primary human monocytes were labeled with Fluo-4 (Molecular Probes, Eugene, OR), and this was followed by incubation on ice for 15 minutes with no antibody or with 5 μg/mL anti-LIR9, anti-LIR7, or isotype-matched control antibody. Unbound antibodies were then washed off, and the bound primary antibody was cross-linked by the addition of donkey antimouse F(ab')2 fragments at 5 μg/mL (Jackson Laboratories, PA). Calcium mobilization was measured at room temperature on the FLIPR-384 Fluorometric Imaging Plate Reader system (Molecular Devices, Menlo Park, CA). Calcium measurements were performed in real time before, during, and for 4 minutes after the addition of the secondary antibody. The assay buffer was Hanks balanced salt solution with calcium and magnesium, no phenol red, 20 mM HEPES (N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid), 1% fetal bovine serum (FBS), and 2.5 mM probenecid.
Cytokine secretion
Primary human monocytes were incubated in a 96-well tray coated with the following antibodies: anti-LIR9 m660, anti-LIR1 m402, anti-LIR2 m421, anti-LIR7 m473, and the isotype-matched control antileucine-zipper m15 antibody. Cells were cultured for 2 days, and the conditioned media were harvested. All the reagents used in this assay were free of endotoxin contamination. Ten different cytokines were analyzed in a multiplex assay system containing fluorescently labeled microsphere beads (Beadlyte Human Multi-Cytokine Detection System 3; Upstate Biotechnology, Lake Placid, NY), and cytokine levels were quantitated using the Luminex 100 system (Luminex, Austin, TX).
Results and discussion
We performed RACE cloning on a large pool of leukocyte RNA and identified a new member of the LIR family, which we named LIR9. Four different forms of LIR9 were identified: 2 membrane-bound molecules, LIR9m1 (also known as ILT11) and LIR9m2, and 2 molecules, LIR9s1 and LIR9s2, which lack a transmembrane domain (Figure1A). The difference between LIR9m1 and LIR9m2 and between LIR9s1 and LIR9s2 resides in the presence or absence of a small exon encoding 12 amino acids. This exon is present near the amino terminus of LIR9m1 and LIR9s1 and is partially contained within the signal peptide of these molecules. After cleavage of the signal peptide, LIR9m1 and LIR9s1 are predicted to have amino termini that are different from those of LIR9m2 and LIR9s2. The insertion of this exon also creates a new N-linked glycosylation site in LIR9m1 and LIR9s1. It is unclear whether these differences will impose any changes on LIR9 function, expression, or stability.
LIR9 amino acid sequences and LIR9 expression in leukocytes and transfected cells.
(A) Alignment of the amino acid sequences of the 4 forms of LIR9 and LIR6b, another activating LIR with 2 immunoglobulinlike domains. Two arrows indicate the predicted cleavage sites for the signal peptides; the first arrow indicates the cleavage site for LIR9m1 and LIR9s1, and the second arrow indicates the cleavage site for LIR9m2 and LIR9s2. The box highlights the 12 amino acid exon that is present in LIR9m1 and LIR9s1. Underlined amino acids are N-linked glycosylation sites. The arginine residue within the transmembrane domain is shown in boldface. (B) Expression of LIR9m (1 and 2) and LIR9s (1 and 2) transcripts in peripheral blood leukocytes and in monocyte-derived dendritic cells as determined by TaqMan quantitative reverse transcription–polymerase chain reaction (RT-PCR). Data are expressed on a relative scale using the LIR9s expression in monocytes as the reference value. (C) LIR9m expression in CD14+ monocytes as determined by 2-color fluorescence-activated cell sorter (FACS) analysis. (D) LIR9s1 is detected in the conditioned media of COS cells transfected with the LIR9s1 cDNA, but not with the LIR9m1 cDNA or with empty vector. Cells were transfected with the indicated plasmids and 2 days later were metabolically labeled with35S-(cysteine-methionine) for 5 hours. Ten microliters conditioned media were loaded per lane, and the gel was exposed to film for 49 hours. Arrow indicates the LIR9s1 protein.
LIR9 amino acid sequences and LIR9 expression in leukocytes and transfected cells.
(A) Alignment of the amino acid sequences of the 4 forms of LIR9 and LIR6b, another activating LIR with 2 immunoglobulinlike domains. Two arrows indicate the predicted cleavage sites for the signal peptides; the first arrow indicates the cleavage site for LIR9m1 and LIR9s1, and the second arrow indicates the cleavage site for LIR9m2 and LIR9s2. The box highlights the 12 amino acid exon that is present in LIR9m1 and LIR9s1. Underlined amino acids are N-linked glycosylation sites. The arginine residue within the transmembrane domain is shown in boldface. (B) Expression of LIR9m (1 and 2) and LIR9s (1 and 2) transcripts in peripheral blood leukocytes and in monocyte-derived dendritic cells as determined by TaqMan quantitative reverse transcription–polymerase chain reaction (RT-PCR). Data are expressed on a relative scale using the LIR9s expression in monocytes as the reference value. (C) LIR9m expression in CD14+ monocytes as determined by 2-color fluorescence-activated cell sorter (FACS) analysis. (D) LIR9s1 is detected in the conditioned media of COS cells transfected with the LIR9s1 cDNA, but not with the LIR9m1 cDNA or with empty vector. Cells were transfected with the indicated plasmids and 2 days later were metabolically labeled with35S-(cysteine-methionine) for 5 hours. Ten microliters conditioned media were loaded per lane, and the gel was exposed to film for 49 hours. Arrow indicates the LIR9s1 protein.
