Abstract
The mutational status of tumor immunoglobulin VHgenes is providing a powerful prognostic marker for chronic lymphocytic leukemia (CLL), with patients having tumors expressing unmutated VH genes being in a less favorable subset. However, the biologic differences correlating with VH gene status that could determine the clinical course of the disease are unknown. Here we show that differing responses to IgM ligation are closely associated with VH gene status. Specifically, 80% of cases with unmutated VH genes showed increased global tyrosine phosphorylation following IgM ligation, whereas only 20% of samples with mutated VH genes responded (P = .0002). There was also an association between response to IgM ligation and expression of CD38 (P = .015). The Syk kinase, critical for transducing B-cell receptor (BCR)– derived signals, was constitutively present in all CLL samples, and there was a perfect association between global phosphorylation and induction of phosphorylation/activation of Syk. Nonresponsiveness to anti-IgM could be circumvented by ligation of IgD (10 of 15 samples tested) or the BCR-associated molecule CD79α (12 of 15 samples tested). These results suggest that multiple mechanisms underlie nonresponsiveness to anti-IgM in CLL and that retained responsiveness to anti-IgM contributes to the poor prognosis associated with the unmutated subset of CLL. The prognostic power of the in vitro response to IgM ligation remains to be determined in a large series, but the simple technology involved may present an alternative or additional test for predicting clinical course.
Introduction
Chronic lymphocytic leukemia (CLL) is the most common adult B-cell malignancy in the Western world and is characterized by the accumulation of monoclonal CD5+ B cells with the appearance of small mature lymphocytes (for a review, see Caligaris-Cappio and Hamblin1). The clinical course of CLL is heterogeneous, with some patients progressing rapidly with early death, whereas others exhibit a more stable, possibly, nonprogressing disease lasting many years. The clinical management of CLL is therefore challenging and considerable effort has been directed toward the identification of clinically useful prognostic markers to guide treatment.
Somatic mutation of the immunoglobulin VH genes in normal B cells is a key event in increasing diversity during maturation of immune responses. The mutation status of the VH genes expressed in CLL cells is a powerful prognostic marker for CLL; patients with unmutated genes have a worse prognosis.2,3The cell surface expression of CD38 may be another potential prognostic marker in CLL. A relatively high level of CD38 surface expression by CLL cells has been shown to be a marker of poor prognosis.3-5 Although originally suggested to correlate with unmutated VH gene status, this is controversial and other studies have shown CD38 expression does not correlate with VH gene status and can vary during disease progression.6-8 Taken together, the studies suggest that VH gene status and CD38 expression are independent prognostic markers for CLL.
Although both VH gene status and CD38 may have clinical utility as prognostic markers in CLL, the biologic differences between CLL subtypes that underlie their very different disease courses are not known. CLL cells have heterogeneous responses to stimulation via cell surface receptors including CD40,9 CD5,10 and the B-cell receptor (BCR).11-13 BCR is a key molecule that triggers signaling pathways that regulate proliferation, differentiation, and apoptosis in B cells. BCR comprises membrane immunoglobulin, the antigen-binding subunit, and a heterodimer of CD79α and CD79β, the signaling subunit (for a review, see Matsuuchi and Gold14). In normal B cells, stimulation of the BCR leads to phosphorylation of tyrosine residues in CD79α and CD79β by the protein tyrosine kinase (PTK) Lyn. Syk is recruited to the immune receptor tyrosine-based activation motif (ITAM) present in phosphorylated CD79α and CD79β. Once bound to the ITAM, Lyn phosphorylates Syk, which, in turn, autophosphorylates leading to additional tyrosine phosphorylation of many more proteins. This generates a signaling cascade downstream of Syk including intracellular calcium mobilization. These downstream signals lead to cellular proliferation or apoptosis depending on cosignals received by the cell and the stage of cellular differentiation (for a review, see Healy and Goodnow15). Hence, tyrosine phosphorylation of Syk plays a critical role in the signaling cascade from the BCR.
Previous studies have found that CLL cells differ in their responses to IgM ligation based on differences in the constitutive levels of Syk11 or phosphorylation of Syk.12 However, at that time no correlation with VH gene mutational status was available. Because differences in BCR function may contribute to differences in biologic behavior, we have analyzed the signaling response to BCR cross-linking in CLL and its relationship to VH gene mutational status and expression of CD38.
Patients, materials, and methods
Patients' cells
Following Southampton and Southwest Hampshire local research ethics committee (LREC) approval, peripheral blood was obtained from 40 patients with classical CLL. Some of the patients had been characterized previously for VH gene mutation status and CD38 expression2 8 and patients were selected to provide a representative selection of VH gene and CD38 status (Table1). Peripheral blood mononuclear cells were isolated by Ficoll-Paque gradient centrifugation (Amersham Pharmacia Biotech, Little Chalfont, Bucks, United Kingdom), washed, and cryopreserved. CLL cells were not further purified to minimize in vitro manipulations. The proportion of contaminating normal B-cell CLL samples determined by fluorescence-activated cell sorting (FACS) using CD19 and CD5 was equal to or less than 0.1%.
If not known, VH gene mutation status was determined as previously described.2 Daudi, Ramos, and Jurkat cells were cultured in RPMI 1640 medium containing 10% (vol/vol) fetal calf serum (FCS), 2 mM glutamine, 1% (wt/vol) sodium pyruvate, and antibiotics. CLL samples were thawed and maintained for 1 hour at 37°C in this medium before the assays were performed. Cell viability was 80% to 100% following thawing and remained unchanged during the experiment.
Flow cytometry
Cells were prepared and analyzed for flow cytometry as previously described8 using a FACSCalibur flow cytometer (Becton Dickinson, Cowley, United Kingdom). CD38 expression was determined as described previously8 and reported as the percentage of positive cells. The expression of CD20 and BCR was analyzed using antibody B-Ly1 and F(ab′)2 rabbit antihuman IgM, respectively (Dako, Glostrup, Denmark) and expression of CD79α and CD79β was analyzed using ZL7-4 and ZL9-2 antibodies, respectively16 (kind gift of Professor Martin Glennie, Tenovus Laboratories, Southampton, United Kingdom). The expression of surface immunoglobulin, CD79β, and CD20 was reported as molecules of equivalent fluorochrome (MEF) values. These were determined using FluoroSpheres (Dako). FluoroSpheres are a mixture of 5 beads with a diameter of 3.2 μm having trapped within them a combination of fluorochromes that enables the beads to be excited by light of any wavelength between 365 and 650 nm. They can thus be used to calibrate all channels of the flow cytometer. The use of these beads enables arbitrary units of mean fluorescence intensity to be transformed to MEF values that can be compared from one run to another.
Tyrosine phosphorylation
Cells were washed in RPMI 1640 medium and resuspended at a density of 2 × 107 cells/250 μL in RPMI 1640 medium and were untreated or incubated for 2 minutes at 37°C with 20 μg/mL goat anti-IgM, goat anti-IgD, or goat immunoglobulins as a control (all from Southern Biotechnology Associates, Birmingham, AL). We used goat IgG antibody because this binds poorly to human Fc receptors17 and has been shown previously to behave similarly to F(ab′)2 fragments in CLL signaling assays.13 For stimulation of CD79α, we used the mouse monoclonal antibody (mAb) ZL7-416 and mouse IgG1 (Southern Biotechnology Associates) as a control. Reactions were stopped by centrifugation and the pellets resuspended in 150 μL lysis buffer (Tris [tris(hydroxymethyl)aminomethane] 20 mM, pH 7.5, EDTA [ethylenediaminetetraacetic acid] 1 mM, NaCl 140 mM, 1:100 dilution of inhibitor cocktail for mammalian tissues [Sigma, Poole, Dorset, United Kingdom], sodium orthovanadate 2 mM) containing 1% (vol/vol) Brij 97 detergent (polyoxythylene 10 oleyl ether [Sigma]) for 60 minutes at 4°C. Cell debris was removed by centrifugation at 12 000g and the supernatant boiled in sample buffer. Proteins were separated by denaturing sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were transferred to nitrocellulose membranes (Schleicher and Schuell, Dassel, Germany) and probed with mouse mAbs against phosphotyrosine 4G10 (Upstate Biotechnology, Lake Placid, NY) followed by horseradish peroxidase–conjugated antimouse antibodies. Bound immunocomplexes were subsequently detected using enhanced chemiluminescence (Perbio, Tattenhall, Cheshire, United Kingdom) and visualized on a Fluor-S Max imager (Biorad, Hemel Hempstead, Herts, United Kingdom). The fold increase in Syk phosphorylation in cells treated with anti-IgM, IgD, or CD79α antibodies relative to control cells was quantified using Quantity One software (Biorad).
