Abstract
The presence or absence of somatic mutations in the expressed immunoglobulin heavy chain variable regions (IgVH) of chronic lymphocytic leukemia (CLL) cells provides prognostic information. Patients whose leukemic cells express unmutated IgVH regions (Ig-unmutated CLL) often have progressive disease, whereas patients whose leukemic cells express mutated IgVH regions (Ig-mutated CLL) more often have an indolent disease. Given the difficulty in performing IgVH sequencing in a routine diagnostic laboratory, this prognostic distinction is currently unavailable to most patients. Pilot gene expression profiling studies in patients with CLL identified genes that were differentially expressed between the Ig-unmutated and Ig-mutated CLL subtypes. Here, we have profiled an expanded cohort of 107 patients and show that ZAP-70 is the gene that best distinguishes the CLL subtypes. Ig-unmutated CLL expressed ZAP-70 5.54-fold more highly than Ig-mutated CLL (P < 10-21). ZAP-70 expression correctly predicted IgVH mutation status in 93% of patients. ZAP-70 expression and IgVH mutation status were comparable in their ability to predict time to treatment requirement following diagnosis. In 7 patients, ZAP-70 expression and IgVH mutation status were discordant: 4 Ig-mutated CLLs had high ZAP-70 expression and 3 Ig-unmutated CLLs had low ZAP-70 expression. Among these ZAP-70 “outliers,” those with Ig-mutated CLL had clinical features that are uncharacteristic of this CLL subtype: 2 required early treatment and 2 used a mutated VH3-21 gene, an IgVH gene that has been associated with progressive disease. We developed reverse transcriptase–polymerase chain reaction and immunohistochemical assays for ZAP-70 expression that can be applied clinically and would yield important prognostic information for patients with CLL.
Introduction
The clinical course of chronic lymphocytic leukemia (CLL) is heterogeneous.1-3 Although some patients have an indolent disease without any need for therapeutic interventions, other patients may succumb rapidly despite intensive treatment. There has been an intense search for good prognostic markers in early-stage disease that might facilitate risk-adapted treatment strategies.4,5 Although cytogenetic abnormalities, especially deletions of 11q and 17p, have been shown to correlate with short survival,6,7 these changes may not be present in early disease and may only be acquired during disease progression.8
The presence or absence of somatic mutations in the variable region of the B-cell–receptor heavy chain gene (IgVH) has been shown to distinguish between 2 disease subsets conferring important prognostic information.9,10 Median survival in patients whose CLL cells express unmutated IgVH genes (Ig-unmutated CLL) ranges between 79 and 119 months.7,9-11 In contrast, patients whose CLL cells express mutated IgVH genes (Ig-mutated CLL) have a distinctly longer median survival, reaching 293 months in one study, and many may never require treatment.11 Several lines of evidence indicate that this survival difference reflects a distinct biologic behavior of the leukemic clone. First, gene expression profiling of CLL samples using DNA microarrays demonstrated differences in the expression patterns of several hundred genes in independent studies.12,13 Many of the differentially expressed genes were also modulated in normal B cells that have been activated by B-cell–receptor signaling, suggesting that the 2 CLL subsets may rely on different signaling pathways for growth and survival.13 Second, immunophenotyping suggests a different activation pattern and/or cell of origin for the 2 disease subtypes.14 Third, Ig-unmutated CLL shows a propensity to acquire distinct karyotypic abnormalities such as deletion of 17p or 11q.7
Given the clinical differences between Ig-mutated and Ig-unmutated CLL, it would be beneficial to incorporate this distinction into the clinical diagnosis of patients with CLL. Most clinical diagnostic laboratories do not have the routine ability to sequence IgVH genes.11 This analysis is time consuming and expensive, making it doubtful that it can be established as a clinical test available to all patients with CLL. Further, the distinction between Ig-mutated and Ig-unmutated CLL is based on the degree of identity between the CLL IgVH sequence and the closest germ line IgVH sequence. However, the optimal cut point for this distinction is not clear. Early studies used a cutoff of 98% sequence identity to allow for germ line immunoglobulin polymorphisms in the human population.9,10,15 In a recent study with 300 patients, a cutoff of 97% sequence identity was optimal for distinguishing patients with CLL that had different overall survival rates.7 However, the 95% confidence interval for this distinction ranged from 96% to 98% sequence identity.
Expression of CD38 as determined by flow cytometry has been shown to have prognostic significance in CLL.16-19 Initially, it was proposed that CD38 might serve as a surrogate marker for IgVH mutational status.9 Subsequent studies have not always shown this relationship.20,21 It has also been suggested that CD38 expression might add to the prognostic information in patients with known IgVH status,20 but 2 large studies that together included more than 500 patients failed to confirm CD38 as an independent prognostic factor in multivariate analysis.7,11 Some of the differences may be due to technical aspects of the CD38 assays and the choice of an optimal cut point for the number of CD38+ cells. The largest study to date found that a cutoff of 7% was best at separating different prognostic groups.7 Another confounding issue is that CD38 expression by the leukemic clone may change during the course of the disease, and an increase of CD38 expression may herald disease progression.1,8
More recently, genomic-scale gene expression profiling has shown that Ig-mutated and Ig-unmutated CLL are readily distinguished from one another by the expression of a few hundred genes.12,13 In one study of 28 patients with CLL, ZAP-70 was found to be more highly expressed in Ig-unmutated CLL than in Ig-mutated CLL and its expression could distinguish these 2 subsets with high statistical significance,13 but a parallel study did not report this finding.12 Because ZAP-70 is also expressed in T cells, this discrepancy may be due to the fact that the latter study did not separate the CD19+ CLL cells from T cells in all cases.12 In the present study, we have expanded this analysis by profiling gene expression in purified CLL samples from 107 patients and show that ZAP-70 expression is the best discriminator of Ig-mutated and Ig-unmutated CLL. Further, we show that ZAP-70 expression identifies patients with a more aggressive clinical course and, therefore, has the potential to be a clinically useful molecular marker of prognosis in CLL.
Patients, materials, and methods
Patients and samples
We studied 107 patients with CLL; 48 (45%) were women and 59 (55%) were men. Median age was 68 years (range, 34-94 years). When diagnosed, 99 (93%) patients were in the low clinical risk category, ie, Binet stage A or Rai stage 0-I. At the time of study, median time from diagnosis was 71 months. Patients were seen at the Royal Bournemouth Hospital, United Kingdom, as previously reported11 or were included in an observational study protocol at the National Institutes of Health, Bethesda, MD. Patients were selected on the basis of the availability of fresh, preferably untreated samples suitable for profiling. Clinical diagnosis of CLL was based on standard morphologic and immunophenotypic criteria, and treatment was initiated for progressive disease according to standard guidelines.22 When the study sample was obtained, 93 patients (87%) were untreated. All patients gave informed consent to the use of blood and tissue samples for research. Peripheral blood mononuclear cells (PBMCs) were obtained by Ficoll gradient centrifugation (ICN Biomedicals, Costa Mesa, CA). B cells were purified using a CD19 magnetic bead system (Miltenyi Biotec, Auburn, CA). Purified cells were stored at –80°C until RNA preparation. CD19 selection typically resulted in more than 98% purity as assessed by flow cytometry. To obtain paraffin cell pellets, PBMCs were washed in phosphate-buffered saline (PBS), pelleted, and resuspended in plasma. Clot formation was initiated with the addition of thrombin. The clot was fixed in 10% formalin and processed by routine techniques. Bone marrow biopsies and aspirate sections were obtained as clinically indicated, fixed in B5 (Advanced Biomedical, Stanwood, WA) and processed by routine techniques.
IgVH gene analysis
Amplification of the IgVH gene was performed as described.10,23,24 In brief: 500 ng mRNA or 1 to 5 μg total RNA was used to generate oligo-dT primed cDNA using Superscript (Invitrogen, Carlsbad, CA). cDNA was amplified by polymerase chain reaction (PCR) using a mixture of 5′ oligonucleotides specific for each leader sequence of the VH1 to VH7 IgVH families23 as forward primers and either a 3′ oligonucleotide complementary to the consensus sequence of the joining region23 or the constant region of the IgM locus24 as reverse primers. For samples that failed to amplify with this combination, IgVH family-specific primers complementary to the 5′ end of framework region 1 (FR1) were used.25 PCR was performed in 50 μL reactions with Taq polymerase (Sigma, St Louis, MO) and 20 pmol of each primer. Cycling conditions were 94°C for 30 seconds, 60°C for 20 seconds, and 72°C for 30 seconds for up to 35 cycles. Products were purified (MinElute PCR Purification Kit; Qiagen, Valencia, CA) and sequenced directly with the appropriate 3′ oligonucleotide using Big Dye Terminator and analyzed using an automated DNA sequencer (Applied Biosystems, Foster City, CA). Nucleotide sequences were aligned to the V-Base sequence directory (http://www.mrc-cpe.cam.ac.uk). Percentage of homology was calculated by counting the number of mutations between the 5′ end of FR1 and the 3′ end of FR3. Sequences with 2% or less deviation from any germ line IgVH sequence were considered unmutated.10
CD38 expression analysis
Whole blood was stained within 24 hours of collection with a panel of antibodies as previously described.26 Five-parameter, 3-color flow cytometry was performed with a fluorescence-activated cell sorter (FACS) Calibur flow cytometer and analyzed with CellQuest software (Becton Dickinson, San Jose, CA). Lymphocytes were gated by forward and side scatter. Isotype controls were run with each patient specimen. CD38+ cells were determined as the percentage of lymphocytes staining more intensely with anti-CD38 (CD38-phycoerythrin [PE]; Becton Dickinson) than with isotype control.
DNA microarray analysis
The DNA microarray methods have been described in detail elsewhere.27,28 Fluorescently labeled cDNA probes were generated from mRNA (Fast Track kit, Invitrogen), using the Cy5 dye to label cDNA from the CLL samples, and the Cy3 dye to label cDNA from a reference pool of mRNA prepared from 9 lymphoma cell lines.28 Lymphochip DNA microarrays containing 13 868 human cDNAs were prepared and used as previously described.13,28 Initial microarray data selection was based on fluorescence signal intensity, with the requirement of 50 relative fluorescent units (RFU) above background in both the Cy3 and Cy5 channels, or 500 RFU above background in either channel alone. Expression data presented in Figure 1 are available at http://llmpp.nih.gov/cll.
