Abstract
Laminins are αβγ heterotrimeric extracellular proteins that regulate cellular functions by adhesion to integrin and nonintegrin receptors. Laminins containing α4 and α5 chains are expressed in bone marrow, but their interactions with hematopoietic progenitors are unknown. We studied human bone marrow cell adhesion to laminin-10/11 (α5β1γ1/α5β2γ1), laminin-8 (α4β1γ1), laminin-1 (α1β1γ1), and fibronectin. About 35% to 40% of CD34+ and CD34+CD38− stem and progenitor cells adhered to laminin-10/11, and 45% to 50% adhered to fibronectin, whereas they adhered less to laminin-8 and laminin-1. Adhesion of CD34+CD38− cells to laminin-10/11 was maximal without integrin activation, whereas adhesion to other proteins was dependent on protein kinase C activation by 12-tetradecanoyl phorbol-13-acetate (TPA). Fluorescence-activated cell-sorting (FACS) analysis showed expression of integrin α6 chain on most CD34+ and CD34+CD38−cells. Integrin α6 and β1 chains were involved in binding of both cell fractions to laminin-10/11 and laminin-8. Laminin-10/11 was highly adhesive to lineage-committed myelomonocytic and erythroid progenitor cells and most lymphoid and myeloid cell lines studied, whereas laminin-8 was less adhesive. In functional assays, both laminin-8 and laminin-10/11 facilitated stromal-derived factor-1α (SDF-1α)–stimulated transmigration of CD34+ cells, by an integrin α6 receptor–mediated mechanism. In conclusion, we demonstrate laminin isoform–specific adhesive interactions with human bone marrow stem, progenitor, and more differentiated cells. The cell-adhesive laminins affected migration of hematopoietic progenitors, suggesting a physiologic role for laminins during hematopoiesis.
Introduction
Hematopoietic cell development occurs within the bone marrow microenvironment, where adhesive interactions between progenitor cells and their extracellular matrix ligands are essential for normal cell proliferation and differentiation and for maintenance of the hematopoietic stem cell.1 Binding of extracellular matrix ligands to cell surface adhesion receptors activates receptor-mediated signal transduction pathways and thereby regulates cellular functions. Evidence for convergence of intracellular signaling pathways initiated by ligand binding to growth factor and cell adhesion receptors indicates that cooperation between these 2 signaling pathways is essential for normal cell development and functions (reviewed by Levesque and Simmons2).
More than 20 different adhesion receptors have been identified on hematopoietic progenitors (reviewed by Verfaillie3). Of the receptors for extracellular matrix molecules, integrins are the most extensively studied. Integrins are dimeric proteins composed of α and β chains. The extracellular domain mediates cell-cell or cell–extracellular matrix interactions, and the cytoplasmic domain provides a link with cytoskeletal proteins and is involved in the transmission of intracellular signals. Different integrins have specific functions in intracellular signal transduction, activation of growth factor receptors, and association with other transmembrane proteins (reviewed by Giancotti and Ruoslahti4).
The role of fibronectin binding to integrins α4β1 and α5β1 in hematopoietic cell–matrix adhesive interactions is well established. Primitive long-term in vivo repopulating cells, multipotent-, and single-lineage clonogenic progenitors5-8 adhere to different domains of fibronectin. Adhesion to fibronectin or its cell-binding fragments stimulates proliferation and migration of stem and progenitor cells9,10 and proliferation of lineage-committed and differentiated cells.11-13 In agreement with the in vitro findings, studies with integrin α4 null chimeric mice have shown impaired embryonal and postnatal development of erythroid, myeloid, and B cells, most likely attributable to impaired cellular interactions of the α4β1 integrin receptor with fibronectin.14,15
Laminins are large extracellular matrix proteins that regulate survival, proliferation, differentiation, and specialized functions of several types of cells. All laminins are heterotrimeric proteins composed of α, β, and γ polypeptide chains. So far, 5 α (α1-5), 3 β (β1-3), and 3 γ (γ1-3) chain variants have been characterized in mammalians, and 15 different heterotrimers have been proposed (reviewed by Colognato and Yurchenco16). Expression of laminin isoforms shows a high degree of developmentally regulated and tissue-specific variation. In vitro studies, gene targeting experiments, and studies of mutated genes have indicated different functions for different laminins (reviewed by Timpl17). Major cell-binding domains of laminins are located in the carboxyterminus of α chains, and consequently the cell-binding activities of laminin isoforms are largely determined by the α chains. Several integrins (α1β1, α2β1, α3β1, α6β1, α6β4, α7β1, α9β1, αvβ3) bind to laminins but with variable binding affinities to different isoforms.18-22
Most studies on hematopoietic cell interactions with laminins have been performed by using laminin-1 (α1β1γ1), isolated from Engelbreth-Holm-Swarm (EHS) tumor, which can be transplanted into a mouse, or from placental laminin containing several laminin isoforms. Mature granulocytes,23,24lymphocytes,25 mononuclear phagocytes,26activated macrophages,27 and eosinophils28adhere to these laminins in vitro. Adhesion to these laminins influences survival and maturation of eosinophils29 and proliferation of macrophages and T lymphocytes.27,30 In contrast to mature blood cells, human bone marrow progenitor cells have not been found to adhere to laminin-1.31
Laminin α5 chain is found in most adult basement membranes, including endothelia.32,33 Laminin α4 chain is synthesized by endothelial and mesenchymal cells and is also expressed at specific sites of loose connective tissue.34 In the bone marrow, laminin α4 and α5 chains, assembled with β1 and γ1 chains to form laminin-8 (α4β1β1) and laminin-10 (α5β1γ1), are present in sinusoidal subendothelial basement membranes, and α4 laminins are in addition located in the intersinusoidal spaces. In contrast, laminin-1 has not been found in bone marrow in vivo,35,36 in agreement with its expression mainly in a subset of epithelial basement membranes.37,38
Because of their expression in bone marrow, the α4 and α5 laminins might affect hematopoietic cell development and functions. To define the role of different laminins for primitive and developing human hematopoietic cells, we isolated CD34+CD38−and CD34+ cell fractions and tested their adhesion to several laminin isoforms and fibronectin. This revealed that both stem and progenitor cells bound to laminin-10/11 nearly as efficiently as to fibronectin. The cells adhered also to laminin-8, although with a lower binding affinity than to laminin-10/11. Both these laminin isoforms promoted migration of CD34+ cells in vitro. The α6β1 integrin was identified as a major laminin receptor for both laminin-10/11 and laminin-8 in these cells. These findings suggest that the interactions with bone marrow laminins could be functionally important during stem and progenitor cell development.
Materials and methods
Reagents and antibodies
Recombinant laminin-8 was produced in a mammalian expression system by triple transfection of human laminin α4 and γ1 chains and mouse laminin β1 chain.21 Human laminin-10/11, purified by immunoaffinity column with monoclonal antibody 4C7 against laminin α5 chain,39 was from Gibco (Täby, Sweden). This laminin contains laminin-10 (α5β1γ1) and laminin-11 (α5β1γ2).40 However, based on amino acid sequencing, placental laminin-10/11 purified by 4C7 contains mainly laminin-10.41 Human plasma fibronectin and mouse laminin-1 were purchased from Gibco. Fluorochrome-conjugated antibodies against human CD34 (anti–HPCA-2, clone 8G12), CD38 (clone HB7), integrin α6 (GoH3), integrin α3 (C3 II.1), and the isotype standards were from Becton Dickinson (San Jose, CA). The MIKd 2 antibody against integrin α3 was from Cymbys Biotechnology (Hants, United Kingdom). Mouse antihuman integrin α3 antibody ASC-6 was from Chemicon (Temecula, CA). The purified GoH3 antibody was from Becton Dickinson and Immunotech (Marseille, France). Mouse antihuman integrin β1 antibody P4C10 was from Gibco. Rat immunoglobulin G (IgG) antibody 193 against mouse laminin α1 chain has been previously described.42
Cell lines
The human hematopoietic cell lines were grown in RPMI 1640 (Gibco) and 10% fetal calf serum (Gibco) in a humidified environment at 37°C and 5% CO2.
Isolation and analysis of bone marrow cell fractions
Bone marrow samples were obtained from healthy volunteers, after informed consent, using guidelines approved by the Ethical Committee, Lund University. Mononuclear cells were isolated by density gradient centrifugation (Ficoll-Paque; Pharmacia, Uppsala, Sweden). CD34+ cells were isolated by 2 passages through magnetic columns (MidiMacs; Miltenyi Biotec, Bergish Gladbach, Germany) by using a hapten-conjugated CD34 antibody (Qbend/10) and an antihapten antibody conjugated to magnetic beads (CD34+ isolation kit; Miltenyi Biotec). CD34 expression was analyzed by immunostaining with a FACSCalibur flow cytometer (Becton Dickinson) by using the CellQuest program (Becton Dickinson) and was usually more than 90%.
For isolation of CD34+CD38− and CD34+CD38+ cell fractions, CD34+cells were first enriched by magnetic columns and thereafter incubated with phycoerythrin (PE)–anti-CD38 antibody and fluorescein isothiocyanate (FITC)–anti-CD34 antibody or isotype-matched control antibodies. The cells were sorted with a FACS VantageSE flow cytometer (Becton Dickinson).43 A total of 3% to 5% of the cells with lowest CD38 expression (CD34+CD38−) and 10% of the cells with highest CD38 expression (CD34+CD38+) were isolated.
For analysis of expression of integrin α3 and α6 chains, CD34+ cells were enriched by the magnetic column and thereafter stained with FITC–anti-CD34, allophycocyanin (APC)–anti-CD38, PE-conjugated GoH3 antibody against integrin α6 chain or, alternatively, PE-conjugated antibody against integrin α3 chain, and corresponding isotype controls. The expression of integrin α3 and α6 chains in CD34+ and CD34+CD38− cells was analyzed by FACSCalibur (Becton Dickinson).
Cell adhesion assay
The 96-well nontissue culture plates (Sigma, Stockholm, Sweden) were coated overnight at 4°C with 50 μL extracellular matrix proteins diluted with phosphate-buffered saline (PBS) (Gibco). The proteins were used at 10 to 30 μg/mL. As negative controls, PBS was used for coating. Thereafter, the wells were washed 3 times with PBS and blocked with 2% heat-denatured fatty acid–free bovine serum albumin (BSA) (Sigma) in PBS for 1 hour in a humidified environment at 37°C and 5% CO2. The wells were washed twice with PBS and once with Iscove modified Dulbecco medium (IMDM) (BioWhittaker, Walkersville, MD). The cells were resuspended in IMDM and added to the wells in a volume of 50 to 100 μL per well. In some experiments, 300 ng/mL stromal-derived factor-1α (SDF-1α) (R&D Systems, Oxon, United Kingdom) was added to the cell suspension, or the cells were incubated with 5 ng/mL 12-tetradecanoyl phorbol-13-acetate (TPA) (Sigma) for 1 to 4 hours before incubation in the wells.
The cell-adhesion assay was performed at 37°C, 5% CO2 in humidified atmosphere for 1 hour. The nonadherent cells were detached by shaking the plate and by 1 to 3 washes with IMDM. The adherent cells were fixed with methanol for 10 minutes and thereafter stained with 0.1% Giemsa (Sigma) for 10 to 30 minutes. Thereafter, the plates were washed with large volumes of deionized water. The adherent cells from the entire bottom area of the wells were counted by using a Zeiss Axioskop2 microscope (Carl Zeiss Mikroscopie, Jena, Germany), and the percentage of adherent cells were counted as follows: (no. of adherent cells/no. of cells plated) × 100. In experiments using cell lines, the adherent cells were fixed with 96% ethanol for 10 minutes and stained with 0.1% crystal violet in water for 30 minutes. Thereafter, the plates were washed with deionized water, and adherent cells were lysed with 0.2% Triton X-100 (Sigma). Absorbance was measured at 595 nm with a DigiScan microplate reader by using DigWin software (Asys Hitech, Eugendorf, Austria). For antibody inhibition experiments, the antibodies (P4C10 at a dilution of 1:50, other antibodies at 25 μg/mL) were added to cell suspension and the cells were incubated at 37°C for 10 minutes before they were added to the wells. The role of divalent cations on cell adhesion was tested by using 10 mM EDTA (ethylenediaminetetraacetic acid).
Progenitor assays
The 96-well tissue culture plates (Falcon, Becton Dickinson) were coated with 10 μg/mL extracellular matrix proteins or PBS as a control, blocked with BSA, and washed as described in the adhesion assay. CD34+ cells were incubated in the wells as described above. Thereafter, the nonadherent cells were collected, and the adherent cells were detached with vigorous pipetting. The cells in adherent and nonadherent fractions were cultured in triplicate (600/mL) in 1 mL 0.8% methylcellulose culture medium containing cytokines (Methocult GF+ H4435; StemCell Technologies, Vancouver, BC, Canada). After 14 days of culture in a humidified environment at 37°C and 5% CO2, the colonies were counted by using an inverted microscope.
Cell migration assay
Transwell inserts with 5 μm pore size (Costar, Cambridge, MA) were coated with extracellular matrix proteins, blocked with BSA, and washed as described for the adhesion assay. For control, the inserts were coated with PBS instead of protein solutions. The cells (200 000 cells in 100 μL per well) were resuspended in migration buffer (IMDM, 0.2% BSA) and added into the upper chambers; 0.6 mL migration buffer containing 100 ng/mL SDF-1α (R&D Systems) was added to the bottom chambers. After incubation for 4 hours at 37°C in a humidified environment containing 5% CO2, the cells from the lower chamber were collected, and adherent cells from the lower surface of the Transwell inserts and the lower chamber were collected after treatment with 0.6 mL trypsin. For antibody perturbation experiments, azide-free rat antihuman antibody GoH3 against integrin α6 chain (Immunotech) or control monoclonal rat IgG antibody was added to the cells in migration buffer, and the cells were incubated for 10 minutes at 37°C before they were added to the Transwell inserts.
Statistical analysis
Results are expressed as mean ± SD of triplicate assays or as indicated. Statistical significance was determined using the unpaired t test.
RNA isolation and RT-PCR
RNA from cells was isolated with Trizol reagent (Gibco). Complementary DNA synthesis was performed by using a cDNA synthesis kit (Gibco). The primers used for the integrin α6 cDNA PCR were 5′-ATCTCTCGCTCTTCTTTCCG-3′ and 5′-GACTCTTAACTGTAGCGTGA-3′, covering the alternatively spliced region present in integrin α6A but not in α6B mRNA.44 Amplified cDNA was analyzed on a 1% agarose.
Results
Expression of laminin receptors in stem and progenitor cells
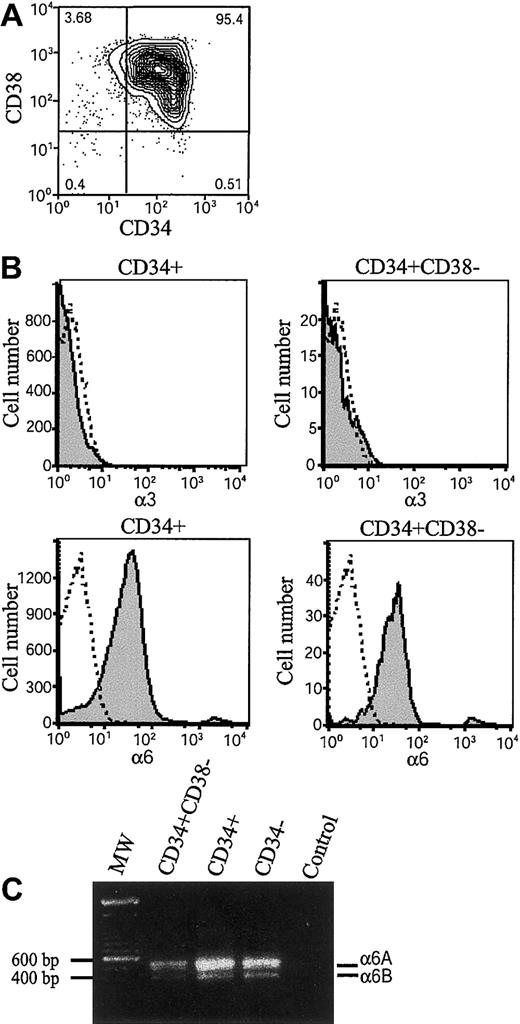
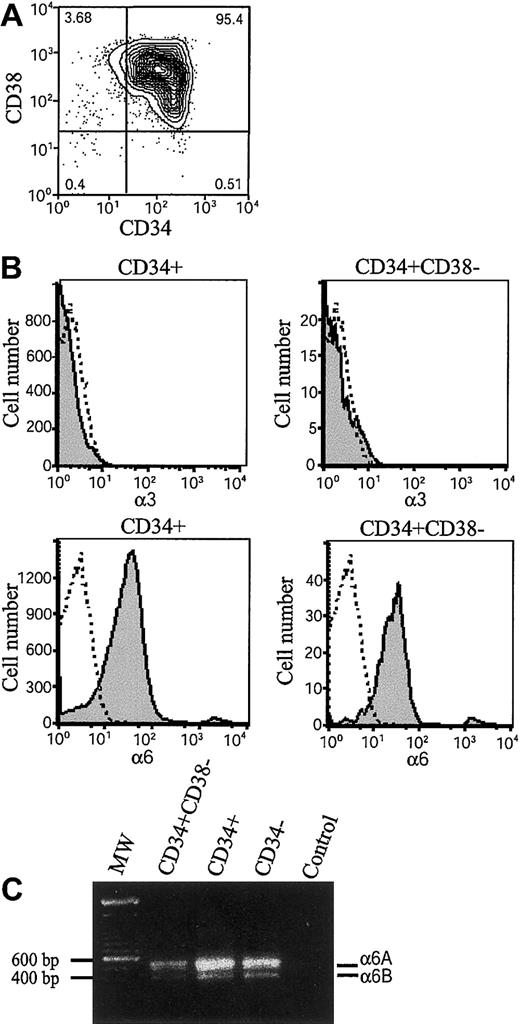
Integrins α6β1, α6β4, and α3β1 have been found to mediate cell adhesion to laminin-10/11 and laminin-8.20-22 35 The β1 integrin chain is ubiquitously expressed in hematopoietic cells, but expression of integrin α6 and α3 chains during early human hematopoiesis has been unclear. We therefore analyzed their expression in bone marrow stem and progenitor cells by flow cytometry (Figure 1). The CD34+ stem and progenitor cells were isolated by magnetic separation (Figure 1A). Immunostaining by using 2 different monoclonal antibodies C3 II.1 (Figure 1B) and MIKd 2 (data not shown) did not show expression of integrin α3 in CD34+ cells or CD34+CD38− cells. In contrast, integrin α6 chain was found in more than 80% of CD34+ cells and 90% of CD34+CD38− cells (Figure 1B), suggesting that integrin α6β1 receptor might mediate adhesion of bone marrow stem and progenitor cells to laminins. In agreement with the high expression of integrin α6 chain in CD34+ cells, more than 80% of CD34+CD38+ cells also expressed integrin α6 chain (not shown).
Expression of integrin α6 chain and integrin α6A and α6B mRNA splice variants in bone marrow stem and progenitor cells.
(A) Fluorescence-activated cell-sorting (FACS) analysis after immunostaining with anti-CD38 and anti-CD34 antibodies of CD34+-enriched bone marrow cells. The vertical and horizontal bars were set on the basis of isotype-matched negative control profiles (99.3% of cells negative). The numbers indicate percentages of cells in each gated area. The purity of CD34+-enriched cells is more than 95%. (B) CD34+ cells and CD34+CD38− cells were gated, and expression of integrin α3 and integrin α6 was studied by antibodies (C3II.1 and GoH3) against integrin α3 (α3) and α6 (α6) (shaded histograms). Immunostaining with isotype-matched control antibodies is shown as open histograms. Shown is 1 representative of 2 experiments. (C) RT-PCR analysis for integrin α6A and α6B mRNA splice variants of bone marrow CD34+CD38− cells, CD34+ cells, and mononuclear CD34− cells. MW indicates molecular weight markers showing positions of 600 bp and 500 bp markers on the gel; Control, PCR reaction without cDNA. The approximately 550 and 420 bp fragments corresponding to integrin α6A and α6B splice variants were seen in all 3 cell populations.
Expression of integrin α6 chain and integrin α6A and α6B mRNA splice variants in bone marrow stem and progenitor cells.
(A) Fluorescence-activated cell-sorting (FACS) analysis after immunostaining with anti-CD38 and anti-CD34 antibodies of CD34+-enriched bone marrow cells. The vertical and horizontal bars were set on the basis of isotype-matched negative control profiles (99.3% of cells negative). The numbers indicate percentages of cells in each gated area. The purity of CD34+-enriched cells is more than 95%. (B) CD34+ cells and CD34+CD38− cells were gated, and expression of integrin α3 and integrin α6 was studied by antibodies (C3II.1 and GoH3) against integrin α3 (α3) and α6 (α6) (shaded histograms). Immunostaining with isotype-matched control antibodies is shown as open histograms. Shown is 1 representative of 2 experiments. (C) RT-PCR analysis for integrin α6A and α6B mRNA splice variants of bone marrow CD34+CD38− cells, CD34+ cells, and mononuclear CD34− cells. MW indicates molecular weight markers showing positions of 600 bp and 500 bp markers on the gel; Control, PCR reaction without cDNA. The approximately 550 and 420 bp fragments corresponding to integrin α6A and α6B splice variants were seen in all 3 cell populations.
The integrin α6 polypeptide chain is expressed in 2 functionally different variants, α6A and α6B, with different cytoplasmic domains generated by alternative mRNA splicing.44 The expression of the integrin α6 mRNA splice variants was analyzed by reverse transcriptase–polymerase chain reaction (RT-PCR) by using primers covering the 130 base pair (bp) alternatively spliced segment. Both the 550 bp and 420-bp cDNA fragments corresponding to α6A and α6B mRNAs, with a higher expression of the larger splice variant, were detected in CD34+CD38−, CD34+, and CD34− fractions. (Figure 1C).
Adhesion of bone marrow stem and progenitor cells to laminin isoforms and fibronectin
We studied adhesion of bone marrow stem and progenitor cells to laminin-1, laminin-8, and laminin-10/11 and compared cell binding to fibronectin, the so far best characterized bone marrow extracellular adhesion protein. For these assays we isolated bone marrow CD34+ progenitor cells and CD34+CD38− cells, consisting of a highly enriched primitive stem and progenitor cell population.45Because integrin receptors can exist in different functional stages with low or high binding capacity for particular ligands, experiments were performed both without and after activation of integrins with the protein kinase C activator TPA, which rapidly up-regulates integrin-ligand binding affinity.46
About 35% to 40% of CD34+ cells and CD34+CD38− cells adhered to laminin-10/11 (Figure 2A,B). Adhesion of both stem and progenitor cell fractions to laminin-10/11 was almost as high as to fibronectin. Adhesion of CD34+CD38− cells to laminin-10/11 was maximal without treatment with TPA, indicating steady-state receptor activation in these cells. In contrast, adhesion of CD34+CD38− cells to fibronectin and laminin-8 and adhesion of CD34+ cells to all studied proteins was dependent on protein kinase C activation, as shown by greatly enhanced cell adhesion after TPA treatment. Laminin-8 and laminin-1 were less adhesive substrates to CD34+ cells than laminin-10/11, indicating isoform-specific differences in the cell-adhesive interactions of laminins with hematopoietic stem and progenitor cells. However, about 25% of CD34+CD38− cells were adhesive to laminin-8, and adhesion of CD34+ cells to laminin-8 also clearly exceeded adhesion to PBS-coated wells, suggesting physiologically significant interactions of bone marrow stem and progenitor cells with both laminin-10/11 and laminin-8.
Adhesion of bone marrow stem and progenitors cells to laminin isoforms and fibronectin.
Adhesion of human bone marrow CD34+ cells (A,D), CD34+CD38− cells (B), and CD34+CD38+ cells (C) to fibronectin (FN), laminin-1 (LN-1), laminin-8 (LN-8), and laminin-10/11 (LN-10/11). PBS indicates cell adhesion to wells coated with PBS instead of proteins; light columns, cell adhesion without treatment with TPA (n = 9); dark columns, cell adhesion after 1 hour of treatment with TPA (PBS, n = 6; other columns, n = 9; values are shown as means ± SDs); asterisks, a significant difference from the corresponding control value (percentage of adherent cells in wells coated with PBS); ns, not significant; *P < .05; **P < .01; ***P < .001. (D) CD34+ cell adhesion to wells coated with 10 μg/mL and 30 μg/mL proteins. The difference in cell adhesion to each protein at 10 μg/mL and 30μg/mL was not significant (P > .05).
Adhesion of bone marrow stem and progenitors cells to laminin isoforms and fibronectin.
Adhesion of human bone marrow CD34+ cells (A,D), CD34+CD38− cells (B), and CD34+CD38+ cells (C) to fibronectin (FN), laminin-1 (LN-1), laminin-8 (LN-8), and laminin-10/11 (LN-10/11). PBS indicates cell adhesion to wells coated with PBS instead of proteins; light columns, cell adhesion without treatment with TPA (n = 9); dark columns, cell adhesion after 1 hour of treatment with TPA (PBS, n = 6; other columns, n = 9; values are shown as means ± SDs); asterisks, a significant difference from the corresponding control value (percentage of adherent cells in wells coated with PBS); ns, not significant; *P < .05; **P < .01; ***P < .001. (D) CD34+ cell adhesion to wells coated with 10 μg/mL and 30 μg/mL proteins. The difference in cell adhesion to each protein at 10 μg/mL and 30μg/mL was not significant (P > .05).
Of the CD34+ cells with high expression of CD38 (CD34+- CD38+), consisting of a more mature progenitor cell population than the CD34+CD38−cells, approximately 35% adhered to laminin-10/11 and fibronectin after cell activation with TPA (Figure 2C). Less than 20% of CD34+CD38+ cells adhered to laminin-8.
Receptors involved in adhesion to laminins
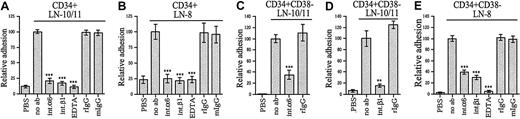
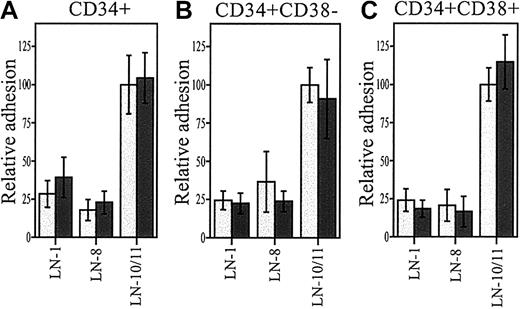
We used function-blocking monoclonal antibodies to analyze the role of integrin α6 and β1 chains in adhesion of stem and progenitor cells to α4 and α5 laminins (Figure3). TPA-induced adhesion of the CD34+ cells to laminin-10/11 and laminin-8 was completely or largely inhibited by antibodies against integrin α6 and β1 chains (Figure 3A,B). Adhesion of CD34+CD38−cells to laminin-10/11 was largely inhibited by an antibody against integrin α6 chain (Figure 3C) and almost completely inhibited by an antibody against integrin β1 chain (Figure 3D). Adhesion of CD34+CD38− cells to laminin-8, studied after TPA treatment (Figure 3E), was also partially inhibited by antibodies against integrin α6 and integrin β1 chains. Binding was fully inhibited with EDTA, indicating that adhesion is dependent on the presence of divalent cations. Hence, although also other laminin receptors might be involved, integrin α6β1 is a ubiquitous receptor for laminin-8 and laminin-10/11 in most CD34+ and CD34+CD38− cells.
Inhibition of adhesion of bone marrow stem and progenitor cells to laminin-10/11 and laminin-8 by monoclonal antibodies against integrin α6 and β1 chains.
Adhesion of CD34+ cells (A,B) and CD34+CD38− cells (C-E) to laminin-10/11 (LN-10/11; A,C,D) and laminin-8 (LN-8; B,E). PBS indicates cell adhesion to wells coated with PBS instead of proteins. Adhesion assay was performed in the presence of antibodies GoH3 against integrin α6 and P4C10 against β1 chain (int.α6 and int.β1, respectively), isotype-matched control rat or mouse monoclonal antibodies (rIgG and mIgG, respectively), or 10 mmol EDTA. No ab indicates cell adhesion to laminin-10/11 or laminin-8 without antibody addition. The results are shown as percentages of adhesion to laminin-10/11 or laminin-8 without antibody addition. There was a significant difference in cell adhesion in the presence of anti-integrin antibodies or EDTA compared with adhesion to laminins without antibody addition (**P < .01; ***P < .001). Shown are means of 1 experiment representative of 2 or more experiments performed in triplicate.
Inhibition of adhesion of bone marrow stem and progenitor cells to laminin-10/11 and laminin-8 by monoclonal antibodies against integrin α6 and β1 chains.
Adhesion of CD34+ cells (A,B) and CD34+CD38− cells (C-E) to laminin-10/11 (LN-10/11; A,C,D) and laminin-8 (LN-8; B,E). PBS indicates cell adhesion to wells coated with PBS instead of proteins. Adhesion assay was performed in the presence of antibodies GoH3 against integrin α6 and P4C10 against β1 chain (int.α6 and int.β1, respectively), isotype-matched control rat or mouse monoclonal antibodies (rIgG and mIgG, respectively), or 10 mmol EDTA. No ab indicates cell adhesion to laminin-10/11 or laminin-8 without antibody addition. The results are shown as percentages of adhesion to laminin-10/11 or laminin-8 without antibody addition. There was a significant difference in cell adhesion in the presence of anti-integrin antibodies or EDTA compared with adhesion to laminins without antibody addition (**P < .01; ***P < .001). Shown are means of 1 experiment representative of 2 or more experiments performed in triplicate.
Adhesion of bone marrow–committed progenitor cells to laminins and fibronectin
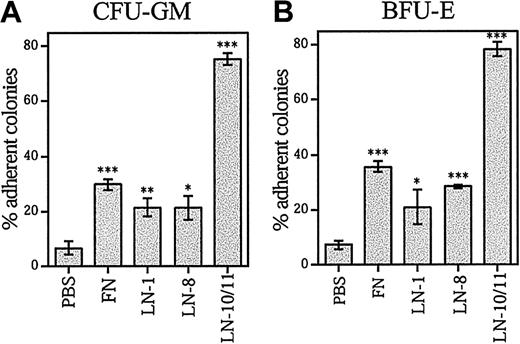
To analyze the adhesion of committed progenitors to laminins and fibronectin, adherent and nonadherent CD34+ cells were separately cultured in methylcellulose in the presence of cytokines. Most myeloid (granulocyte, macrophage colony-forming units [CFU-GMs]) (Figure 4A), erythroid (erythroid burst-forming units [BFU-Es]) (Figure 4B), and multipotent (granulocyte, erythroid, macrophage, megakaryocyte CFUs (CFU-GEMMs]) (data not shown) progenitors were adherent to laminin-10/11, whereas less than 40% of the myeloid and erythroid colony-forming cells (CFCs) were adherent to fibronectin and other laminin isoforms. This result shows that the hematopoietic progenitor cells efficiently adhere to laminin-10/11 without prior integrin activation, whereas they adhered less to laminin-1 and -8 or fibronectin.
Adhesion of clonogenic progenitor cells CFU-GM and BFU-E to laminins and fibronectin.
Bone marrow CD34+ cells were plated in 96-well plates coated with PBS, fibronectin (FN), laminin-1 (LN-1), laminin-8 (LN-8), or laminin-10/11 (LN-10/11). After incubation for 1 hour at a 37°C, 5% CO2, humidified environment, the nonadherent and adherent cells were collected separately and plated in methylcellulose progenitor assay. The percentages of adherent progenitors were calculated as follows: (no. of adherent CFCs/no. of adherent CFCs + no. of nonadherent CFCs) × 100. The total numbers of CFCs adherent to wells coated with PBS were 3672 ± 730, fibronectin 8664 ± 990, laminin-1 4690 ± 340, laminin-8 8408 ± 522, and laminin-10/11 22 553 ± 1635; the values are mean ± range of 2 measurements for laminin-8 and mean ± SD of 3 measurements for other proteins and PBS. Asterisks indicate a significant difference from the control value (percentage of adherent colonies in wells coated with PBS). *P < .05; **P < .01; ***P < .001.