LIR9 transcripts are present mostly in tissues of the hematopoietic system, including bone marrow, spleen, lymph node, and peripheral leukocytes (data not shown). Among leukocytes, monocytes and neutrophils express the highest levels of LIR9 transcripts (Figure 1B). At the protein level, LIR9m is expressed in CD14+ monocytes (Figure 1C), but not in T cells, B cells, or NK cells.
The LIR9s1 and LIR9s2 splice variants contain a signal peptide and lack a transmembrane domain, suggesting that these molecules might be secreted. To determine whether LIR9s1 could be secreted, we cloned the LIR9s1 cDNA including the native signal peptide into a mammalian expression vector and transfected this plasmid into COS cells. Supernatants from LIR9s1-transfected cells, but not control cells, contain a protein of approximately 35 kDa, which is the predicted size for glycosylated LIR9s1 (Figure 1D). Based on these results and on cell surface staining, we concluded that LIR9 is expressed as a membrane-bound and a secreted protein. The function of LIR9 in vivo is expected to be modulated by the ratio of LIR9s/LIR9m in the environment around the cells.
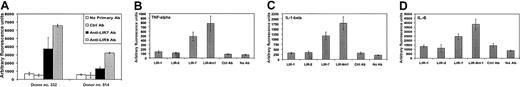
Cross-linking LIR9 on the surfaces of monocytes induces the mobilization of calcium (Figure 2A), a known signaling mediator that is released during cell activation. In addition, triggering of LIR9 on monocytes induces the secretion of interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and IL-6 (Figure 2B). Secretion of these cytokines was detected as early as 12 hours after the cross-linking of LIR9 on the surfaces of monocytes (L.B. and T.K., unpublished data, 2002). IL-1β, TNF-α, and IL-6 are normally released in the early stages of inflammatory responses, suggesting that LIR9 might play a role in modulating monocyte function in inflammatory settings. In addition, it has been shown that LIR2 and LIR7 are up-regulated in monocytes and neutrophils infiltrating the synovia of patients with early rheumatoid arthritis.14 Evaluation of LIR9 expression in diseased tissues, particularly in conditions that have an inflammatory component, might help to further delineate LIR9 function and to define its potential as a therapeutic target.
Cross-linking of LIR9m on the surfaces of monocytes induces calcium mobilization and cytokine secretion.
(A) Monocytes from 2 different donors were labeled with Fluo-4 and then incubated with anti-LIR9, anti-LIR7, an isotype-matched control antibody, or no antibody. Calcium mobilization was measured on a fluorometric plate reader as a response to cross-linking of the primary antibodies with donkey antimouse F(ab')2 fragments. Data shown are representative of 2 of 4 different donors. Among the 4 donors, the average -fold changes and standard deviations in calcium flux relative to the nonantibody control were the following: control antibody, 0.91 ± 0.07; anti-LIR7, 3.10 ± 0.79; and anti-LIR9, 5.54 ± 1.57. (B) Monocytes were incubated on a 96-well tray coated with the antibodies against LIR1, LIR2, LIR7, LIR9, isotype-matched control antibody, or no antibody. Two days later, conditioned media were harvested and cytokine levels were measured using the Luminex assay system as described in “Materials and methods.” Data show the averages and standard deviations of 4 replicates of each sample. Data shown are representative of 3 experiments on different donors.
Cross-linking of LIR9m on the surfaces of monocytes induces calcium mobilization and cytokine secretion.
(A) Monocytes from 2 different donors were labeled with Fluo-4 and then incubated with anti-LIR9, anti-LIR7, an isotype-matched control antibody, or no antibody. Calcium mobilization was measured on a fluorometric plate reader as a response to cross-linking of the primary antibodies with donkey antimouse F(ab')2 fragments. Data shown are representative of 2 of 4 different donors. Among the 4 donors, the average -fold changes and standard deviations in calcium flux relative to the nonantibody control were the following: control antibody, 0.91 ± 0.07; anti-LIR7, 3.10 ± 0.79; and anti-LIR9, 5.54 ± 1.57. (B) Monocytes were incubated on a 96-well tray coated with the antibodies against LIR1, LIR2, LIR7, LIR9, isotype-matched control antibody, or no antibody. Two days later, conditioned media were harvested and cytokine levels were measured using the Luminex assay system as described in “Materials and methods.” Data show the averages and standard deviations of 4 replicates of each sample. Data shown are representative of 3 experiments on different donors.
We thank G. Sapina, K. Schooley, B. Greenfield, L. Peterson, V. Rajurs, T. Stevens, and D. Hirschstein for technical assistance. We also thank D. Cosman, J. Derry, K. Mohler, and D. Williams for critically reviewing the manuscript.
Prepublished online as Blood First Edition Paper, September 26, 2002; DOI 10.1182/blood-2002-05-1432.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Luis Borges, Amgen, Inc, 51 University St, Seattle, WA 98101; e-mail: borgesl@amgen.com.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal