Dendritic cells (DCs) genetically engineered to overexpress CD95 (Fas) ligand (CD95L-DC) were proposed as tools to induce peripheral tolerance to alloantigens. Herein, we observed that CD95L-DC obtained after retroviral gene transfer in bone marrow (BM) precursors derived from CD95-deficient (lpr/lpr) mice elicit much stronger allospecific type 1 helper T-cell and cytotoxic T-cell activities than control DCs upon injection in vivo, although they induce lower T-cell responses in vitro. Indeed, a single injection of CD95L-DC prepared from C57BL/6 mice was sufficient to prime bm13 recipients for acute rejection of C57BL/6 skin allografts that were otherwise tolerated in the context of this single weak major histocompatibility complex (MHC) class I incompatibility. Massive neutrophil infiltrates depending on interleukin (IL)–1 signaling were observed at sites of CD95L-DC injection. Experiments in IL-1 receptor–deficient mice or in animals injected with depleting anti-Gr1 monoclonal antibody (mAb) established that neutrophil recruitment is required for the development of vigorous T-cell responses after injection of CD95L-DC in vivo.
Introduction
CD95 (Fas)–mediated apoptosis of activated T lymphocytes is critically involved in the homeostasis of the T-cell pool1,2 and the maintenance of peripheral tolerance to self antigens.3 Moreover, it has been proposed that the immune privilege status of particular anatomic sites could be related to local expression of CD95L4-6 and that expression of CD95L by tumor cells might protect them from immune attack.7-9 On this basis, it has been considered that expression of CD95L on allo- or xenografts might promote their acceptance by deleting host T cells specific for transplanted antigens. Indeed, CD95L expression on Sertoli cells was suggested to be directly responsible for testis allograft survival.4,10 It was then reported that implantation of syngeneic muscle cells transfected with CD95L together with allogeneic grafted pancreatic islets allowed long-term survival of the transplanted islets.11 More recently, CD95L overexpression on allogeneic endothelium was shown to inhibit transplant-associated intimal hyperplasia.12 However, several of these observations have been refuted13-15 so that the role of CD95L in conferring immune privilege is currently a matter of controversy. Furthermore, chemoattraction of neutrophils leading to a massive inflammatory reaction has emerged as a major consequence of CD95L overexpression. As a matter of fact, neutrophil infiltration leading to graft destruction was observed after implantation of pancreatic islets in which the CD95L gene was overexpressed.16 Likewise, CD95L transgenic islet β cells or heart allografts were shown to be more rapidly rejected than their wild-type counterparts, in association with a massive influx of neutrophils in the transplant.17 18
In order to promote deletion of allospecific T cells without inducing inflammation at the graft level, it has been proposed to condition allograft recipients with antigen-presenting cells overexpressing CD95L prior to transplantation. Indeed, allogeneic macrophages transduced with murine CD95L induced profound alloantigen-specific T-cell unresponsiveness.19 Dendritic cells (DCs) represent a suitable cell type for such an approach as indicated by the report of Matsue et al showing that injection of an ovalbumin-pulsed DC line transfected with murine CD95L induced antigen-specific T-cell hyporesponsiveness.20 In the transplantation setting, Min et al reported significant enhancement of heart allograft survival in mice repeatedly injected with high doses of donor-type bone marrow (BM)–derived DCs transfected with human CD95L.21
Herein, DCs genetically engineered to overexpress CD95L were derived from BM precursors of CD95-deficient lpr/lpr mice, as rapid apoptosis was observed when wild-type mice were used as BM donors. We assessed the allostimulatory capacities of CD95L-DC in 2 models involving either a single major histocompatibility complex (MHC) class I or MHC class II disparity.22 23 Unexpectedly, CD95L-DCs were found to elicit stronger TH1-type and cytotoxic T-cell (CTL) responses than control DCs in vivo. These observations led us to investigate the role of neutrophils during the induction phase of alloreactive T-cell responses triggered by CD95L-DCs and to revisit the consequences of the injection of CD95L-DCs on the fate of a subsequent tissue allograft.
Materials and methods
Mice
C57BL/6 (H-2b) and BALB/c (H-2d) breeders were purchased from Harlan Nederland (Horst, The Netherlands). C57BL/6.C-H-2-bm12 (bm12) that differ from C57BL/6 wild-type (WT) mice with a point mutation in the I-Aβ chain and C57BL/6-lpr/lpr Fas-deficient (lpr/lpr) mice were obtained originally from Jackson Laboratories (Bar Harbor, ME) and bred in our animal facility. C57BL/6-lpr/lpr-C57BL/6.C-H-2bm12 (lpr/lpr bm12) mice were generated in our animal facility by successive backcrossing of bm12 and lpr/lpr mice. Mice were tested by polymerase chain reaction (PCR) (see “PCR detection of the lpr/lpr mutation”) for lpr genotype based on the retrovirus insertion in the CD95 gene.24 The bm12 phenotype was determined by negative selection by flow cytometry using anti–MHC II (I-Ab, 25-9-17) monoclonal antibodies (mAbs). C57BL/6.C-H-2bm13 (bm13) mice that differ from C57BL/6 WT with an H-2D mutation were obtained from the Pasteur Institute of Brussels from breeding pairs initially provided by the Nederland Cancer Institute (Amsterdam, The Netherlands). C57BL/6 interleukin (IL)–1 receptor (R)–deficient mice were provided by Immunex, Seattle, WA. Animals were maintained and treated according to institutional guidelines.
Reagents and cell lines
The recombinant murine granulocyte macrophage–colony stimulating factor (rmGM-CSF) used for the DC generation was produced as previously described.25 The ascitic preparation of anti–GR1 rat IgG2b mAb (RB6-8C526) used for the in vivo depletion was obtained from Dr O. Leo (UniversitéLibre de Bruxelles, Gosselies, Belgium). The RB6-8C5 clone was kindly provided by Dr R. Coffman (DNAX Research Institute, Palo Alto, CA). The anti–DNP rat IgG2a mAb LO-DNP-16 was purchased from LO-IMEX (Université Catholique de Louvain, Belgium). Lipopolysaccharide (LPS) from Escherichia coli (serotype 0111:B4) was purchased from Sigma-Aldrich (Bornem, Belgium). The endotoxin level of ascites was evaluated < 5 endotoxin units (EU)/mL, as determined by the Coatest Limulus Amebocyte Lysate assay (BioWhittaker). The murine mastocytoma P815 cell line transfected with murine Fas cDNA was generously provided by Professor P. Vassalli and Dr K. Matsuura (University of Geneva, Switzerland) and maintained in Dulbecco modified Eagle medium (DMEM) containing 4.5 g/L glucose and l-glutamine (BioWhittaker, Verviers, Belgium) supplemented with 5% heat-inactivated fetal bovine serum (FBS) (SB0012; BioWhittaker), 100 U/mL penicillin, and 100 μg/mL streptomycin (BioWhittaker). The PhoenixECO ecotropic packaging cell line was provided by Dr G. P. Nolan (Stanford, CA) and was grown in the same medium.