Adhesion of clonogenic progenitor cells CFU-GM and BFU-E to laminins and fibronectin.
Bone marrow CD34+ cells were plated in 96-well plates coated with PBS, fibronectin (FN), laminin-1 (LN-1), laminin-8 (LN-8), or laminin-10/11 (LN-10/11). After incubation for 1 hour at a 37°C, 5% CO2, humidified environment, the nonadherent and adherent cells were collected separately and plated in methylcellulose progenitor assay. The percentages of adherent progenitors were calculated as follows: (no. of adherent CFCs/no. of adherent CFCs + no. of nonadherent CFCs) × 100. The total numbers of CFCs adherent to wells coated with PBS were 3672 ± 730, fibronectin 8664 ± 990, laminin-1 4690 ± 340, laminin-8 8408 ± 522, and laminin-10/11 22 553 ± 1635; the values are mean ± range of 2 measurements for laminin-8 and mean ± SD of 3 measurements for other proteins and PBS. Asterisks indicate a significant difference from the control value (percentage of adherent colonies in wells coated with PBS). *P < .05; **P < .01; ***P < .001.
Adhesion of hematopoietic cell lines to laminins and fibronectin
Hematopoietic cell lines of the B-lymphocytic (CO, BJAB, NALM/6, CA-46, DAUDI, DG-75, KM-3), plasma cell (LP-1), early myeloid (KG-1), erythroid-megakaryocytic (K562, HEL), promyelocyte (NB-4, HL-60) or monocyte-macrophage (Monomac, U-937) lineages were used in adhesion assays. The assays were performed without and after cell activation with TPA. Fourteen of the 15 cell lines were adhesive to fibronectin (Table 1), whereas adhesion to laminins was highly isoform-specific. Thirteen of the cell lines adhered to laminin-10/11. Only 4 cell lines adhered to laminin-8, and 2 adhered to laminin-1. Thus, laminin-10/11, like fibronectin, was a ubiquitous adhesive protein for differentiated precursors of both B-lymphocytic, erythroid, megakaryocytic, and myelomonocytic cell lineages, whereas adhesion to laminin-8 and laminin-1 was restricted to a few cell lines.
The effect of laminins and fibronectin on migration of CD34+ cells
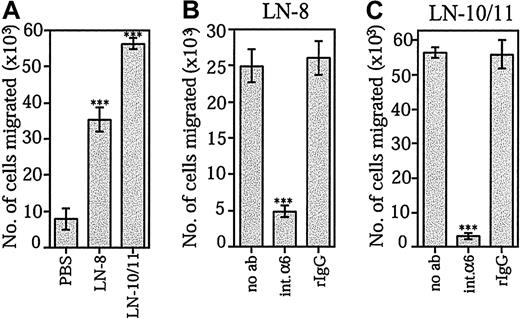
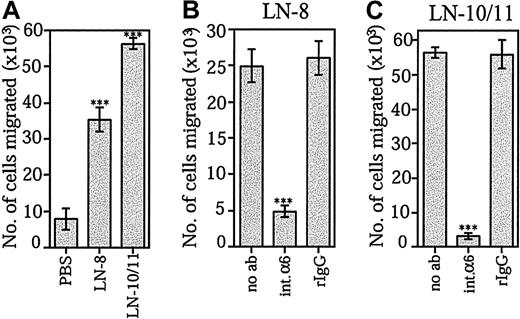
The ability of laminin-10/11 and laminin-8 to promote SDF-1α–stimulated transmigration of bone marrow CD34+progenitor cells was studied by using Transwell inserts coated with the extracellular matrix proteins. CD34+ cell migration was greatly enhanced through membranes coated with both laminin-10/11 and laminin-8 (Figure 5A). Migration stimulated by each laminin isoform was similar when protein concentrations at 30 μg/mL (data not shown) or at 10 μg/mL were used. In agreement with previous reports, fibronectin was found to promote SDF-1α–stimulated migration of CD34+ cells (not shown). Migration of CD34+ cells through Transwell inserts coated by both laminin-8 (Figure 5B) and laminin-10/11 (Figure 5C) was effectively inhibited by the GoH3 antibody against integrin α6 chain.
Effects of laminin-8 and laminin-10/11 on SDF-1α–stimulated migration of bone marrow progenitor cells across Transwell inserts.
(A) Migration of CD34+ cells across Transwell inserts coated with PBS, laminin-8 (LN-8), or laminin-10/11 (LN-10/11). Shown are means ± SDs of 1 experiment representative of 2 experiments performed in triplicate. (B,C) Inhibition of migration of CD34+ cells across Transwell inserts coated with laminin-8 (B) and laminin-10/11 (C) by monoclonal antibody GoH3 against integrin α6 chain. Migration was studied in the absence of antibodies (no ab), in the presence of the GoH3 antibody against integrin α6 (int.α6), and in the presence of irrelevant rat monoclonal antibody (rIgG). Asterisks indicate a significant difference from the control value (migration across Transwell inserts coated with PBS in panel A; migration in the absence of antibodies in panels B and C). ***P < .001.
Effects of laminin-8 and laminin-10/11 on SDF-1α–stimulated migration of bone marrow progenitor cells across Transwell inserts.
(A) Migration of CD34+ cells across Transwell inserts coated with PBS, laminin-8 (LN-8), or laminin-10/11 (LN-10/11). Shown are means ± SDs of 1 experiment representative of 2 experiments performed in triplicate. (B,C) Inhibition of migration of CD34+ cells across Transwell inserts coated with laminin-8 (B) and laminin-10/11 (C) by monoclonal antibody GoH3 against integrin α6 chain. Migration was studied in the absence of antibodies (no ab), in the presence of the GoH3 antibody against integrin α6 (int.α6), and in the presence of irrelevant rat monoclonal antibody (rIgG). Asterisks indicate a significant difference from the control value (migration across Transwell inserts coated with PBS in panel A; migration in the absence of antibodies in panels B and C). ***P < .001.
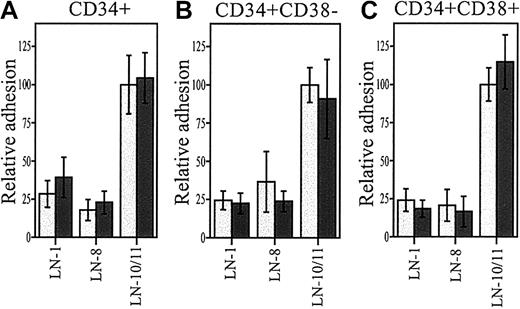
Adhesion of stem and progenitor cells to laminins in the presence of SDF-1α
Protein kinase C signal transduction pathway has been found to be involved in SDF-1α/CXCR-4 signaling.47 We therefore studied whether SDF-1α, like TPA, enhances adhesion of progenitor cells to laminins. SDF-1α did not stimulate adhesion of CD34+CD38− or the more mature progenitor cells to laminin-8 or laminin-10/11 (Figure6).