Constitutive levels of Syk
Untreated lysed cell samples boiled in sample buffer from tyrosine phosphorylation studies (see “Tyrosine phosphorylation”) were run on denaturing SDS-polyacrylamide gels and transferred to nitrocellulose membranes. Separate gels were probed with mouse mAbs against human Syk 4D10.1 (Upstate Biotechnology) and then detected as described. Ramos and Jurkat cell lines were used as controls.
Immunoprecipitation
Brij-97–lysed supernatants were precleared for 30 minutes with protein G–coated Sepharose beads (Amersham Pharmacia Biotech) followed by incubation with 2 μg specific antibody for 2 hours at 4°C. Packed protein G-Sepharose beads (15 μL) blocked with 5% (wt/vol) bovine serum albumin were added to the sample, which was then incubated for a further 60 minutes at 4°C. The beads were washed 4 times with cold lysis buffer and boiled in sample buffer. The precipitated proteins were separated by SDS-PAGE and specific proteins detected by immunoblotting. To detect Syk phosphorylation, immunoprecipitations were performed using antiphosphotyrosine antibody 4G10 followed by immunoblotting with anti-Syk 4D10.1. Samples that had at least 2 times more phosphorylated Syk following anti-IgM, IgD, or CD79α stimulation were considered to be responsive. To detect Tyr525/526 phosphorylated Syk, immunoprecipitations were performed using a rabbit polyclonal antibody against Tyr525/526 phosphorylated Syk (New England Biolabs, Hitchin, Herts, United Kingdom) followed by immunoblotting with mouse anti-Syk 4D10.1. To detect Lyn, immunoprecipitations were preformed using rabbit polyclonal antibody against Lyn (sc-15; Santa Cruz Biotechnology, Santa Cruz, CA) or rabbit control antibodies (ChromPure Rabbit IgG, whole molecule; Jackson Immunoresearch Laboratories, West Grove, PA).
Statistics
Statistical analysis was performed using the SPSS for Windows program version 10 (SPSS UK, Woking, Surrey, United Kingdom). Chi-squared analysis was used for comparison of mutation status or CD38 with signaling responsiveness. Values for MEF were calculated using the software program, TallyCAL (Dako). Scattergrams of CD20, surface Ig, and CD79β MEF levels were prepared using the GraphPad Prism (version 3) software (San Diego, CA), and comparisons with mutation, CD38, and signaling status made using the Mann-Whitney test. The comparison of Syk expression and signaling status was made using the Mann-Whitney test.
Results
Patients' immunophenotype and VH gene status
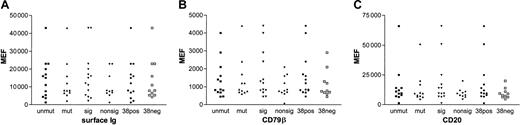
All patients with CLL studied were untreated, most were Binet stage A (Table 1). CLL cells from all patients studied expressed CD5, CD19, surface IgM (sIgM), and CD23. The levels of sIgM, CD79β, and CD20, expressed as MEF values, were variable (Table 1). Of the cases studied here, 15 of 40 (38%) had unmutated VHgenes and 17 of 38 (45%) were positive for CD38 expression (Table2). There was no significant correlation between VH gene status or CD38 expression and cell surface expression of IgM, CD20, or CD79β (Figure1).
Expression of cell surface molecules.
Scattergrams showing expression as mean equivalent fluorochrome values of surface immunoglobulin (A), CD79β (B), and CD20 (C) comparing mutated (mut, ▴) with unmutated (unmut, ▪), signaling (sig, ▾) with nonsignaling (nonsig, ♦), and CD38+ (38pos, ●) with CD38− (38neg, ■) groups. None of the comparisons shows a significant difference. Only samples for which the expression of all surface molecules were known were included in the analysis.