Generation of bone marrow–derived DCs
To generate DCs from bone marrow cultures, we used a modified protocol described by Lutz et al.27 Briefly, bone marrow was flushed from the femurs and tibiae of mice, disintegrated by vigorous pipetting, filtered through a nylon mesh, and depleted of red blood cells with ammonium chloride. At day 0, bone marrow progenitors were seeded in a 6-well plate at the rate of 1 × 106 per well in 4 mL of RPMI 1640 (BioWhittaker) medium containing 10% heat-inactivated FBS (SB0012; BioWhittaker), 20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 2 mM glutamine, 1 mM nonessential amino acids (BioWhittaker), sodium pyruvate (BioWhittaker), 2-mercaptoethanol, and 20 ng/mL of rmGM-CSF. At day 3, another 4 mL of complete medium containing 20 ng/mL rmGM-CSF was added to each well. At days 6 and 8, half of the culture supernatant was collected and centrifuged, and the cell pellet was resuspended in 4 mL fresh medium supplemented with 20 ng/mL rmGM-CSF and given back into the original well. On day 10, DCs were harvested by gentle pipetting. When indicated, LPS was added at 100 ng/mL for the last 48 hours of the DC culture. For phenotypic analysis, DCs were incubated with the biotinylated mAbs directed against CD80 (1G10) CD86 (GL1) or MHC class II (I-Ab,25-9-17) surface molecules in the presence of 2.4G2 supernatant, a rat mAb directed against the mouse FcRII/IIIγ (CD32/CD16) receptor. Binding of the mAbs was revealed by a second incubation with phycoerythrin (PE)–labeled streptavidin (Pharmingen, San Diego, CA). CD40 expression was revealed with unlabeled anti-CD40 mAb (HM40-3) and fluorescein isothiocyanate (FITC)-conjugated F(ab′)2 mouse anti–rat IgG (Jackson ImmunoResearch, West Grove, PA). The DC culture purity was evaluated with an FITC-conjugated anti-CD11c mAb (HL3) in the presence of 2.4G2 supernatant. All mAbs were purchased from Becton Dickinson Pharmingen (Mountain View, CA), and cells were analyzed on a FACScalibur flow cytometer (Becton Dickinson).
Cloning of retroviral vector constructs
For retrovirus production the retroviral vector MFG, derived from Moloney murine leukemia virus, was used. This vector does not contain a drug-resistance marker, nor does it express any potential antigenic protein other than the inserted cDNA.28 The P1A and CD95L cDNAs were obtained by PCR. The amplification products were sequenced before insertion into the MFG vector. P1A gene was amplified from P1HTR cells and cloned in pMFG/Nco1-BamH1. The P1A recombinant retrovirus was used to generate the “control” transduced DCs. The CD95/CD95L cytotoxic activity was reported to be more effective in mFasL.2 mice than in mFasL.1 mice.29 We therefore decided to generate an mFasL.2 recombinant retroviral vector. The cDNA encoding mFasL.2, the murine CD95L gene, was obtained by reverse transcriptase–polymerase chain reaction (RT-PCR) on RNA from activated T cells of BALB/c origin. After cloning in pCR2, the mCD95L gene was excised from the plasmid as aBspH1-BamH1 fragment and cloned in pMFG/Nco1-BamH1. The eGFP (enhanced green fluorescent protein) gene was obtained as aNco1-Bcl1 fragment by digestion of peGFP-C1 (Clonetech Westburg, Leusden, The Netherlands) and ligated in pMFG/Nco1-BamH1.
Retrovirus production and DC transduction
Ten million PhoenixECO producer cells were transfected with 40 μg of retroviral vector DNA by the calcium phosphate precipitation method.30 Cells were incubated in complete DMEM medium supplemented with 25 μM chloroquine (Sigma-Aldrich) at 37°C for 10 hours. The medium was renewed with Opti-MEM (Gibco BRL, Merelbeke, Belgium) after 14 hours, and the retrovirus-containing medium was harvested 48 hours after transfection. The retroviral supernatants were filtered (0.22-μm pore size), snap-frozen, and stored at −80°C. On days 1, 2, and 3 after the start of the bone marrow cell culture, the medium was removed and replaced with 2 mL viral supernatant containing 8 μg/mL polybrene (Sigma-Aldrich). The cells were transduced by centrifugation of the 6-well plates for 2 hours at 2400 rpm and at room temperature. The retroviral supernatant was removed, and the cells were resuspended in cytokine-containing medium. To evaluate our retroviral transduction efficiency, we used eGFP as a reporter system. Transduction efficiency was monitored by flow cytometry on day 10 of the DC culture. We consistently obtained up to 85% of green fluorescent DCs.
Apoptosis assay
The untransfected and mCD95-transfected P815 target cells were labeled with 5 μCi (0.185 MBq)/mL of [3H]-thymidine (ICN, Asse-Relegem, Belgium) during an overnight incubation at 37°C and 5% C02. Labeled target cells were harvested, washed, and seeded in 96-well round-bottom plates (Greiner, Wemmel, Belgium) at a density of 10 000 cells/well. Effector cells were washed and added to the target cells at the indicated ratios. After 18 hours of incubation at 37°C and 5% C02, intact nuclei were harvested on Unifilter plates, and the radioactivity was measured on a microplate beta counter (Topcount; Packard Instrument, Meriden, CT). Data were expressed as percentages of cytotoxicity calculated by the following formula: [1-(cpm with effector/cpm without effector)] × 100. When indicated, recombinant mouse Fas/human Fc chimera (mFas-hFc TNFRSF6; R&D systems, Minneapolis, MN) was added to the culture at 10 μg/mL.
Flow cytometry of peritoneal exudate cells (PECs)
Sixteen hours after intraperitoneal injection of 8 × 105 transduced DCs, mice were killed by carbon dioxide asphyxiation. PECs were harvested with 8 mL cold Ca and Mg-free Hanks balanced salt solution (HBSS) medium containing red phenol (BioWhittaker). PECs were washed and characterized by flow cytometry using FITC-conjugated anti–CD11b mAb (M1/70) and biotinylated anti–GR1 mAb (RB6-8C5) plus PE-labeled streptavidin (BD Pharmingen) in the presence of 2.4G2 mAb. Cytospins of freshly isolated PECs were incubated with May-Grünwald-Giemsa staining solution to identify polymorphonuclear leukocytes.
Production of cytokines in mixed lymphocyte culture
106 responder T cells from draining popliteal and inguinal lymph nodes (LNs) of naive or primed (5 days after the immunization) mice were seeded in 48-well flat-bottom plates (NUNC, Roskilde, Denmark) with 3 × 105 irradiated (20 Gy) allogeneic DCs or 2.5 × 106 splenocytes in 1 mL culture medium. Supernatants were harvested after 24 hours of culture for determination of IL-2 levels and after 72 hours for interferon (IFN)–γ, IL-5, and IL-4 detection. Culture medium for mixed lymphocyte cultures (MLCs) was RPMI 1640 supplemented with 5% heat-inactivated FBS (1 578 075, Greiner), 20 mM HEPES, 2 mM glutamine, 1 mM nonessential amino acids, sodium pyruvate, and 2-mercaptoethanol. Quantification of cytokines in MLC supernatants was made using commercially available enzyme-linked immunosorbent assay (ELISA) (Duoset; R&D systems, Minneapolis, MN) for IFN-γ, IL-2 and IL-4, and Opt EIA set (Pharmingen) for IL-5. The detection limits were 15 pg/mL for IL-2, IL-4, and IL-5, and 30 pg/mL for IFN-γ.
Generation of CTL responses
5 × 106 popliteal and inguinal lymph node responder cells were cultured with 5 × 106 irradiated (20 Gy) allogeneic spleen cells in 24-well plates (NUNC, Roskilde, Denmark). Cultures were incubated at 37°C and 5% CO2 in humidified air for 5 days. Target cells were prepared in 24-well plates by incubation of 2 × 106 spleen cells per well with 4 μg of Concanavalin A (Sigma-Aldrich) in 2 mL complete RPMI medium containing 10% heat-inactivated FBS (5SB0007; BioWhittaker) for 2 days and pulsed overnight with 10 μCi (0.37 MBq) of [3H]-thymidine. Effector cells were harvested, washed, and plated at various E:T ratios in 96-well round-bottom plates (NUNC) containing 5 × 103 radio-labeled target cells. After 4 hours of incubation at 37°C and 5% C02, cultures were harvested on Unifilter plate and residual radioactivity was measured on a microplate beta counter (Topcount; Packard Instrument, Meriden, CT).
Skin transplantation
Skin grafts were prepared from tails of sex-matched mice and grafted onto the flanks of the recipients as previously described.31 Petroleum gauze was placed over the grafts, and sticking plaster was applied around the trunk. The bandages were removed after 7 days, and the grafts were monitored daily until day 30. Skins were considered rejected when complete epithelial breakdown had occurred. For histologic analysis, tissue sections (5 μm) of unboned feet were stained with hematoxylin and eosin after fixation in 10% neutral formalin solution and paraffin embedding.
PCR detection of the lpr/lpr mutation
Inguinal, popliteal, or mesenteric LNs of DC-primed mice were frozen in liquid nitrogen after collection. For the lpr genotype of lpr/lpr-bm12 mice, tail pieces were digested by proteinase K (Sigma-Aldrich). DNA was extracted using the NucleoSpin Tissue kit (Macherey-Nagel, Düren, Germany). PCR amplification of DNA was carried out with forward and reverse specific primers (Life Technologies, Paisley, United Kingdom). Briefly, PCR was performed on a Biometra thermocycler (Clonetech, Westburg) as follows:(a) denaturation, 4 minutes at 94°C; (b)amplification, 27 cycles for β-actin and 35 for the lpr mutation and wild-type gene, 30 seconds at 94°C; 20 seconds at 55°C for β-actin; and 20 seconds at 58°C for the lpr mutation and WT gene, 30 seconds at 72°C; and (c) extension, 10 minutes at 72°C. Of each sample, 15 microliters were run on a 2% agarose gel stained with ethidium bromide. For semiquantitative PCR, DNA bands were digitalized under UV and quantified with Multi-Analyst PC software (Bio-Rad Laboratories, Hercules, CA). DNA levels were normalized against β-actin and expressed as a ratio of lpr/β actin. To differentiate heterozygous and homozygous lpr/lpr mice, PCR amplification of DNA was carried out with 2 couples of primers (Life Technologies). For detection of the lpr mutation (retroviral insertion in the CD95 gene), forward: AAGCCGTGCCCTAGGAAACA (upstream of the insert); reverse: AGCAGCTCGCAACGTGAACG (in the retrovirus insert), the expected fragment size was 359 bp. For detection of the wild-type gene: forward: AAGCCGTGCCCTAGGAAACA (upstream of the insert); reverse: AGTAATGGGCTCAGTGCAGC (downstream of the insert), the expected fragment size was 195 base pair (bp). Primers for the β-actin were: forward: TGGAATCCTGTGGCATCCATGAAAC; reverse: TAAAACGCAGCTCAGTAACAGTCCG; the expected fragment size was 349 bp.
Statistical analysis
Statistical analysis was performed using the 2-tailed Mann-Whitney nonparametric test and when indicated, the 2-tailed Student t test. Graft survival curves were compared by the log-rank test.
Results
DCs overexpressing CD95L function as killer DCs in vitro
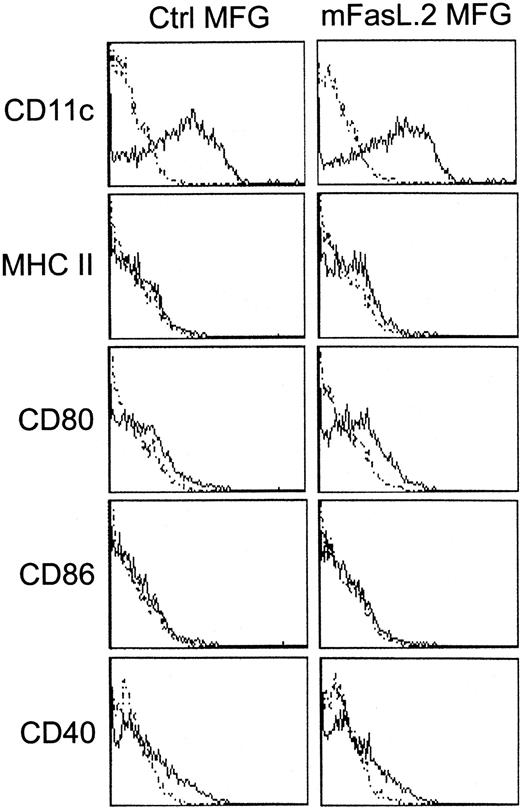
In a first set of experiments, DCs were generated from wild-type C57BL/6 BM-progenitors during a 10-day culture in the presence of mGM-CSF and were submitted to mFasL.2 MFG retroviral transduction. This resulted in massive cell death as more than 90% of BM cells were annexin V– and propidium iodide–positive 48 hours after the first CD95L transduction, compared with 5% after the control transduction. Suicidal or fratricidal death was most likely involved, since more than 85% viability of CD95L-transduced DCs was obtained at the end of the culture when CD95-deficient lpr/lpr mice were used as BM donors. After transduction of lpr/lpr BM progenitors with either mFasL.2 or control retrovirus and culture in granulocyte-macrophage colony-stimulating factor (GM-CSF), around 85% of the cells were CD11c+ GR1low DCs with an immature phenotype as indicated by low expression of MHC class II, CD80, CD86, and CD40 (Figure 1).
Phenotypic analysis of CD95L-transduced BM-derived DCs.
Lpr/lpr BM-derived DCs were analyzed by flow cytometry on day 10 of the culture with mGM-CSF. The solid-line histograms represent the surface expression of the indicated markers. Thin dotted histograms show either unstained controls, PE-streptavidin, or FITC-secondary mAbs staining. Lpr/lpr BM-derived DCs either were untreated or transduced with control or CD95L MFG retrovirus. Those flow cytometry analysis are representative of at least 3 experiments.
Phenotypic analysis of CD95L-transduced BM-derived DCs.
Lpr/lpr BM-derived DCs were analyzed by flow cytometry on day 10 of the culture with mGM-CSF. The solid-line histograms represent the surface expression of the indicated markers. Thin dotted histograms show either unstained controls, PE-streptavidin, or FITC-secondary mAbs staining. Lpr/lpr BM-derived DCs either were untreated or transduced with control or CD95L MFG retrovirus. Those flow cytometry analysis are representative of at least 3 experiments.
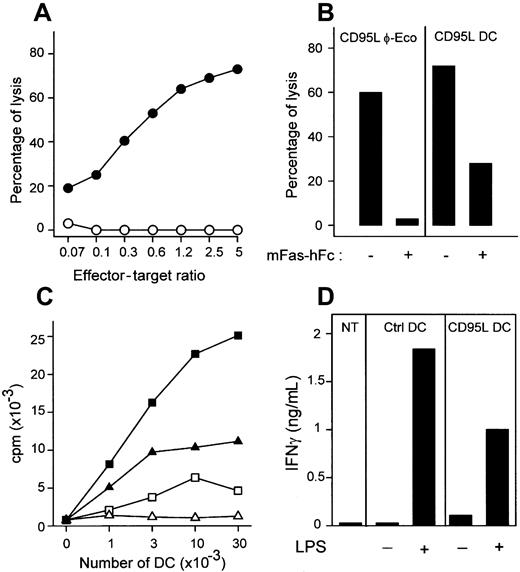
DCs transduced with CD95L induced a dose-dependent lysis of CD95+ cells that was dependent on CD95-CD95L interaction, since it was blocked by the addition of mFas-hFc fusion protein (Figure2). As expected from their immature phenotype, DCs transduced with control vector induced only low T-cell proliferation in mixed leukocyte culture, and this was further reduced when CD95L-DCs were used as stimulators. Whatever the retroviral vector used, immature DCs did not induce significant production of IFN-γ. As expected, both the proliferative response and the production of IFN-γ elicited by CD95L-DCs after maturation with LPS were lower than when control DCs were used as stimulators.
CD95L-transduced DCs are cytotoxic and down-regulate allogeneic MLC in vitro.
(A) mCD95-transfected P815 cells were incubated with CD95L-DCs (●) or control DCs (○). Data are representative of 15 experiments. (B) CD95-P815 cells were cocultured with CD95L-DCs (right panel) or CD95L-transfected PhoenixECO cells (left panel) at an effector/target cell ratio of 2.5:1, in the presence or not of mFas-hFc. Percentages of lysis are representative of 2 experiments. (C) Triplicate culture of 2 × 105 bm12 LN cells and lpr/lpr CD95L (without LPS, ▵; with LPS, ▴) or control DCs (without LPS, ■; with LPS, ▪) was incubated for 3 days. Results are representative of 3 experiments. (D) 106 BALB/c LN cells were seeded with 3 × 105 irradiated C57BL/6 lpr/lpr CD95L or control DCs activated or not with LPS. Supernatants were collected after 72 hours for IFN-γ quantification. Similar data were obtained in 3 experiments.
CD95L-transduced DCs are cytotoxic and down-regulate allogeneic MLC in vitro.
(A) mCD95-transfected P815 cells were incubated with CD95L-DCs (●) or control DCs (○). Data are representative of 15 experiments. (B) CD95-P815 cells were cocultured with CD95L-DCs (right panel) or CD95L-transfected PhoenixECO cells (left panel) at an effector/target cell ratio of 2.5:1, in the presence or not of mFas-hFc. Percentages of lysis are representative of 2 experiments. (C) Triplicate culture of 2 × 105 bm12 LN cells and lpr/lpr CD95L (without LPS, ▵; with LPS, ▴) or control DCs (without LPS, ■; with LPS, ▪) was incubated for 3 days. Results are representative of 3 experiments. (D) 106 BALB/c LN cells were seeded with 3 × 105 irradiated C57BL/6 lpr/lpr CD95L or control DCs activated or not with LPS. Supernatants were collected after 72 hours for IFN-γ quantification. Similar data were obtained in 3 experiments.
DCs overexpressing CD95L induce vigorous TH1 and CTL responses in vivo and prime for acute allograft rejection
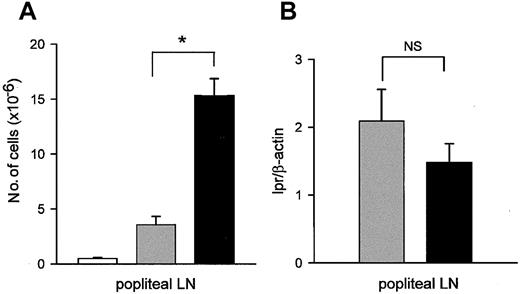
The next series of experiments were designed to determine whether lpr/lpr CD95L-DCs would inhibit alloreactive responses in vivo. We first observed that injection of CD95L-DC in the footpad of bm12 mice was followed by swelling of the draining popliteal LNs with a significant increase in cellularity as compared to LN draining at the site of injection of control DCs (Figure3A). In parallel, we assessed the presence of DCs in LNs using a semiquantitative PCR for the lpr/lpr mutation. As shown in Figure 3B, similar levels of donor-type DNA were found after injection of CD95L-DCs or control DCs, suggesting that CD95L overexpression did not influence DC migration.
CD95L-DCs induce strong T-cell proliferation in vivo.
(A) bm12 mice received one footpad injection of 106 lpr/lpr DCs transduced with CD95L (black bars) or control retrovirus (gray bars), or were untreated (white bars). The total number of popliteal LN cells was determined 5 days later. Results were expressed as mean number of cells ± SEM (*P < .02). (B) bm12 mice were injected with 106 lpr/lpr CD95L (black bar) or control DCs (gray bar). DNA was extracted from popliteal LNs 5 days later, and level of lpr mutation was measured by PCR. Results are expressed as mean of lpr versus β-actin signals ± SEM. Each group contains 4 individual mice. NS indicates not significant.
CD95L-DCs induce strong T-cell proliferation in vivo.