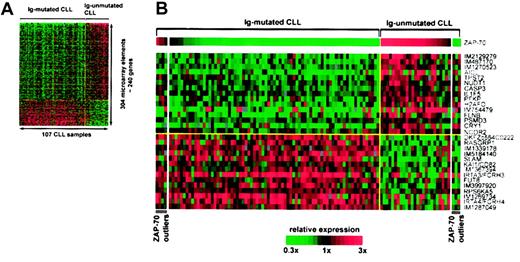
DNA microarray analysis identifies genes that are differentially expressed between Ig-mutated and Ig-unmutated CLL. The relative level of gene expression is depicted according to the color gradient at the bottom. For each array element, the scale is centered at the best distinction cutoff between the CLL subtypes. Red indicates higher expression, and green indicates lower expression of a given gene in the respective subgroup. (A) Expression profile of CLL subtype distinction genes. Relative expression levels of 304 Lymphochip array elements representing approximately 240 genes that were differentially expressed between Ig-mutated and Ig-unmutated CLL at a significance of P < .001 are shown. Columns represent individual patients (n = 107), and rows represent individual array elements. (B) ZAP-70 is the best CLL subtype distinction gene. The 30 most differentially expressed (P < .00001) genes between Ig-mutated and Ig-unmutated CLL are shown. Patients are grouped by their IgVH mutation status and, within subtypes, are arranged by the relative expression level of ZAP-70. Patients discordant for IgVH mutation status and ZAP-70 expression (ZAP-70 outliers) appear at the left (Ig-mutated CLL, ZAP-70 expression above cutoff) and right (Ig-unmutated CLL, ZAP-70 expression below cutoff) ends of the spectrum. Genes are labeled with the gene symbols. For unnamed genes the IMAGE clone numbers (IM) are given. The upper half contains genes more highly expressed in Ig-unmutated CLL and the lower half contains genes more highly expressed in Ig-mutated CLL. In each group, genes are arranged from top to bottom in descending order of statistical significance.
DNA microarray analysis identifies genes that are differentially expressed between Ig-mutated and Ig-unmutated CLL. The relative level of gene expression is depicted according to the color gradient at the bottom. For each array element, the scale is centered at the best distinction cutoff between the CLL subtypes. Red indicates higher expression, and green indicates lower expression of a given gene in the respective subgroup. (A) Expression profile of CLL subtype distinction genes. Relative expression levels of 304 Lymphochip array elements representing approximately 240 genes that were differentially expressed between Ig-mutated and Ig-unmutated CLL at a significance of P < .001 are shown. Columns represent individual patients (n = 107), and rows represent individual array elements. (B) ZAP-70 is the best CLL subtype distinction gene. The 30 most differentially expressed (P < .00001) genes between Ig-mutated and Ig-unmutated CLL are shown. Patients are grouped by their IgVH mutation status and, within subtypes, are arranged by the relative expression level of ZAP-70. Patients discordant for IgVH mutation status and ZAP-70 expression (ZAP-70 outliers) appear at the left (Ig-mutated CLL, ZAP-70 expression above cutoff) and right (Ig-unmutated CLL, ZAP-70 expression below cutoff) ends of the spectrum. Genes are labeled with the gene symbols. For unnamed genes the IMAGE clone numbers (IM) are given. The upper half contains genes more highly expressed in Ig-unmutated CLL and the lower half contains genes more highly expressed in Ig-mutated CLL. In each group, genes are arranged from top to bottom in descending order of statistical significance.
Western blot and immunohistochemistry
Twenty million CD19+ purified CLL cells were lysed in 1 mL lysis buffer containing 1% Triton X-100. Protein concentration was determined by Bradford assay. Per sample, 12 μg protein was separated on an acrylamide gel and transferred to nitrocellulose. Western blots were incubated with a mouse monoclonal antibody to ZAP-70 (clone 2F3.2; Upstate Biotechnology, Lake Placid, NY) in PBS with 4% milk. Loading and transfer efficiency were assessed by immunostaining with a control antibody against β-tubulin (Sigma). Blots were developed by chemiluminescence (Amersham, Piscataway, NJ).
Immunohistochemistry was performed on deparaffinized sections by standard protocols. In brief, the deparaffinized slides were placed in 10 mM citrate buffer (pH 6.0) containing 0.1% Tween 20 and were microwaved for 40 minutes at 700 watts. Antigens were localized using an avidin-biotin-peroxidase method with 3,3′-diaminobenzidine as a chromogen and performed using an automated immunostainer (Ventana Medical Systems, Tucson, AZ) according to the manufacturer's protocol. ZAP-70– (SUDHL-6) and ZAP-70+ (Jurkat) cell lines were used to establish optimal staining conditions. ZAP-70 staining in PBMC pellets and aspirate sections was in general brighter than in bone marrow biopsies as is observed for many antigens. T cells, identified by CD3 staining, served as internal positive controls; residual myeloid cells served as internal negative controls. Anti-CD3 and anti-CD20 antibodies were obtained from Dako (Carpinteria, CA). An independent pathologist (R.E.D.) scored all slides in a blinded fashion.
Statistical analysis
A 2-group t statistic on log 2–transformed mRNA expression ratios was used to measure the ability of each array element to discriminate between the 2 IgVH mutation subtypes of CLL univariately. To create a test for this subtype distinction based on ZAP-70 mRNA expression, we divided the patients into 2 groups on the basis of a cut point of ZAP-70 expression that minimized the classification errors. Time to treatment measured from diagnosis was estimated by the Kaplan-Meier method and was compared by the log-rank test.
Quantitative RT-PCR
An aliquot of the same mRNA used for the DNA microarray study was diluted to approximately 0.5 ng/μL. Diluted mRNA (5 μL) per reaction was used for quantitative reverse transcriptase (RT)–PCR using TaqMan reagents and analyzed in real time on an ABI Prism 7700 Sequence Detector as recommended by the manufacturer (Applied Biosystems). All samples were run in triplicates. Amplification of the sequence of interest was compared with a reference probe (β-2-microglobulin) and normalized against a standard curve of Jurkat cell mRNA. Primers and probes for β-2-microglobulin and Cyclin D1 have been described29 and for ZAP-70 these were 5′-CGCTGCACAAGTTCCTGGT-3′ (forward primer), 5′-GACACCTGGTGCAGCAGCT-3′ (reverse primer), and 5′-CATTGCTCACAGGGATCTCCTCCCTCT-3′ (FAM-probe). All primers and probes were obtained from Synthegen (Houston, TX).
Results
CLL subtype distinction genes
In a pilot gene expression profiling study of 28 CLL samples, approximately 175 genes were found to be differentially expressed between Ig-unmutated and Ig-mutated CLL.13 In this study, we used Lymphochip DNA microarrays to profile gene expression in CD19+-purified CLL samples from a larger cohort of 107 patients to identify the genes that most accurately discriminate between the CLL subtypes, which could potentially be used in a clinical test for this distinction. Using a conventional cutoff of 98% sequence identity to the nearest germ line IgVH sequence, 79 cases (74%) were classified as Ig-mutated CLL and 28 cases (26%) were classified as Ig-unmutated CLL. Of the Ig-unmutated CLL samples, 21 (75%) were 100% identical to a germ line IgVH sequence and 7 (25%) were 98% to 99% identical. The Ig-unmutated and Ig-mutated CLL samples differentially expressed approximately 240 genes (304 microarray elements) with high statistical significance (P < .001) (Figure 1A). These differentially expressed genes included many that were identified in pilot gene expression profiling studies of CLL.12,13 ZAP-70 was by far the best subgroup distinction gene in the present analysis (Figure 1B). ZAP-70 expression was, on average, 5.54-fold higher in Ig-unmutated CLL than in Ig-mutated CLL and distinguished the subtypes with a statistical significance of P < 10-21.
ZAP-70 and CD38 as surrogate markers ofIgVH mutation status
IgVH mutation status confers important prognostic information,7,9-11,20,30,31 but IgVH sequencing is not suitable for most clinical laboratories. We, therefore, investigated whether ZAP-70 mRNA expression could be used as a surrogate marker for this distinction. To do this, we determined a cut point based on ZAP-70 expression levels that would optimally distinguish most Ig-unmutated CLL samples from most Ig-mutated CLL samples (Figure 2A). Using this cut point, 93% of the CLL samples could be classified into the correct CLL subtype. Seven samples (7%) were discordant for IgVH mutation status and ZAP-70 expression (Figure 2A). Among the 79 Ig-mutated CLL samples, 4 showed ZAP-70 expression levels comparable to Ig-unmutated CLL. Among the 28 Ig-unmutated CLL samples, 3 had very low ZAP-70 expression. We searched for other genes whose expression patterns could be combined with ZAP-70 expression to create a multivariate classifier that would perform better than ZAP-70 expression alone; no such gene was found (data not shown).
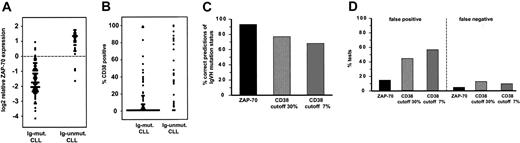
The value of ZAP-70 mRNA and CD38 protein expression as surrogate markers of IgVH mutation status in CLL. (A) Distribution of ZAP-70 mRNA expression levels as measured by DNA microarrays in CLL subtypes. The dashed line indicates the optimal cutoff for distinguishing Ig-unmutated and Ig-mutated CLL. (B) Distribution of CD38 protein expression as measured by flow cytometry and expressed as the percentage of CLL cells more intensely stained with CD38 than with an immunoglobulin isotype control. (C) ZAP-70 mRNA expression is a better predictor of IgVH mutation status than CD38 protein expression. (D) ZAP-70 as a surrogate marker of IgVH mutation status is associated with lower false-positive and false-negative rates than CD38.
The value of ZAP-70 mRNA and CD38 protein expression as surrogate markers of IgVH mutation status in CLL. (A) Distribution of ZAP-70 mRNA expression levels as measured by DNA microarrays in CLL subtypes. The dashed line indicates the optimal cutoff for distinguishing Ig-unmutated and Ig-mutated CLL. (B) Distribution of CD38 protein expression as measured by flow cytometry and expressed as the percentage of CLL cells more intensely stained with CD38 than with an immunoglobulin isotype control. (C) ZAP-70 mRNA expression is a better predictor of IgVH mutation status than CD38 protein expression. (D) ZAP-70 as a surrogate marker of IgVH mutation status is associated with lower false-positive and false-negative rates than CD38.
CD38 surface expression has been shown to be a surrogate marker of IgVH mutation status in some studies.9 Flow cytometric analysis of CD38 expression was available on 104 patients in our study. Early studies considered CLL cases with 30% or more CD38-expressing cells to be CD38+.14 On the basis of this criterion, 31 (30%) of our CLL samples were CD38+. More recently, CD38+ CLL cases were defined as those with 7% or more CD38-expressing cells, based on overall survival analysis in 200 patients.7 In the present series, 49 patients (47%) were CD38+ by this criterion. As expected, CD38 expression tended to be higher in Ig-unmutated CLL samples, but there was a considerable overlap in CD38 expression between the CLL subtypes (Figure 3B). Overall, CD38 predicted IgVH mutation status correctly in 77% of patients when a cutoff of 30% was used and in 67% when a cutoff of 7% was applied (Figure 2C). Thus, considerably more patients were misclassified by CD38 expression than by ZAP-70 expression. Furthermore, CD38 expression yielded more false-positive and false-negative assignments than did ZAP70 expression (Figure 2D).