Expression of cell surface molecules.
Scattergrams showing expression as mean equivalent fluorochrome values of surface immunoglobulin (A), CD79β (B), and CD20 (C) comparing mutated (mut, ▴) with unmutated (unmut, ▪), signaling (sig, ▾) with nonsignaling (nonsig, ♦), and CD38+ (38pos, ●) with CD38− (38neg, ■) groups. None of the comparisons shows a significant difference. Only samples for which the expression of all surface molecules were known were included in the analysis.
Signaling through IgM
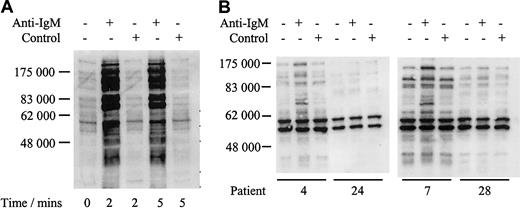
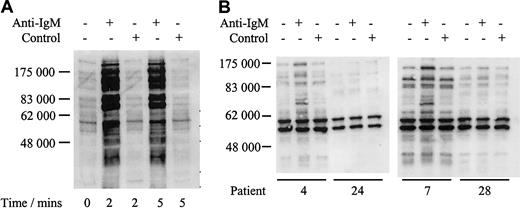
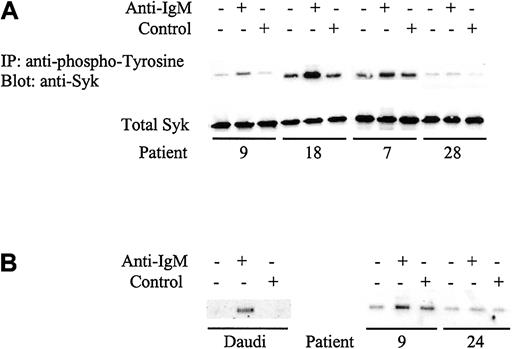
We analyzed responsiveness to IgM ligation by measuring increases in global protein tyrosine phosphorylation. Daudi cells were used as a positive control and showed a rapid increase in global tyrosine phosphorylation when treated with anti-IgM, but not with control antibody (Figure 2A), as reported.12 In contrast, the response of CLL samples was variable with only a proportion of cases responding to anti-IgM stimulation shown by a visible increase in band intensity (Figure 2B; Table 2). For example patients 4 and 7 showed increased global tyrosine phosphorylation, whereas patients 24 and 28 displayed no response. Nonresponsiveness was not simply due to delayed phosphorylation in some samples because there was no evidence for increased global tyrosine phosphorylation in any of 4 nonresponding samples, which were incubated with anti-IgM for up to 2 hours (data not shown). In addition, IgM responsiveness was repeatable in duplicate fresh and frozen samples from 6 patients studied (3 responsive to anti-IgM treatment and 3 nonresponsive, data not shown).
Analysis of tyrosine phosphorylated proteins in Daudi cells and CLL samples.
Immunoblot analysis of tyrosine phosphorylated proteins in Daudi cells (A) or representative CLL samples (B) incubated with anti–human IgM or isotype control antibody. Tyrosine phosphorylated proteins were detected by direct immunoblotting using antibody 4G10. Proteins were separated on a 10% SDS-PAGE gel. The molecular mass of protein standards is shown. Data for all CLL samples are summarized in Table2.
Analysis of tyrosine phosphorylated proteins in Daudi cells and CLL samples.
Immunoblot analysis of tyrosine phosphorylated proteins in Daudi cells (A) or representative CLL samples (B) incubated with anti–human IgM or isotype control antibody. Tyrosine phosphorylated proteins were detected by direct immunoblotting using antibody 4G10. Proteins were separated on a 10% SDS-PAGE gel. The molecular mass of protein standards is shown. Data for all CLL samples are summarized in Table2.
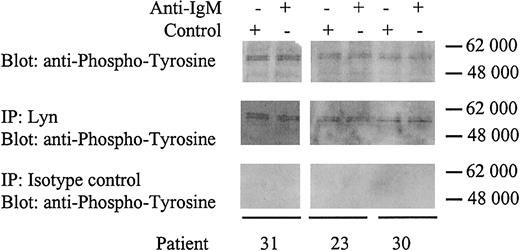
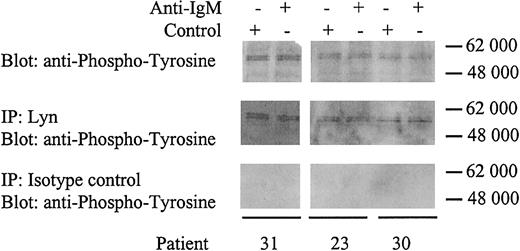
All CLL samples expressed 2 heavily phosphorylated proteins of approximately 55 kDa regardless of whether the cell could signal through the BCR (Figure 2B). Human Lyn occurs as 2 isoforms with molecular weights of 53 and 56 kDa. To determine whether the proteins in CLL cells were phosphorylated Lyn, we performed immunoprecipitations using a Lyn-specific antibody (Figure 3). The tyrosine phosphorylated proteins immunoprecipitated by the anti-Lyn antibody were identical to the approximately 55-kDa proteins detected by direct immunoblotting using a phosphotyrosine-specific antibody. Therefore, Lyn appears to be constitutively phosphorylated in CLL cells independent of anti-IgM stimulation. Lyn phosphorylation apparently did not alter following IgM ligation and was present in responsive and nonresponsive CLL samples.
Identification of Lyn.
CLL samples were incubated with anti-IgM or control antibody for 2 minutes. Samples were either immunoblotted for tyrosine phosphorylation (top panels), or immunoprecipitated (IP) with rabbit anti-Lyn antibody and immunoblotted for tyrosine phosphorylation (middle panels), or immunoprecipitated with rabbit isotype control antibody and immunoblotted for tyrosine phosphorylation (bottom panels). Proteins were separated on a 4% to 15% gradient SDS-PAGE gel. The molecular mass of protein standards is shown.
Identification of Lyn.
CLL samples were incubated with anti-IgM or control antibody for 2 minutes. Samples were either immunoblotted for tyrosine phosphorylation (top panels), or immunoprecipitated (IP) with rabbit anti-Lyn antibody and immunoblotted for tyrosine phosphorylation (middle panels), or immunoprecipitated with rabbit isotype control antibody and immunoblotted for tyrosine phosphorylation (bottom panels). Proteins were separated on a 4% to 15% gradient SDS-PAGE gel. The molecular mass of protein standards is shown.
Analysis of PTK Syk
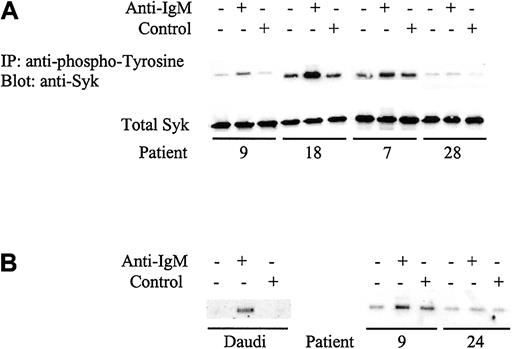
The nonresponsiveness of CLL cells to IgM ligation has been suggested previously to correlate with reduced constitutive expression of Syk11 or to a failure to induce Syk phosphorylation.12 To resolve this discrepancy, we first analyzed the constitutive expression of Syk in CLL samples. Syk was readily detected in all samples analyzed (data not shown). Although there was variability in the level of expression between samples, samples with the lowest levels retained responsiveness to anti-IgM and there was no significant difference in the mean levels between mutated and unmutated CLL (P = .3). Because constitutive Syk expression did not correlate with responsiveness, we next measured Syk phosphorylation by immunoprecipitating tyrosine phosphorylated proteins and immunoblotting for Syk. As with global tyrosine phosphorylation, increases in Syk phosphorylation were detected only in a proportion of CLL samples (Figure 4A). Further, there was a perfect correlation between responsiveness to anti-IgM measured by visible increases in global tyrosine phosphorylation and specific tyrosine phosphorylation of Syk (Table 2). Examples of patients 7 and 28 are shown in Figures 2B and 4A. Hence, specific measurement of phosphorylated Syk by densitometry enabled quantification of the signaling response in all cases, rather than the arbitrary method of a visible increase in global tyrosine phosphorylation. When 6 patients were retested for phosphorylation of Syk following anti-IgM treatment, all gave reproducible results to that shown in Table 2 (data not shown).
Analysis of Syk phosphorylation following anti-IgM treatment.
(A) CLL samples were incubated with anti-IgM or control antibody for 2 minutes. Tyrosine-phosphorylated Syk was analyzed by immunoprecipitation (IP) with phosphotyrosine-specific antibody followed by blotting with the Syk-specific antibody (top panel). Total Syk expression was analyzed by direct immunoblotting (bottom panel). Representative results are shown. Table 2 provides summary of data for all samples. (B) Daudi cells or CLL samples were incubated with anti-IgM or control antibody for 2 minutes. Tyrosine phosphorylation on Tyr525/526 of Syk was detected by immunoprecipitation using the Syk phospho-Tyr525/526–specific antibody followed by direct immunoblotting for Syk. Representative results are shown for analysis of 6 CLL samples. Patient 9 is an unmutated sample that showed global tyrosine phosphorylation following anti-IgM treatment, whereas patient 24 is a mutated sample that did not show phosphorylation with anti-IgM.
Analysis of Syk phosphorylation following anti-IgM treatment.
(A) CLL samples were incubated with anti-IgM or control antibody for 2 minutes. Tyrosine-phosphorylated Syk was analyzed by immunoprecipitation (IP) with phosphotyrosine-specific antibody followed by blotting with the Syk-specific antibody (top panel). Total Syk expression was analyzed by direct immunoblotting (bottom panel). Representative results are shown. Table 2 provides summary of data for all samples. (B) Daudi cells or CLL samples were incubated with anti-IgM or control antibody for 2 minutes. Tyrosine phosphorylation on Tyr525/526 of Syk was detected by immunoprecipitation using the Syk phospho-Tyr525/526–specific antibody followed by direct immunoblotting for Syk. Representative results are shown for analysis of 6 CLL samples. Patient 9 is an unmutated sample that showed global tyrosine phosphorylation following anti-IgM treatment, whereas patient 24 is a mutated sample that did not show phosphorylation with anti-IgM.
Syk is phosphorylated on multiple tyrosine residues and these modifications can activate or inhibit Syk function. In particular, phosphorylation of tyrosine residues 525 and 526 is required for Syk activation.18 Immunoprecipitations were performed using an antibody that specifically detects Tyr525/526 phosphorylated Syk (Figure 4B). In the 6 cases studied, we readily detected increased Tyr525/526 phosphorylation of Syk in Daudi cells and in responsive (patients 9, 11, and 18) but not nonresponsive (patients 24, 26, and 30) CLL samples.
We then assessed responsiveness to IgM ligation in all cases and compared the results with the VH gene mutational status and with expression of CD38, each important prognostic markers for CLL. There was a statistically significant association between anti-IgM responsiveness and unmutated VH genes (P = .0002; Table 2); 12 of 15 (80%) of samples with unmutated VH genes responded to anti-IgM, whereas only 5 of 25 (20%) of samples with mutated VH genes responded. In addition, there was also an association between CD38 expression and anti-IgM responsiveness, although this was not as strong as the correlation with mutations status: 11 of 17 (65%) of CD38+and 5 of 20 (25%) of CD38− samples responded to anti-IgM (P = .015). Also, it was interesting to note that CD38 expression did associate with anti-IgM responsiveness within samples with mutated VH genes (P = .001), because 0 of 15 (0%) of CD38− samples and 4 of 7 (57%) of CD38+ samples responded to anti-IgM; however, no correlation was seen in samples with unmutated VHgenes. There was no significant association with levels of expression of surface IgM, CD20, or CD79β (Figure 1).
Differential signaling via BCR components
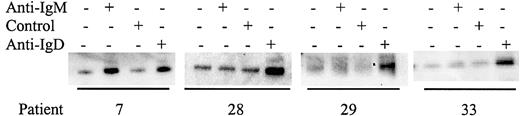
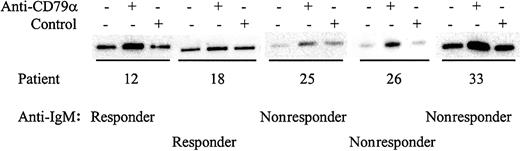
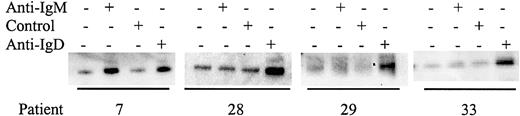
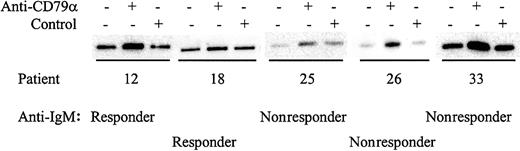
Although some CLL samples were resistant to stimulation with anti-IgM antibodies, it was possible that nonresponsiveness could be circumvented by stimulation of other BCR components. All samples expressed IgM, CD79α, and IgD (data not shown) and we therefore determined whether stimulation of CLL samples with antibodies to these molecules increased tyrosine phosphorylation of Syk (Figures5-6; results summarized in Table 3). Of the 15 samples tested that did not respond to anti-IgM, 10 (66%) were responsive to anti-IgD (Figure 5). Similarly, of 15 samples tested, 12 (80%) were responsive to anti-CD79α (Figure 6). The 3 samples that did not respond to anti-CD79α were nonresponsive to either anti-IgM or anti-IgD. Therefore, there are various patterns of differential responsiveness to BCR ligation in CLL suggesting that multiple molecular defects underlie failure to respond to anti-IgM. Most CLL samples that are nonresponsive to anti-IgM retain responsiveness to anti-IgD and CD79α. A smaller proportion of CLL samples fail to respond to anti-IgM or anti-IgD but do respond to anti-CD79α, or fail to respond to ligation of any of the BCR components tested. By contrast, CLL samples that were responsive to anti-IgM were also sensitive to anti-CD79α and anti-IgD.
Analysis of Syk phosphorylation following anti-IgD treatment.
CLL samples were incubated with anti-IgM, anti-IgD, or control antibody for 2 minutes. Tyrosine-phosphorylated Syk was analyzed by immunoprecipitation with phosphotyrosine-specific antibody followed by blotting with the Syk-specific antibody. Representative results are shown.
Analysis of Syk phosphorylation following anti-IgD treatment.
CLL samples were incubated with anti-IgM, anti-IgD, or control antibody for 2 minutes. Tyrosine-phosphorylated Syk was analyzed by immunoprecipitation with phosphotyrosine-specific antibody followed by blotting with the Syk-specific antibody. Representative results are shown.
Analysis of Syk phosphorylation following anti-CD79α treatment.
CLL samples were incubated with anti-CD79α or control antibody for 2 minutes. Tyrosine-phosphorylated Syk was analyzed by immunoprecipitation with phosphotyrosine-specific antibody followed by blotting with the Syk-specific antibody. Representative results are shown.
Analysis of Syk phosphorylation following anti-CD79α treatment.
CLL samples were incubated with anti-CD79α or control antibody for 2 minutes. Tyrosine-phosphorylated Syk was analyzed by immunoprecipitation with phosphotyrosine-specific antibody followed by blotting with the Syk-specific antibody. Representative results are shown.
Discussion
CLL is a heterogeneous disease and recent evidence suggests VH gene mutation status may have prognostic utility, guiding clinical management. The biologic differences that correlate with these prognostic markers and underlie the variable clinical behavior of CLL are not known. Here we have shown that the capacity to signal through membrane IgM correlates closely with VH gene status.
We used Syk phosphorylation as a measure of responsiveness to BCR stimulation, because Syk tyrosine phosphorylation plays a key role in downstream signaling, including activation of PLCγ2, SLP-65, and calcium mobilization. The constitutive levels of Syk expression do not differ between anti-IgM responsive and nonresponsive CLL, and we found a perfect correlation between Syk phosphorylation and increases in global tyrosine phosphorylation following anti-IgM stimulation. Importantly, there was a strong correlation observed between VH gene status and anti-IgM–induced phosphorylation, with cases expressing unmutated VH genes mostly showing induced phosphorylation of PTK Syk. CD38 expression has been reported to be associated with a poor prognosis,3-5 but most studies indicate only a weak correlation with unmutated VHgenes.6-8 Taking our whole patient group, we have confirmed the previously reported correlation between CD38 expression and responsiveness to signaling via sIgM.13 Within the unmutated subset, signaling capacity appeared to be independent of CD38 expression. However, in the mutated subset, the few cases that did signal tended to express CD38. It is possible that the ability to signal reflects possession of either of the poor prognostic features, unmutated VH genes or expression of CD38. However, more cases will be required to determine if signaling can replace or improve VH gene mutational status as a prognostic factor.
The BCR has a profound impact on the behavior of B cells, including induction of proliferation or apoptosis, depending on the stage of development, and on the context and strength of stimulation (for a review, see Healy and Goodnow15). In the CD38+ subset of CLL, ligation of sIgM has been reported to result in accelerated apoptosis.19 In contrast, ligation of sIgD resulted in cell survival.19 At present, our data relate only to Syk phosphorylation and tyrosine phosphorylation, leaving open the question of whether signaling in the unmutated cases leads to apoptosis. What is clear in the mutated subset is that there is a differential capacity to transduce signals from the BCR, dependent on which component of the BCR complex is targeted. The fact that the apparent deficit in signaling via sIgM can be bypassed by stimulating via sIgD or CD79α demonstrates for the first time that it is the molecular organization of the BCR complex that differs among the subsets, rather than downstream pathways.
A hierarchy of factors appears to be involved in failure to signal via sIgM. Most CLL samples that fail to respond to anti-IgM retain responsiveness to anti-IgD and anti-CD79α, indicating a specific deficit in signal transduction from IgM to CD79α/β. Less frequently, CLL samples were nonresponsive to anti-IgM and anti-IgD but did respond to anti-CD79α. Therefore, the signal transduction pathway downstream from CD79α is intact in these cells, but cannot be engaged by either immunoglobulin molecule. A third group of CLL samples failed to respond to ligation of any of the BCR components tested, indicating a defect further downstream in the signaling pathway. Although anti-IgM responsiveness did not correlate with the cell surface expression of CD79β, mutation of CD79β or expression of alternate RNA splice products have been described in CLL previously.20-22 It remains to be determined whether these alterations, or defects in other BCR components, account for the specific patterns of differential responsiveness to BCR stimulation observed in CLL.
The features of the cases, mostly with mutated VH genes, which were unresponsive to signaling, are reminiscent of B cells that have undergone receptor desensitization, following chronic stimulation by antigen.23 This anergic state has been well documented in mice where the desensitized BCR remains able to bind antigen, but fails to transduce signals.23 As with our observations in CLL, the murine desensitized receptors remain responsive to signaling via CD79α/β.23 The apparently differential desensitization of sIgM and sIgD in CLL may relate to early findings that the kinetics and intensity of phosphorylation differ between the 2 receptors on a single cell.24 Although BCR of both isotypes can apparently undergo receptor desensitization,23 the level of engagement by antigen required for each may differ.
The nature of antigen involved in mediating the proposed desensitized state is unclear, although the biased usage of the V4-34 gene in the mutated subset might point to either a viral antigen25 or an autoantigen.26 27 It appears that less malignant behavior is associated with the anergic state, perhaps suggesting that the unmutated cases with more competent BCRs are better able to receive signals for maintenance or proliferation. Clearly there are many steps between BCR signaling and cellular response that need to be uncovered in vitro, and their relevance to conditions in vivo determined, before we can connect these proximal events to tumor progression.
We thank Professor Martin Glennie and Dr Mark Cragg (Tenovus Laboratory, Southampton, United Kingdom) for supplying antibodies, particularly for CD79 analysis. We would also like to thank Zadie Davis, Jenny Orchard, and Mo Tiller for technical assistance.
Prepublished online as Blood First Edition Paper, September 26, 2002; DOI 10.1182/blood-2002-06-1822.
Supported by Tenovus United Kingdom and Cancer Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Freda Stevenson, Molecular Immunology Group, Tenovus Research Laboratory, Southampton University Hospitals Trust, Tremona Road, Southampton SO16 6YD, United Kingdom; e-mail:fs@soton.ac.uk.