(A) bm12 mice received one footpad injection of 106 lpr/lpr DCs transduced with CD95L (black bars) or control retrovirus (gray bars), or were untreated (white bars). The total number of popliteal LN cells was determined 5 days later. Results were expressed as mean number of cells ± SEM (*P < .02). (B) bm12 mice were injected with 106 lpr/lpr CD95L (black bar) or control DCs (gray bar). DNA was extracted from popliteal LNs 5 days later, and level of lpr mutation was measured by PCR. Results are expressed as mean of lpr versus β-actin signals ± SEM. Each group contains 4 individual mice. NS indicates not significant.
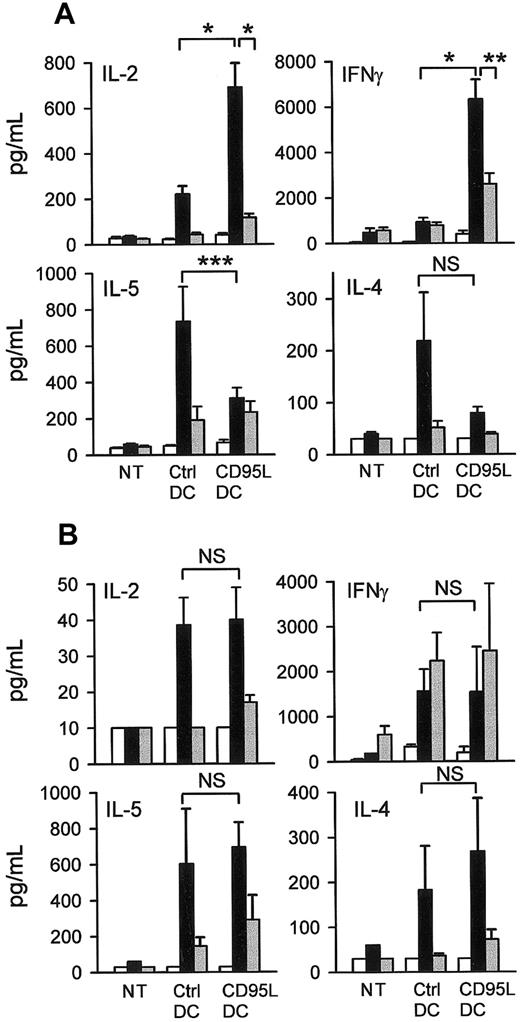
To characterize the T-cell responses induced in vivo by C57BL/6 lpr/lpr CD95L-DCs or control DCs, MLCs were prepared between responder LN T cells from bm12 (MHC class II mismatch) or bm13 (MHC class I mismatch) mice inoculated with the transduced DCs and donor-type or third-party splenocytes as stimulators. Consistent with previous studies,32 33 the response elicited by control DCs in MHC class II incompatible mice was TH2 skewed as indicated by a high production of IL-4 and IL-5 and a low production of IL-2 and IFN-γ (Figure 4A). In contrast, the in vivo response to CD95L-DC injection in the same strain combination was characterized by a dominant induction of IL-2 and IFN-γ and a low production of IL-5 and IL-4 (Figure 4A). This TH1-skewed response elicited by CD95L-DCs was specific for the donor alloantigen, as it was not observed with third-party stimulators. The absence of TH1-type cytokine hyperproduction induced by CD95L-DCs in CD95-deficient lpr/lpr bm12 recipients confirmed that this TH1-skewed response was dependent on CD95-CD95L interactions (Figure 4B).
CD95L-DCs promote a TH1 response in MHC class II–disparate mice.
CD95L-DCs exacerbated TH1 cytokines production in bm12 but not in lpr/lpr bm12 mice. 5 days after the subcutaneous footpad injection of 3 × 105 CD95L or control DCs draining popliteal and inguinal LN cells from bm12 (A) or lpr/lpr bm12 mice (B) were cultivated with bm12 (syngeneic, white bars), C57BL/6 lpr/lpr (allogeneic, black bars), or BALB/c (third-party, gray bars) spleen cells for 3 days. Supernatants were collected after 24 hours for IL-2 measurement and after 72 hours for IL-4, IL-5, and IFN-γ quantification. Results were expressed as mean ± SEM of 11 to 21 mice per groups of bm12 mice (*P ≤ .0001; **P = .0015; *** P = .03) and 3 mice per groups of lpr/lpr bm12 mice (NS: not significant with a 2-tailed Student t test). NT indicates nontreated mice.
CD95L-DCs promote a TH1 response in MHC class II–disparate mice.
CD95L-DCs exacerbated TH1 cytokines production in bm12 but not in lpr/lpr bm12 mice. 5 days after the subcutaneous footpad injection of 3 × 105 CD95L or control DCs draining popliteal and inguinal LN cells from bm12 (A) or lpr/lpr bm12 mice (B) were cultivated with bm12 (syngeneic, white bars), C57BL/6 lpr/lpr (allogeneic, black bars), or BALB/c (third-party, gray bars) spleen cells for 3 days. Supernatants were collected after 24 hours for IL-2 measurement and after 72 hours for IL-4, IL-5, and IFN-γ quantification. Results were expressed as mean ± SEM of 11 to 21 mice per groups of bm12 mice (*P ≤ .0001; **P = .0015; *** P = .03) and 3 mice per groups of lpr/lpr bm12 mice (NS: not significant with a 2-tailed Student t test). NT indicates nontreated mice.
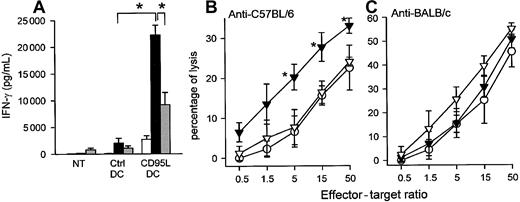
When similar experiments were repeated in MHC class I incompatible bm13 mice as recipients, we found that the injection of CD95L-DC significantly enhanced the production of IFN-γ by donor-reactive T cells as compared with the injection of control DCs. In this strain combination, neither IL-4 nor IL-5 were detectable in the MLC supernatants whatever the DC injected. Importantly, the injection of CD95L-DCs but not control DCs significantly increased the donor-specific CTL activity generated by LN T cells in MLCs (Figure5). As control, we found that the anti–third-party CTL response was not modified by the injection of CD95L-DCs. As the single MHC class I incompatibility in the (C57Bl/6 → bm13) strain combination is not sufficient to trigger skin allograft rejection, this strain combination was adequate to assess the priming effect of CD95L-DCs in a transplantation setting. As shown in Figure 6, a single footpad inoculation of as few as 3 × 105 C57Bl/6 lpr/lpr CD95L-DCs was sufficient to prime bm13 mice for acute rejection of C57Bl/6 skin allograft, whereas DCs transduced with a control vector was not so.
CD95L-DCs induced increased allospecific TH1-type response and cytotoxic activity in an MHC class I disparate model.
(A) Four to 6 bm13 mice received 3 × 105 lpr/lpr control or CD95L-DCs in footpad. MLCs were performed 5 days later with draining LN cells and either syngeneic (bm13; white bars), allogeneic (lpr/lpr; black bars), or third-party (BALB/c; gray bars) spleen cells. Results were expressed as mean ± SEM (*P < .03). Similar results were obtained in 2 experiments. NT indicates nontreated mice. (B) Donor-type– (left panel) or third-party– (right panel) specific CTL activity was evaluated after the same control (○) or CD95L (▴) DC treatment. ▵ represent CD95L-DCs with RB6-8C5 (anti-Gr1) mAbs treatment. Results are mean percentage of lysis ± SEM of 3 to 4 individual mice per group (*P < .02 compared with the control-DC group and *P < .004 compared with anti-GR1–treated group with the 2-tailed Student ttest). Similar results were obtained in 3 separate experiments.
CD95L-DCs induced increased allospecific TH1-type response and cytotoxic activity in an MHC class I disparate model.
(A) Four to 6 bm13 mice received 3 × 105 lpr/lpr control or CD95L-DCs in footpad. MLCs were performed 5 days later with draining LN cells and either syngeneic (bm13; white bars), allogeneic (lpr/lpr; black bars), or third-party (BALB/c; gray bars) spleen cells. Results were expressed as mean ± SEM (*P < .03). Similar results were obtained in 2 experiments. NT indicates nontreated mice. (B) Donor-type– (left panel) or third-party– (right panel) specific CTL activity was evaluated after the same control (○) or CD95L (▴) DC treatment. ▵ represent CD95L-DCs with RB6-8C5 (anti-Gr1) mAbs treatment. Results are mean percentage of lysis ± SEM of 3 to 4 individual mice per group (*P < .02 compared with the control-DC group and *P < .004 compared with anti-GR1–treated group with the 2-tailed Student ttest). Similar results were obtained in 3 separate experiments.
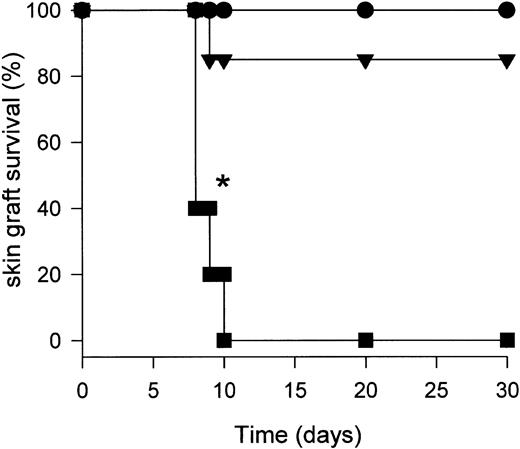
CD95L-DCs induce skin allograft rejection.
Ten bm13 mice were inoculated subcutaneously into the footpad with either 3 × 105 control (▴), CD95L (▪) DCs or were untreated (●). After 5 days, C57BL/6 skin allografts were performed and graft survival was monitored daily (*P < .001 for CD95L-DC–treated mice compared with control-DC injected or untreated mice).
CD95L-DCs induce skin allograft rejection.
Ten bm13 mice were inoculated subcutaneously into the footpad with either 3 × 105 control (▴), CD95L (▪) DCs or were untreated (●). After 5 days, C57BL/6 skin allografts were performed and graft survival was monitored daily (*P < .001 for CD95L-DC–treated mice compared with control-DC injected or untreated mice).
Neutrophils infiltrate sites of CD95L-DC injection
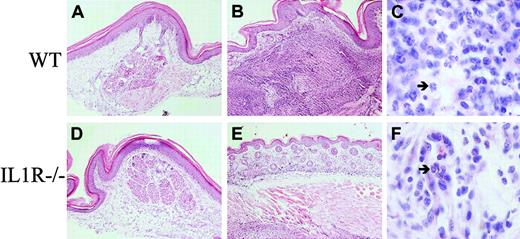
CD95L was reported as a potent chemoattractant of neutrophils in vitro and in vivo.34,35 Indeed, histologic examination of footpads 5 days after injection of CD95L-DCs revealed a major thickening of the dermis, which was massively infiltrated with neutrophils (Figure 7B-C). A highly invasive neutrophil infiltration also was observed in the underneath muscle cells layer. The injection of control DCs did not modify the skin structure and resulted only in a minor mononuclear cell infiltration in the dermis (Figure 7A). A dominant neutrophil recruitment also was observed in peritoneal cavities of bm12 mice injected 16 hours before with lpr/lpr CD95L-DC, as revealed by flow cytometry (Figure 8A) and May-Grünwald-Giemsa staining (Figure 8B, panels i-ii). In mice injected with CD95L-DCs, 67% ± 10.7% (mean ± SEM, n = 8) of the peritoneal exudate cells were Gr1+CD11b+neutrophils with characteristic nuclear morphology (Figure 8B, panel i), whereas only 2.5% ± 2.1% of these cells were found in mice injected with control DCs (n = 6). The neutrophil recruitment induced by CD95L-DCs was strictly dependent on CD95-CD95L interactions as it was not observed after injection in CD95-deficient lpr/lpr bm12 mice (Figure 8A). In agreement with previous reports,36 37 we found that the neutrophil influx triggered by injection of CD95L-DCs in footpads was dramatically reduced in IL-1R−/− mice. In those animals, the only change in the dermis consisted in a moderate infiltrate of mononuclear cells and eosinophils (Figure 7E-F). In parallel, we observed that the influx of GR1+CD11b+ neutrophils after intraperitoneal injection of CD95L-DCs was drastically decreased in IL1-R−/− mice (mean ± SEM of GR1+ CD11b+ cells: 22% ± 7% in IL1R−/− mice versus 67% ± 10% in C57BL/6 wild-type mice).
Neutrophilic infiltration at the footpad injection site of CD95L-DC injection is IL-1 dependent.
C57BL/6 WT (A-C) and IL-1 R−/− (D-F) mice were injected subcutaneously with 3 × 105 bm12-lpr control (A, D) or CD95L-DCs (B-F). Footpad skin sections were realized 5 days later and stained with hematoxylin and eosin. Panels A and D show the histology of a conserved skin structure with negligible cell infiltration (original magnification × 100); panel B, an altered skin structure with dermis thickening and strong inflammation extended until the muscle cells layer (original magnification × 100); and panel C, a massive neutrophil recruitment (arrow) into the dermis (original magnification × 1000). Panel E shows a conserved skin histology with much less inflammatory cells (original magnification × 100), and panel F, mononuclear cells plus eosinophils (arrow) infiltrate located only into the dermis (original magnification × 1000). Representative sections from 3 to 6 individual mice were selected.
Neutrophilic infiltration at the footpad injection site of CD95L-DC injection is IL-1 dependent.