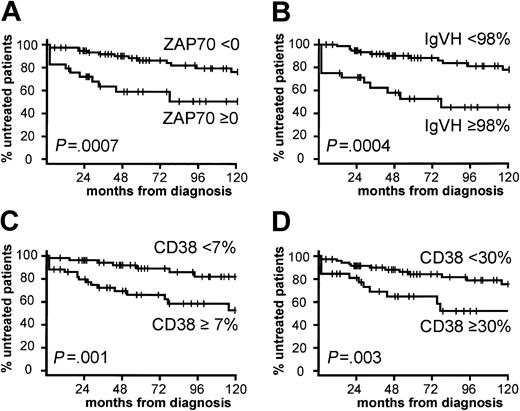
Effect of prognostic features on the clinical course of CLL. Rate of disease progression as assessed by the treatment-free time interval measured in months from diagnosis for (A) ZAP-70 mRNA expression, (B) IgVH mutation status, (C) CD38 protein expression with cutoff of 7%, and (D) CD38 protein expression with cutoff of 30%.
Effect of prognostic features on the clinical course of CLL. Rate of disease progression as assessed by the treatment-free time interval measured in months from diagnosis for (A) ZAP-70 mRNA expression, (B) IgVH mutation status, (C) CD38 protein expression with cutoff of 7%, and (D) CD38 protein expression with cutoff of 30%.
IgVH mutation status confers important prognostic information for patients with CLL.7,9-11,20,30,31 In addition, several groups have reported the prognostic value of CD38 expression without correlating it with IgVH mutational status.8,16-19,32,33 We compared IgVH mutation status, ZAP-70 mRNA expression, and CD38 surface expression for their ability to predict time to disease progression, as judged by treatment requirement. At the time of last follow-up, 29 patients (27%) had been treated. The patients with CLL were divided into 2 groups based on the ZAP-70 expression cutoff described earlier (Figure 2A), and these groups differed significantly in their time to treatment following diagnosis (P = .0007) (Figure 3A). ZAP-70 expression and IgVH mutation status were comparable in their ability to define patients with CLL, who were different with respect to disease progression (Figure 3A-B). CD38 expression also distinguished patients who required treatment early versus late (Figure 3C-D), albeit less well than either IgVH mutation status or ZAP-70 expression. In accord with a previous study,7 we found that a 7% cutoff for CD38 expression was better than a 30% cutoff in predicting time to disease progression (Figure 3C-D).
Important prognostic information in CLL can also be derived from cytogenetic analysis.6 Notably, deletions of 11q and 17p are associated with rapidly progressive disease and short survival, whereas deletions of 13q and trisomy 12 are associated with good and intermediate prognosis, respectively. Cytogenetic analysis was available in 94% of our patients. Among patients with low or absent ZAP-70 expression, 13q deletion was present in 59% of patients. Other common abnormalities were trisomy 12 in 18% and normal cytogenetics in 13%. Deletion of 11q was detected in only 2 (3%) patients in this group. In patients with high ZAP-70 expression, trisomy 12 was the most common abnormality, present in 38% of patients followed by 11q deletion and normal cytogenetics, each of which were found in 21% of patients in this group. The biased distribution of cytogenetic abnormalities between the 2 groups is consistent with reported differences between cytogenetic findings in Ig-mutated versus Ig-unmutated CLL.11,34
Patients discordant forIgVH mutational status and ZAP-70expression have distinct biologic and clinical characteristics
As mentioned earlier, 7 patients were discordant for ZAP-70 expression and IgVH mutation status, and we will refer to these patients as ZAP-70 outliers. All ZAP-70 outliers fulfilled the diagnostic criteria for CLL, and the cytogenetic abnormalities in the leukemic cells of these patients were typical of CLL (Table 1).
The 4 ZAP-70 outliers with mutated immunoglobulin genes expressed ZAP-70 at levels comparable to those of Ig-unmutated CLL cases (Figure 4A). However, these CLLs expressed other CLL subtype distinction genes at levels typical of Ig-mutated CLL (Figure 1B; left 4 samples). Because ZAP-70 was uniquely discordant in these cases and is highly expressed in T cells, we examined whether contamination of these CLL samples with T cells might be responsible. This possibility was unlikely given that each CLL sample was purified by magnetic sorting to more than 98% homogeneity. These CLL samples did not express other T-cell–specific genes (eg, T-cell–receptor α, CD3 γ, etc) at higher levels than other CLL samples, thereby ruling out T-cell contamination as a confounding influence (data not shown).