Adhesion of stem and progenitor cells to laminins in the presence of SDF-1α.
Adhesion of CD34+ (A), CD34+CD38−(B), and CD34+CD38+ cells to laminin-1 (LN-1), laminin-8 (LN-8), and laminin-10/11 (LN-10/11) in the presence of 300 ng/mL SDF-1α (dark columns) and without SDF-1α (light columns). The differences in cell adhesion in the presence or absence of SDF-1α were not significant (P > .05; LN-1, n = 3; LN-8, n = 9; LN-10/11, n = 12). Data are expressed as means ± SDs.
Adhesion of stem and progenitor cells to laminins in the presence of SDF-1α.
Adhesion of CD34+ (A), CD34+CD38−(B), and CD34+CD38+ cells to laminin-1 (LN-1), laminin-8 (LN-8), and laminin-10/11 (LN-10/11) in the presence of 300 ng/mL SDF-1α (dark columns) and without SDF-1α (light columns). The differences in cell adhesion in the presence or absence of SDF-1α were not significant (P > .05; LN-1, n = 3; LN-8, n = 9; LN-10/11, n = 12). Data are expressed as means ± SDs.
Discussion
Laminin α4 and α5, 2 recently characterized laminin chains, are widely expressed in tissues and are major components of basement membranes of blood vessels. Gene deletion of laminin α5 chain results in embryonally lethal phenotype with multiple defects,48whereas α4 chain null mutant mice have abnormal motoneuron synapses, impaired angiogenesis, extensive bleeding, and anemia in neonatal stage.49,50 In vitro studies on several types of cells, including epithelial and endothelial cell lines, have established an important cell-adhesive role for laminin-10/11 20,51 and laminin-8.21,22 We have previously shown that mouse multipotent hematopoietic FDCP-mix cells adhere to laminin-10/11,35 suggesting that these laminins might also interact with hematopoietic progenitors. Recent studies have shown binding of monoblastic, T-, and B-lymphoid cell lines and CD4+ lymphocytes to laminin-8 and laminin-10/11, platelets to laminin-8, and mouse integrin β2–deficient granulocytes to laminin-10/11,41 52-55 suggesting specific roles for different laminin isoforms in interactions with lineage-differentiated and mature hematopoietic cells. However, the cellular interactions of α4 and α5 laminin isoforms with human hematopoietic stem and progenitor cells have been unclear.
Here we demonstrate that defined stem and progenitor cell populations from normal human bone marrow adhere to bone marrow laminin isoforms, suggesting a significant role for these laminins for early hematopoietic development. CD34+CD38− cells represent only 0.05% to 0.1% of the nucleated human bone marrow cells and are highly enriched in stem cells, defined by their ability to maintain long-term hematopoiesis on irradiated stroma in vitro56 or to reconstitute long-term multilineage hematopoiesis in myeloablated recipients in vivo.57Our present results show that CD34+CD38− cells adhere to laminin-10/11 with nearly similar binding affinity as to the so far best characterized bone marrow adhesion protein, fibronectin. The CD34+CD38− cells adhered also to laminin-8, suggesting a biologically significant interaction. Our results raise the possibility that the most primitive long-term repopulating stem cells are adhesive to laminins. It will therefore be interesting to use experimental stem cell transplantation models or long-term stroma culture–based assays to test whether the long-term reconstituting stem cells adhere to laminin isoforms.
The CD34+ marker, expressed in 1% to 2% of bone marrow mononuclear cells, defines a cell population consisting of multipotent, lineage-committed, and lineage-differentiated progenitor cells, which express early myeloid, erythroid-megakaryocytic, and T- and B-lymphocytic markers. A large proportion, 40% to 50%, of CD34+ cells adhered to laminin-10/11 and fibronectin, and nearly 15% adhered to laminin-8 after maximal cell activation with TPA. The CD34+ cell fraction with high CD38 expression (CD34+CD38+), which contains more mature progenitor cells, also adhered to laminins and fibronectin. We therefore studied by colony assays whether the committed progenitors were adherent to these proteins. Most multipotent, myeloid (CFU-GM), and erythroid (BFU-E) progenitors were adherent to laminin-10/11. A much lower fraction of committed myeloid and erythroid progenitors were adherent to fibronectin, laminin-8, and laminin-1. However, maximal adhesion of CD34+ and CD34+CD38+progenitor cells to fibronectin and laminins was dependent on prior protein kinase C activation. Because the cells were not exposed to TPA before colony assays, the lower binding of CFU-GMs and BFU-Es to fibronectin than laminin-10/11 may be due to different activation stages of the receptors for individual matrix proteins in the progenitors.
Integrins can exist in different functional states with low or high binding capacity to particular ligands.58 Such conformational changes can be triggered by extracellular signals, including divalent cations, activating antibodies, or physiologically by ligand binding to the receptor. An important physiologic mechanism is activation of integrins by intracellular signals, induced by physiologic agonists. Adhesion of hematopoietic progenitor cells to fibronectin and activation of integrin receptors are modulated by a variety of cytokines and chemokines, and such modulation might be a major regulatory mechanism influencing stem and progenitor cell proliferation, transendothelial or stromal migration, and homing.2,10,59 60 To activate integrin receptors, we used phorbol ester TPA, which activates the protein kinase C pathway and mimics the effect of physiologic agonists. Adhesion of most stem and progenitor cell fractions to laminin-10/11 and laminin-8 was stimulated by TPA, suggesting that specific cytokines and chemokines modulate progenitor cell adhesion to laminins.
Most studied lineage-differentiated cells lines expressing B-lymphocytic, erythroid-megakayocytic, monocyte-macrophage, or myeloid differentiation markers adhered to fibronectin, and most adhered also to laminin-10/11, in agreement with reported findings.41In line with previous studies with cell lines,22 41laminin-8 and laminin-1 were less adhesive to lineage-differentiated cells than laminin-10/11. The cell lines studied have been established from patients with hematologic malignancies, and the findings may thus reflect biologically relevant interactions of primary leukemic and lymphoma cells with their physiologic environment. However, lineage-differentiated cell populations from healthy donors should be used to study the interactions of nontransformed cells with laminin isoforms present in bone marrow, vascular endothelia, lymph nodes, and thymus.
Integrins α6β1, α6β4, and α3β1 have been identified as receptors for both laminin-10/11 and laminin-8.21,22,35,51 In addition, laminin-10/11 binds cells via nonintegrin receptors, α-dystroglycan on endothelial, muscle, and epithelial cells,18,61 and Lutheran and LW glycoproteins on erythroid cells.62 The β1 integrin chain is ubiquitously expressed in hematopoietic cells, whereas integrin α6 receptor chain expression has been found in lineage-differentiated precursors and mature cells, including erythroid, megakaryocytic, monocyte-macrophage lineage cells, and a subset of lymphoid cells. Expression of integrin α3 chain has been reported in hematopoietic cell lines41 but is unclear for most hematopoietic cell subsets, including stem and progenitor cells. Integrin α3 chain mRNA in mouse stem and progenitor cells was shown in one study by PCR, but the proportion of cells expressing it is not known.63
The present results using FACS analysis showed that integrin α3 chain is not expressed in human CD34+ cells or the CD34+CD38− cell fraction. In contrast, integrin α6 chain was found in most of both CD34+ and CD34+CD38− cells. This is in agreement with the reported high expression of integrin α6 chain in mouse stem and progenitor cells.64 The present findings by using functionally active antibodies indicate that integrin α6β1 is a major laminin receptor in bone marrow stem and progenitor cells. Furthermore, the inhibition of cell adhesion with antibodies against the corresponding receptor confirmed the specificity of ligand-receptor interaction.65 66
We detected integrin α6 mRNA expression also by RT-PCR in CD34+ and CD34+CD38− cells, in agreement with the immunostaining results. The 2 described integrin α6 mRNA variants, generated by alternative mRNA splicing, could be amplified in both cell fractions. The 2 splice variants have different cytoplasmic domains and different functional properties. In adult tissues, the larger variant, integrin α6A, is assocated with the β1 subunit in lymphocytes, macrophages, and platelets, whereas both α6Aβ1 and α6Bβ1 variants have been found in endothelial cells (reviewed by de Melker et al67,68). Transfection and gene deletion studies have shown that the 2 integrin α6 variants can equally associate with the integrin β1 subunit and have similar ligand binding specificity and affinity. However, the α6A, in contrast to α6B, triggers protein kinase C–dependent activation of mitogen-activated protein (MAP) kinases and is more active than α6B in promoting migration of cells, as also shown by impaired in vitro migration of α6A-deficient lymphocytes on laminin.69 The presence of the integrin α6A splice variant in CD34+ and CD34+CD38−cells indicates that interactions of laminins with integrin α6 receptor could be involved in hematopoietic stem and progenitor cell migration and mobilization.
Small numbers of stem and progenitor cells are present in peripheral blood during steady-state hematopoiesis, suggesting that continuous stem cell mobilization and homing into bone marrow is a physiologic process.70 The mechanisms involved in stem cell mobilization and homing are not yet clear. It is apparently a multistep process directed by chemoattractants and mediated by cell-adhesive interactions with stromal cells and matrix components of the bone marrow environment.71 In vitro studies have shown that migration of human bone marrow CD34+ cells through bone marrow endothelial cell layer involves interaction of several cell surface adhesion molecules including β1 and β2 integrins, platelet endothelial cell adhesion molecule-1 (PECAM-1), andO-glycosylated proteins.72 In vivo experiments using blocking antibodies and gene-deleted mice suggested that the integrin β1 chain and integrin α4β1 receptor in hematopoietic stem and progenitor cells, and stromal vascular cell adhesion molecule-1 (VCAM-1), are involved in the homing and mobilization of stem cells and progenitors.73-76 However, studies with chimeric mice with homozygous deletions of integrin α chains have not settled the role of individual integrin α chains in stem cell homing, and it is possible that multiple receptors are involved.14,77,78
In vitro studies have shown a migration promoting activity for both laminin-10/11 and laminin-8 in tumor cell lines22,79 and mouse integrin β2–deficient granulocytes.55 We therefore here analyzed the effect of laminin-8 and laminin-10/11 on transmigration of CD34+ cells stimulated with the chemokine SDF-1α.80 Laminin-10/11 and laminin-8 both promoted transmigration of CD34+ cells by a mechanism involving integrin α6 receptor, as shown by inhibition of migration by antibodies against integrin α6. Our findings raise the possibility that α4 and α5 laminins are involved in mobilization and homing of hematopoietic progenitor cells.
SDF-1α, acting via the CXCR-4 receptor on CD34+ cells, has been shown to mediate homing of human progenitors in mouse bone marrow in a mechanism dependent on activation of protein kinase C signal transduction pathways.47 SDF-1α activates integrin α4β1 and α5β1 receptors and enhances migration of CD34+ cells stimulated by fibronectin.60SDF-1α has been reported to stimulate47,60 but also to suppress81 adhesion of CD34+ progenitors to fibronectin. In line with the observed expression of CXCR-4 receptor in less than 20% of freshly isolated bone marrow CD34+CD38− cells,82 SDF-1α was not found to stimulate adhesion of this cell fraction to laminins. Expression of CXCR-4 was reported in 56% of bone marrow CD34+ cells,82 but adhesion of the more mature progenitor cells to laminin-8 and laminin-10/11 was not either stimulated by SDF-1α.
The present study shows that laminin isoforms present in bone marrow and blood vessel walls are adhesive substrates to bone marrow stem and progenitor cells and influence progenitor cell migration in vitro. The adhesive and migration promoting effects of both laminins are largely mediated by integrin receptors containing the α6 chain. These findings suggest that cell-adhesive interactions of laminins mediated by integrin α6 receptor might be important during hematopoiesis. Deletion of integrin α6 chain leads to severe skin blistering, cerebral malformations, and neonatal death.83 Therefore, analysis of hematopoiesis in integrin α6 chain null mouse embryos and in vivo experiments using transplantation models might be useful in defining the role of laminins and integrin α6 receptor for stem and progenitor cell proliferation, homing, and migration.
Prepublished online as Blood First Edition Paper, September 19, 2002; DOI 10.1182/blood-2002-03-0796.
Supported by grants from Avtal om Läkarutbildning och Forskning (Government Public Health Grant), Crafoord Foundation, Georg Danielsson's Foundation, John Persson's foundation, Swedish Cancer Society, Swedish Natural Science Research Council, University Hospital of Lund Foundation, and Tobias Foundation.
K.T. has declared a financial interest in BioStratum, and J.K. was employed by BioStratum, whose product (recombinant laminin-8) was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Marja Ekblom, University of Lund, BMC B12, 221 84 Lund, Sweden; e-mail:marja.ekblom@medkem.lu.se.