C57BL/6 WT (A-C) and IL-1 R−/− (D-F) mice were injected subcutaneously with 3 × 105 bm12-lpr control (A, D) or CD95L-DCs (B-F). Footpad skin sections were realized 5 days later and stained with hematoxylin and eosin. Panels A and D show the histology of a conserved skin structure with negligible cell infiltration (original magnification × 100); panel B, an altered skin structure with dermis thickening and strong inflammation extended until the muscle cells layer (original magnification × 100); and panel C, a massive neutrophil recruitment (arrow) into the dermis (original magnification × 1000). Panel E shows a conserved skin histology with much less inflammatory cells (original magnification × 100), and panel F, mononuclear cells plus eosinophils (arrow) infiltrate located only into the dermis (original magnification × 1000). Representative sections from 3 to 6 individual mice were selected.
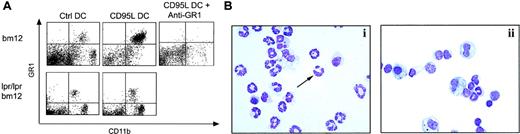
Intraperitoneal neutrophil recruitment by CD95L-DCs.
(A) Bm12 or lpr/lpr bm12 mice were injected intraperitoneally with either 8 × 105 control or CD95L-DCs. PECs were analyzed 16 hours later by flow cytometry. 200 μg of RB6-8C5 (anti-Gr1) mAbs were inoculated 4 hours before CD95L-DC treatment. (B) PEC cytospin preparation from CD95L-DC– (panel i) or control DC– (panel ii) injected mice were stained with May-Grünwald-Giemsa solution to identify polymorphonuclear leukocytes (arrow). Original magnification, × 400. Experiments were repeated at least 5 times with similar results.
Intraperitoneal neutrophil recruitment by CD95L-DCs.
(A) Bm12 or lpr/lpr bm12 mice were injected intraperitoneally with either 8 × 105 control or CD95L-DCs. PECs were analyzed 16 hours later by flow cytometry. 200 μg of RB6-8C5 (anti-Gr1) mAbs were inoculated 4 hours before CD95L-DC treatment. (B) PEC cytospin preparation from CD95L-DC– (panel i) or control DC– (panel ii) injected mice were stained with May-Grünwald-Giemsa solution to identify polymorphonuclear leukocytes (arrow). Original magnification, × 400. Experiments were repeated at least 5 times with similar results.
Involvement of neutrophils in the induction of allospecific TH1 and CTL responses by CD95L-DC
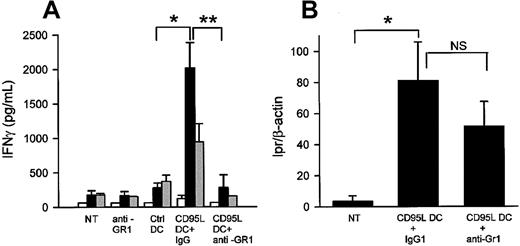
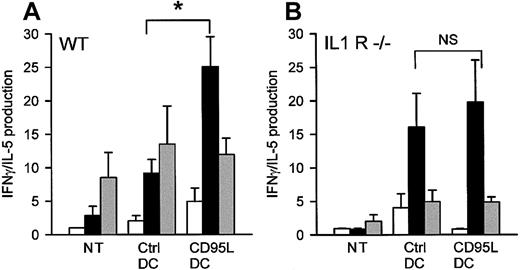
To investigate the role of neutrophils in the allospecific T-cell responses induced by CD95L-DCs, we depleted neutrophils by injection of the RB6-8C5 mAb specific for Gr1.38 39 As shown in Figure8A, mice treated with RB6-8C5 mAb were indeed free of Gr1+CD11b+ neutrophils in the peritoneal cavity after intraperitoneal injection of CD95L-DCs. We then analyzed the helper T-cell responses induced in bm12 mice by intraperitoneal inoculation of CD95L-DCs after injection of either 250 μg of RB6-8C5 or the same amount of control rat IgG mAb given on days −1, 0, 1, 2, and 3. MLCs were performed with mesenteric T cells 5 days after DC injection. As shown in Figure 9A, the hyperproduction of IFN-γ elicited by the injection of CD95L-DCs was abolished by previous neutrophil depletion but not by injection of the control rat mAb. This was not related to impaired DC migration, as the levels of lpr mutation detected in the mesenteric LNs after CD95L-DC injection were not affected by neutrophil depletion. We also evaluated the role played by neutrophils in the increased MHC class I–specific cytotoxic activity elicited by C57BL/6 lpr/lpr CD95L-DC in bm13 mice. As shown in Figure 5B, neutrophil depletion reduced the cytotoxic activity to the level observed after injection of control DCs. Furthermore, when we analyzed the cytokine profile of donor-reactive T cells in draining LNs after subcutaneous injection of lpr/lpr bm12 DCs into C57BL/6 mice, we found an increased IFN-γ/IL-5 production ratio in wild-type but not in IL1-R−/− mice, a finding consistent with the involvement of neutrophils in the TH1 skewing of the response induced by CD95L-DCs (Figure10).
Involvement of neutrophils in the allospecific TH1 response in MHC class II disparate mice.
(A) The anti-Gr1 neutrophil depletion inhibits the CD95L-dependent allospecific TH1 response. Bm12 mice were intraperitoneally injected with 5 × 105 lpr/lpr control or CD95L DCs and treated or not treated (NT) with control IgG or RB6-8C5 mAbs. After 5 days mesenteric LN cells were cultivated with syngeneic bm12 (white bars), allogeneic lpr/lpr (black bars), or third-party BALB/c (gray bars) spleen cells for 3 days. Supernatants were collected after 72 hours for IFN-γ quantification. Each group contains 3 to 7 individual mice. Results were expressed as mean ± SEM (*P = .0025; **P = .036). (B) Anti-GR1 mAbs treatment do not deplete the injected DC. DNA of mesenteric LNs was extracted, and the lpr/lpr mutation was detected by semiquantitative PCR. Results are means of lpr versus β-actin signals ± SEM of 4 mice/group (*P = .02). NS indicates not significant.
Involvement of neutrophils in the allospecific TH1 response in MHC class II disparate mice.
(A) The anti-Gr1 neutrophil depletion inhibits the CD95L-dependent allospecific TH1 response. Bm12 mice were intraperitoneally injected with 5 × 105 lpr/lpr control or CD95L DCs and treated or not treated (NT) with control IgG or RB6-8C5 mAbs. After 5 days mesenteric LN cells were cultivated with syngeneic bm12 (white bars), allogeneic lpr/lpr (black bars), or third-party BALB/c (gray bars) spleen cells for 3 days. Supernatants were collected after 72 hours for IFN-γ quantification. Each group contains 3 to 7 individual mice. Results were expressed as mean ± SEM (*P = .0025; **P = .036). (B) Anti-GR1 mAbs treatment do not deplete the injected DC. DNA of mesenteric LNs was extracted, and the lpr/lpr mutation was detected by semiquantitative PCR. Results are means of lpr versus β-actin signals ± SEM of 4 mice/group (*P = .02). NS indicates not significant.
TH1 versus TH2 response is not increased by CD95L-DCs in IL1R−/− mice.