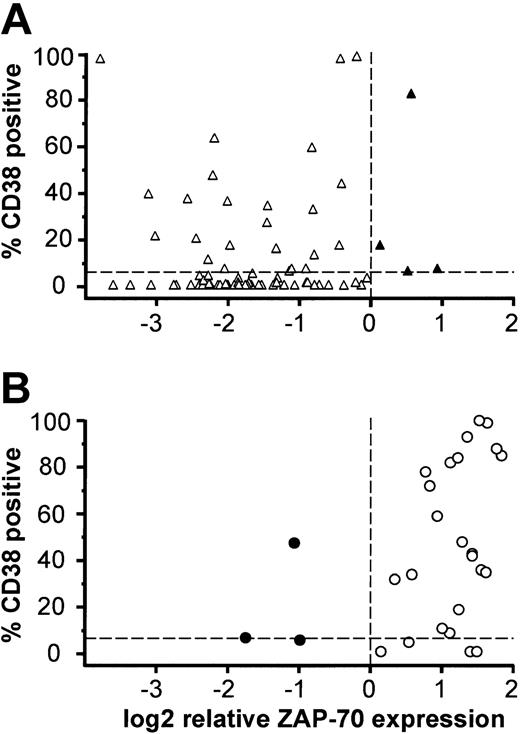
ZAP-70 outliers show a distinct CD38 expression pattern.ZAP-70 mRNA expression and CD38 status is shown for (A) 78 patients with Ig-mutated CLL and (B) 29 patients with Ig-unmutated CLL. Open symbols represent CLL samples concordant for mutational status and ZAP-70 expression, and filled symbols represent ZAP-70 outliers. The dashed lines indicate the cutoff between Ig-mutated and Ig-unmutated CLL.
ZAP-70 outliers show a distinct CD38 expression pattern.ZAP-70 mRNA expression and CD38 status is shown for (A) 78 patients with Ig-mutated CLL and (B) 29 patients with Ig-unmutated CLL. Open symbols represent CLL samples concordant for mutational status and ZAP-70 expression, and filled symbols represent ZAP-70 outliers. The dashed lines indicate the cutoff between Ig-mutated and Ig-unmutated CLL.
The 3 ZAP-70 outliers with unmutated immunoglobulin genes expressed ZAP-70 at low levels, comparable to those observed in most Ig-mutated CLL cases (Figure 4B). In addition, the expression of other CLL subtype distinction genes in these cases was inconsistent, and not characteristic of either CLL subtype (Figure 1B; right 3 samples). One of these cases had an atypical immunophenotype, with weak CD5 expression, bright CD20, FMC7, and CD79b expression (patient U2; Table 1). Nonetheless, the leukemic cells of this patient had trisomy 12, which is characteristic of CLL. Because in some instances it can be difficult to distinguish leukemic mantle cell lymphoma from CLL, we performed quantitative RT-PCR for cyclin D1, which was negative in all 3 patients (data not shown). Taken together, we conclude that these patients have CLL with an unusual gene expression profile that is not typical of either Ig-mutated or Ig-unmutated CLL.
Interestingly, most of the ZAP-70 outliers were also discordant for CD38 expression compared with other CLL patients, based on a cutoff of 7% CD38-expressing cells (Figure 4A-B). Among the Ig-mutated CLL cases that were ZAP-70 outliers, all were CD38+, whereas most other Ig-mutated CLL cases were CD38– (Figure 4A). Among the 3 Ig-unmutated ZAP-70 outliers, 1 was CD38– and 1 was on the borderline between CD38+ and CD38–, whereas most other Ig-unmutated CLL cases were CD38+ (Figure 4B). These findings suggested that the ZAP-70 outliers include cases with unique biologic characteristics.
Two of the 4 Ig-mutated ZAP-70 outlier patients had rapid disease progression and required early treatment, which is unusual for this CLL subtype. Intriguingly, the CLL cells of 2 of these patients expressed a mutated VH3-21 gene, and these were the only cases in our series that used this IgVH gene. This finding is notable because expression of a mutated VH3-21 gene has been associated with progressive disease and may represent a biologically distinct subset of Ig-mutated CLL.35
ZAP-70 assays for potential clinical application
We have demonstrated that ZAP-70 mRNA expression, as measured by DNA microarrays, can be used to assign most patient with CLL to the correct IgVH mutational subtype and can identify patient subsets that have distinct treatment requirements. A clinical test based on ZAP-70 expression would, therefore, be a useful adjunct in patient management.
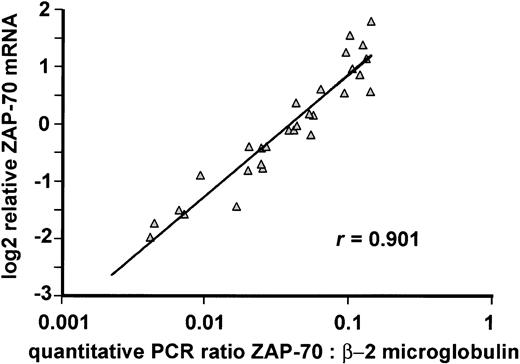
To this end, we devised a quantitative RT-PCR assay for ZAP-70 expression and compared the quantitation of ZAP-70 mRNA levels by this method with the results from the DNA microarray analysis (Figure 5). The 2 assays showed an excellent correlation over a wide range of ZAP-70 mRNA levels. These results confirm the quantitative nature of the DNA microarray measurements and suggest that a quantitative RT-PCR assay might be suitable for measuring ZAP-70 expression in a clinical setting. However, this assay requires that the leukemic cells be purified, because ZAP-70 is expressed highly in T cells.
Quantitative RT-PCR could serve as a clinical test of ZAP-70 mRNA expression. Real time quantitative RT-PCR was performed in 29 CLL samples representing the ZAP-70 mRNA expression spectrum defined by the DNA microarray analysis. ZAP-70 expression is shown relative to the expression of β-2-microglobulin in the same sample. The Pearson coefficient for correlation between the 2 methods was r = 0.901.
Quantitative RT-PCR could serve as a clinical test of ZAP-70 mRNA expression. Real time quantitative RT-PCR was performed in 29 CLL samples representing the ZAP-70 mRNA expression spectrum defined by the DNA microarray analysis. ZAP-70 expression is shown relative to the expression of β-2-microglobulin in the same sample. The Pearson coefficient for correlation between the 2 methods was r = 0.901.
We also investigated the possibility that ZAP-70 protein levels could be used for a clinical diagnostic test. Western blots of lysates of CD19+ CLL cells revealed high levels of ZAP-70 protein expression in Ig-unmutated CLL samples compared with the relatively low levels in Ig-mutated CLL samples (Figure 6A). Because this assay quantitates ZAP-70 protein in a population of cells, it was critical to remove T cells given their high ZAP-70 protein expression.
ZAP-70 protein expression can distinguish CLL subtypes. (A) ZAP-70 protein expression was assessed by Western blotting in whole cell lysates of normal PBMCs, or CD19+-purified leukemic cells from blood of patients with Ig-unmutated and Ig-mutated CLL. The data are representative of Western blot analysis of 60 patient samples analyzed. Equal loading is demonstrated by probing for β-tubulin. (B) ZAP-70 can be detected by immunohistochemistry in clinical samples. PBMCs (top 2 rows; original magnification, × 200) were embedded in a fibrin clot, fixed, and processed by standard techniques. PBMCs and routine bone marrow trephine biopsies (bottom 2 rows; original magnification, × 100-200) were stained with CD20, demonstrating involvement by B-CLL, and CD3, which stains interspersed T cells. Insets show areas of the respective sample at an original magnification of × 1000. ZAP-70 was positive in T cells and Ig-unmutated CLL cells.
ZAP-70 protein expression can distinguish CLL subtypes. (A) ZAP-70 protein expression was assessed by Western blotting in whole cell lysates of normal PBMCs, or CD19+-purified leukemic cells from blood of patients with Ig-unmutated and Ig-mutated CLL. The data are representative of Western blot analysis of 60 patient samples analyzed. Equal loading is demonstrated by probing for β-tubulin. (B) ZAP-70 can be detected by immunohistochemistry in clinical samples. PBMCs (top 2 rows; original magnification, × 200) were embedded in a fibrin clot, fixed, and processed by standard techniques. PBMCs and routine bone marrow trephine biopsies (bottom 2 rows; original magnification, × 100-200) were stained with CD20, demonstrating involvement by B-CLL, and CD3, which stains interspersed T cells. Insets show areas of the respective sample at an original magnification of × 1000. ZAP-70 was positive in T cells and Ig-unmutated CLL cells.
To test if ZAP-70 protein expression might be detectable by immunohistochemistry, we studied peripheral blood and routine bone marrow biopsy samples from patients with CLL (Figure 6B). In all samples, T cells stained strongly for ZAP-70, as expected. In samples from Ig-mutated CLL patients, the leukemic cells were negative or weak for ZAP-70 staining, whereas the interspersed T cells were strongly positive. In samples from Ig-unmutated CLL, both the leukemic cells and the T cells had readily detectable staining. We analyzed ZAP-70 expression in 100 clinical samples from 43 patients. In a blinded analysis, a pathologist (R.E.D.) assigned 37 (86%) of the 43 patients correctly to the IgVH mutation subtype on the basis of ZAP-70 staining by immunohistochemistry. The predictive values of positive (n = 21) and negative tests (n = 22) were 71% and 100%, respectively. Two misclassified patients were ZAP-70 outliers in the DNA microarray analysis and used the mutated VH3-21 IgVH gene. Thus, in these patients the immunohistochemistry was in accord with the mRNA measurement. Interestingly, in 2 further misclassified cases ZAP-70 was positive only in a subset of the leukemic cells comprising less than 25% of the sample. This finding suggests clonal heterogeneity in these patients and could be of biologic significance. The 2 remaining misclassified cases were low for ZAP-70 mRNA expression and, therefore, were false positive by immunohistochemistry.
Discussion
We show here that ZAP-70 mRNA expression is an excellent surrogate marker for the distinction between the Ig-mutated and Ig-unmutated CLL subtypes and can identify patient groups with divergent clinical courses. ZAP-70 expression assigned 93% of the patients studied to the correct immunoglobulin mutation subtype. No other gene represented on our microarrays was as good as ZAP-70 in making this CLL subtype distinction, nor could any other gene improve the predictive power of ZAP-70. High ZAP-70 expression identified a clinically progressive form of CLL. In our series, this group of patients had a median time to treatment following diagnosis of 6.4 years. By contrast, patients whose leukemic cells had low ZAP-70 expression had an indolent disease with a median time to treatment of more than 10 years.
These observations suggest that measurement of ZAP-70 expression would be a useful clinical test that could help guide treatment decisions. In this regard, it is important that the measurement of ZAP-70 expression had a relatively low false-positive rate compared with CD38. Early treatment might be beneficial for patients whose CLL cells have high ZAP-70 expression. This potential would have to be tested in a clinical trial in which patients were stratified for early or late treatment on the basis of ZAP-70 expression. By contrast, patients whose CLL cells have low ZAP-70 expression might be managed best by delaying treatment for as long as possible. Other prognostic markers such as chromosomal abnormalities (ie, 11q or 17p deletion) should also be taken into consideration when designing protocols for stratifying the treatment of CLL patients.
The mechanisms underlying the differential expression of ZAP-70 in CLL remain obscure. ZAP-70 is expressed primarily in T cells and natural killer (NK) cells,36 but it is also detectable in mast cells37 and basophils.38 In T cells, ZAP-70 becomes phosphorylated and active as a tyrosine kinase as a result of T-cell–receptor signaling, and has been shown to be essential for T-cell activation.39 In addition, ZAP-70 may participate in other signaling pathways in T cells, NK cells, and mast cells.37,40-43 The first evidence of ZAP-70 expression in B cells came from an earlier gene expression profiling study that demonstrated ZAP-70 expression in Ig-unmutated CLL, as confirmed in the present study, and also in some B-cell lines that are unrelated to CLL.13
At present, it is unclear whether ZAP-70 expression in B cells reflects a particular stage of B-cell differentiation or instead is a consequence of signaling events and/or disease progression. Analysis of the ZAP-70 outliers may be instructive in this regard. It is intriguing that 5 of 7 of the ZAP-70 outliers were also discordant for CD38 expression. In addition to being a differentiation marker, CD38 surface expression can be induced by a variety of stimuli, including phorbol esters,44 interferons,45 and retinoic acid.46 CD38 surface expression can vary over time in the same patient with CLL and, in some patients, increased CD38 expression can be associated with disease progression.20 These observations suggest that CD38 and possibly also ZAP-70 expression may not be fixed attributes of CLL leukemic cells and may be altered by extrinsic or intrinsic signaling events.
Several of the ZAP-70 outlier patients that had mutated immunoglobulin genes had clinical features that distinguished them from other patients with Ig-mutated CLL. Among the 4 Ig-mutated patients whose CLL cells expressed ZAP-70 and CD38 in the present study, 2 required early treatment, which is uncharacteristic of the Ig-mutated CLL subtype. Notably, the leukemic cells from 2 of these patients expressed mutated VH3-21 genes, and this particular IgVH gene has previously been associated with progressive disease among patients with Ig-mutated CLL.35 These considerations suggest that ZAP-70 expression in CLL may reflect an activation state of the malignant clone that is associated with progressive disease in some patients. In this model, patients with Ig-unmutated CLL are responding to activation signals that lead to disease progression and ZAP-70 expression. Alternatively, it is conceivable that ZAP-70 may play a functional role in CLL disease progression because it is a tyrosine kinase that can signal downstream of many surface receptors. In this regard, it is interesting that Ig-unmutated CLL shows a higher degree of tyrosine phosphorylation following B-cell–receptor (BCR) stimulation than Ig-mutated CLL,47,48 and ZAP-70 can associate with the BCR.47 Further work will be needed to define the functional role of ZAP-70 in CLL cells in vivo.
The combined use of gene expression profiling and IgVH sequencing led to the proposal that CLL is a single disease with 2 common variants, Ig-unmutated and Ig-mutated CLL.12,13 The present analysis is in broad agreement with this view, but the ZAP-70 outlier patients suggest that CLL cannot be neatly divided into 2 categories on the basis of IgVH sequencing and gene expression. The notion that there are additional variants of CLL is supported by the observation that patients whose CLL cells express mutated VH3-21 genes have a progressive clinical course, unlike most other patients with Ig-mutated CLL.35 Given the fact that an individual IgVH gene can be associated with a distinct clinical behavior, it is possible that self or environmental antigens play a role in the disease process. In addition, chromosomal abnormalities clearly also change the clinical characteristics of the malignant clone.6,7 As we understand the pathogenesis of CLL further, we may identify the molecular and cellular mechanisms that cause some patients to have a progressive clinical course whereas others have indolent disease, and this knowledge will be used to refine our diagnosis of CLL subtypes.
These considerations notwithstanding, a clinical test for ZAP-70 expression could be an important adjunct in patient management. In multivariate analysis, IgVH mutational status is a prognostic marker of overall survival that is independent of other factors, including chromosomal abnormalities and white blood cell count.7,11 Currently, IgVH sequencing is not available to most patients with CLL, and it is unlikely that IgVH sequencing will be turned into a routine clinical laboratory test given its expense and technical challenges. Because ZAP-70 expression can assign most patients to the correct Ig-mutation subtype, a clinical test for ZAP-70 expression would be a useful surrogate for this distinction.
How might ZAP-70 expression be evaluated in a clinical diagnostic laboratory? We demonstrated a strong correlation between the ZAP-70 mRNA levels measured by DNA microarray and by quantitative RT-PCR. Quantitative PCR assays are already in use in clinical diagnostic laboratories to measure, for example, HIV-1 viral load. Quantitative PCR assays have exceedingly low variation in measurement and thus would be ideal for accurately discriminating the CLL subtypes according to ZAP-70 expression. A difficulty of this assay, however, is that it requires purification of the leukemic cells. Nonetheless, it is conceivable that a rapid magnetic purification of CD19+ leukemic cells could be performed in an automated fashion in a clinical laboratory. We also demonstrated that ZAP-70 protein expression, as detected by an immunohistochemical assay, correlated well with immunoglobulin mutational status. This assay is easy to perform and does not require purification of the CLL cells. However, this assay is only semiquantitative in nature and may make more false assignments among CLLs that express ZAP-70 at intermediate levels. These issues should be resolved by testing the RT-PCR assay for ZAP-70 mRNA expression and the immunohistochemical assay for ZAP-70 protein expression for their ability to predict survival in the context of a prospective clinical trial of CLL.
Prepublished online as Blood First Edition Paper, February 20, 2003; DOI 10.1182/blood-2002-10-3306.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We appreciate the help of Therese White and Nicole Drbohlav Grant in collecting clinical study samples and the friendly support provided by the staff of the pathology and immunohistochemistry laboratories.