3 × 105 lpr/lpr bm12 CD95L or control DCs were inoculated subcutaneously into the footpad of C57BL/6 wild-type (WT) or C57BL/6 IL-1R−/− mice. Five days later, draining LN cells were cocultured with syngeneic (C57BL/6; white bars), allogeneic (lpr/lpr bm12; black bars), or third-party (BALB/c; gray bars) spleen cells for 3 days. Supernatants were collected after 72 hours for IFN-γ and IL-5 measurement. Results are expressed as mean of IFN-γ/IL-5 production ± SEM of 6 to 8 mice for the WT group and 4 to 7 mice for the IL-1R−/− group (*P < .02). NT indicates nontreated mice.
TH1 versus TH2 response is not increased by CD95L-DCs in IL1R−/− mice.
3 × 105 lpr/lpr bm12 CD95L or control DCs were inoculated subcutaneously into the footpad of C57BL/6 wild-type (WT) or C57BL/6 IL-1R−/− mice. Five days later, draining LN cells were cocultured with syngeneic (C57BL/6; white bars), allogeneic (lpr/lpr bm12; black bars), or third-party (BALB/c; gray bars) spleen cells for 3 days. Supernatants were collected after 72 hours for IFN-γ and IL-5 measurement. Results are expressed as mean of IFN-γ/IL-5 production ± SEM of 6 to 8 mice for the WT group and 4 to 7 mice for the IL-1R−/− group (*P < .02). NT indicates nontreated mice.
Discussion
Overall, the data presented in this paper indicated that, when resistant to CD95 engagement, DCs overexpressing CD95L induce vigorous TH1 type as well as cytotoxic T-cell responses that depend on neutrophils recruitment in vivo. In the transplantation setting this property of CD95L-DCs results in priming for allograft rejection instead of the anticipated tolerogenic effect.
Our observations contrasts with previous reports providing evidence that such CD95L-DCs are immunosuppressive.20,21 In these earlier studies, DCs transduced with the CD95L gene coexpress a functional CD95 that might predispose them to CD95-mediated apoptosis. Indeed, although unmanipulated DCs or transformed DCs were previously found to be resistant to CD95 engagement,40-42 we found that the overexpression of CD95L obtained by gene transfer at an early stage of DC differentiation promotes their apoptosis. The down-regulation of the T-cell responses observed in previous studies could therefore be related to indirect presentation of alloantigens derived from apoptotic cells by host immature DCs.43 This possible drawback was circumvented in our experiments by the use of CD95-deficient DCs. The first cells to be considered as targets for the injected CD95L-DCs are host T lymphocytes. Indeed, the consequences of CD95 engagement on T cells are not univocal. Depending on the activation status and on the naive versus memory phenotype of the T cells, they either undergo apoptosis or receive costimulatory and proliferation signals.3,44,45 CD95-mediated costimulation was reported to be effective in both CD4+ and CD8+ naive T cells46 and could therefore be involved in the capacity of CD95L-DCs to prime both helper T-cell responses against MHC class II and CTL responses against MHC class I alloantigens. However, CD95L overexpression did not enhance and actually inhibited the capacity of DCs to activate alloreactive T cells in vitro, suggesting that the allostimulatory potential of CD95L-DCs in vivo might depend on their action on other cell types than T cells.
Neutrophils are known to be recruited at sites of CD95L overexpression and to contribute to destruction of tumors and allografts overexpressing CD95L.47-50 It was demonstrated that the soluble form of CD95L has chemotactic ability for neutrophils in vitro,34 but the full-length transmembrane CD95L form appears as the predominant neutrophil chemoattractant in vivo.35 Caspase activation elicited by CD95 engagement was shown to be involved in the proinflammatory properties of CD95L by promoting the processing and secretion of IL-1β.37 We observed that CD95L-DCs indeed induced a major neutrophil influx at the site of their injection, either in the footpad or in the peritoneal cavity, and we confirmed that IL-1 mediates this phenomenon as it was dramatically reduced in IL-1 receptor–deficient mice. Furthermore, the reduced production of IFN-γ elicited by CD95L-DCs in the latter animals as well as in mice depleted of neutrophils by injection of anti–Gr1 mAb revealed that neutrophil recruitment is critically involved in the TH1 alloimmune response induced in vivo by DCs overexpressing CD95L. Neutrophils were previously found to promote TH1 polarization of CD4+ T-cell responses in experimental models of infection with Legionella pneumophilia51 or Toxoplasma gondii.52 Herein, we found that neutrophils also participate in the induction of CD8+ cytotoxic T-cell responses against MHC class I alloantigen expressed on CD95L-DCs. The influence of neutrophils on CD4+ and CD8+T-cell responses might be related to their release of TH1 polarizing factors such as IL-12 and IFN-γ, as well as chemokines active on TH1-type cells such as monokine induced by IFN-γ (MIG) and MIP-1α.52,53 It is also possible that neutrophils enhance T-cell responses in vivo by promoting maturation of the injected DCs via their production of TNF-α and IL-1β.54 Host DCs also might be activated by these mediators as well as by CD95 engagement41 and could contribute to the alloimmune response via the indirect pathway of antigen presentation. Thus, neutrophil influx appears as a critical factor governing the ultimate consequences of CD95L-CD95 interactions on T-cell responses. Interestingly, the expression of CD95L in immune privilege sites is often associated with the production of inhibitor of neutrophilic inflammation such as transforming growth factor-β (TGF-β).55,56 Likewise, Chen et al demonstrated that cotransfection of the TGF-β gene in tumor cells overexpressing CD95L was sufficient to facilitate tumor growth, whereas single transfectants expressing only CD95L were readily destroyed.57 The suppressive versus immunogenic properties of cells overexpressing CD95L might indeed depend of a number of factors, including the tissue microenvironment, the release of soluble CD95L, and the level of expression of the transgene.58 59
Because of their potent immunostimulatory properties in vivo, it is unlikely that CD95L-DCs will find applications in transplantation as initially proposed. We suggest instead to consider CD95L-DC as a possible tool to prime antitumor responses in cancer immunotherapy.
We thank Marie-Line Vanderhaeghen, Claude Habran, and Carlo Heirman for technical assistance; Philippe Saas for providing us transfectants; Olivier Denis for providing us the bm13 mice, and Sandrine Florquin for the IL-1R−/− mice.
V.F. is a research associate at the “Fonds National de la Recherche Scientifique.”
Prepublished online as Blood First Edition Paper, October 17, 2002; DOI 10.1182/blood-2002-07-2042.
Supported by the Fonds National de la Recherche Médicale of Belgium, a Pôle d'Attraction Inter-universitaire (PAI) of Belgium, and the Biotechnology Program of the European Union. S.B. is supported by the Fonds de la Recherche Industrielle et Agricole of Belgium.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Véronique Flamand, Laboratory of Experimental Immunology, Université Libre de Bruxelles-Campus Erasme, 808 route de Lennik, B-1070 Brussels, Belgium; e-mail:vflamand@ulb.ac.be.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal