In 65 patients with hemophagocytic lymphohistiocytosis (HLH), we found an as yet undescribed heterogeneity of defects in cellular cytotoxicity when assay conditions were modified by the incubation time, the presence of mitogen, or interleukin-2 (IL-2). The standard 4-hour natural killer (NK) test against K562 targets was negative in all patients. In patients deficient in type 1 (n = 21), type 2 (n = 5), and type 4 (n = 8) HLH, negative NK function could be reconstituted by mitogen, by IL-2, or by prolongation of the incubation time (16 hours), respectively. Most patients (n = 31) displayed the type 3 defect, defined by a lack of any cellular cytotoxicity independent of assay variations. The characteristic hypercytokinemia also concerned counterregulatory cytokines, such as proinflammatory interferon-γ (IFN-γ), simultaneously elevated with suppressive IL-10 in 38% of types 1–, 2–, and 4–deficient patients and in 71% of type 3–deficient patients. Elevated IFN-γ alone correlated with high liver enzymes, but sCD95-ligand and sCD25 did not—though these markers were expected to indicate the extent of histiocytic organ infiltration. Outcome analysis revealed more deaths in patients with type 3 deficiency (P = .017). Molecular defects were associated with homozygously mutated perforin only in 4 patients, but other type 3 patients expressed normal transcripts of effector molecules for target-cell apoptosis, including perforin and granzyme family members, as demonstrated by RNase protection analysis. Thus, target-cell recognition or differentiation defects are likely to explain this severe phenotype in HLH. Hyperactive phagocytes combined with NK defects may imply defects on the level of the antigen-presenting cell.
Introduction
Hemophagocytic lymphohistiocytosis (HLH) constitutes a severe sepsislike illness with massive hypercytokinemia and with proliferating and organ-infiltrating phagocytes, causing cytopenia of different hematopoietic lineages.1,2 Various genetic or acquired immunodeficiencies may be involved.3-5In addition to high levels of interleukin-6 (IL-6), IL-8, interferon-γ (IFN-γ), IL-10, macrophage inflammatory protein–1α (MIP-1α), macrophage–colony-stimulating factor (M-CSF), and tumor necrosis factor-α (TNF-α), very high plasma concentrations of sCD25, sCD95-ligand,5,6 and IL-187 are characteristic. By contrast, the inflammatory marker IL-1β is not elevated.8 Another common denominator in HLH is defective natural killer (NK) cell activity or a total lack of cellular cytotoxicity.9-14 Impaired NK function has also been described in the patients' parents, and the mothers appear to be more frequently affected.15 Thus, NK deficiency could be a marker or a susceptibility gene for this hemophagocytic syndrome. The already-described null mutation of the perforin gene in consanguineous families with HLH16 17 is compatible with this concept. The current study aimed at a fine analysis of defects in cellular cytolysis and an understanding of the function of overexpressed sCD25, sCD95-ligand, IFN-γ, and IL-10. Results demonstrate that patients with the most severe cases of HLH have totally negative cellular cytotoxicity. This group contains many patients with simultaneously elevated IFN-γ and IL-10 levels in the plasma.
Patients, materials, and methods
Patients
Sixty-five patients with HLH (71% were aged 2 years or younger; 62% males, 38% females) were diagnosed from different clinical centers in Germany and Europe on the basis of the diagnostic guidelines proposed by the Histiocyte Society.18 Sixteen patients were Turkish; 4 of them had a homozygous null allele mutation at amino acid position 374 in exon 3. An additional diagnostic marker for HLH was high to very high sCD25 plasma level.14
Pediatric controls
Healthy pediatric sibling donors were tested for cellular cytotoxicity before allogeneic matched bone marrow transplantation (BMT). The functional assays were part of immune functional testings before BMT and were applied to monitor functional reconstitution after BMT.
Preparation of nonadherent lymphocytes
Ficoll-Hypaque–isolated mononuclear cells were prepared from 1 mL heparinized peripheral patient blood collected at diagnosis to isolate more than 2 × 106 nonadherent lymphocytes (NALs). Adherent cells were depleted by overnight incubation in RPMI 1640 medium containing 25 mM HEPES (N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid), L-glutamine, and 10% prescreened, endotoxin-free fetal calf serum (FCS) (less than 2 ng/mL; Boehringer Mannheim Batch 68030402). These NALs served as the effector population for cytotoxicity assays.
Cytotoxicity assay
Sedimented K562 target cells were labeled with 20 μCi (0.74 MBq) sodium chromium Cr 51 (Behring-Werke, Marburg, Germany) for 3 hours, washed, and plated with 104 target cells/well in 96-well round-bottomed microtiter plates. Effector cells were added to obtain effector/target (E/T) cell ratios ranging between 100:1 and 30:1. Three to 4 dilutions of E/T ratios were tested from each patient isolate. Mitogen-activated killing was tested by adding phytohemagglutinin (PHA; Gibco-BRL, Karlsruhe, Germany) at 0.2% (vol/vol). Supernatants were removed and tested for isotope release using a Betaplate scintillation counter (Wallac GmbH, Perkin-Elmer, Freiburg, Germany). Spontaneous release was not higher than 9% of the maximum release in the presence of 3% Triton X100. Cytotoxicity was quantified by calculating the percentage specific lysis from the following formula: cpm (tested) − cpm (spontaneous release)/[(maximum release) − cpm (spontaneous release)] × 100. The lytic units (LU) were calculated from a linear regression analysis of the cytotoxic activity measured at different E/T cell ratios; 1 LU is defined as the reciprocal value of the amount of effector cells necessary to achieve 25% lysis of 107target K562 cells.19
Surface markers
Leukocyte subpopulations were determined by flow cytometric analysis (FACScalibur, BD Europe and CellQuest software) using monoclonal antibodies directed against T , B, and NK cells (CD2, clone Leu5b; CD3, clone Leu4; CD4, clone Leu3a; CD8, clone Leu2a; TCR alpha/beta, clone WT31; CD56, clone Leu19; CD57, Leu7; CD19, Leu12; all were from Becton Dickinson Europe, Heidelberg, Germany.
Generation of lymphokine-activated killers by high-dose IL-2
NK cell function was activated according to a lymphokine-activated killer (LAK) protocol by incubating NAL in 103 IU/mL recombinant IL-2 (rIL-2; Proleukin; Cetus) in RPMI 1640 (Gibco-BRL, Karlsruhe, Germany), 25 mM HEPES, and 10% FCS for 72 hours. For RNA isolation, LAK was propagated for another 7 days using IL-2.
Determination of plasma cytokines and soluble receptors
Plasma was collected from blood samples following Ficoll-separation, aliquoted, and frozen for cytokine determinations. Enzyme-linked immunosorbent assay (ELISA) for IFN-γ were purchased from Genzyme through R&D (Heidelberg, Germany) (DuoSet), IL-10 from BioSource (Hamburg, Germany) and Diaclone (Besançou, France), purchased through Hölzel (Cologne, Germany). Soluble CD25 was determined by an automated chemiluminescent assay (EURO/DPC Glyn Rhonwy, United Kingdom, purchased via DPC-Biermann, Bad Nauheim, Germany). Soluble CD95-ligand (sCD178) and plasma concentrations of IL-18 were quantified by the respective ELISA (MBL, Nagoya, Japan) distributed through Beckman-Coulter (Krefeld, Germany).
Perforin mutation analysis
Genomic DNA was prepared from leukocytes, and cDNA was generated using primers as described by Stepp et al.16 Fragments of exon 2 (3164-3918) and exon 3 (4882-6179) were analyzed using ssDNA sequencing.
RNase protection analysis
Lymphoblasts activated with 103 IU/mL recombinant human IL-2 (rhIL-2) were subjected to RNA analysis using TRIzol preparation. The RiboQuant RNase protection assay for a selection of killer-specific and apoptosis-related gene products, APO-3, was purchased from PharMingen (San Diego, CA). Probes were prepared by synthesizing 32P-labeled RNA strands from plasmid DNA fragments containing gene-specific sequences of 100 to 300 bp in length using T7-polymerase and then were hybridized with highly purified RNA preparations overnight, incubated in RNase, proteinase K cocktails. Following purification, the RNA probe hybridized samples of approximately 103 to 104cpm per sample were subjected to gel electrophoresis. The radioactive bands are visualized by x-ray film exposure at −70°C for 4 to 24 hours. All assays were repeated 2 or more times, giving the same pattern of results.
Statistics
Mean and median values were calculated for values in Figure1 (cytotoxicity of representative patients for the different groups) and Figure2 (cytokine plasma concentrations of all patients), respectively. Results were attributed to patients of either type 1, 2, 3, or 4 NK deficiency. Spearman rank correlation coefficients (SCC) with corresponding scatter plots were calculated, scatter plots were drawn for all parameters in the patients with HLH, and the subclassifications were defined by their distinct NK-deficiency states. Contingency table analyses were applied to interpret differences among groups, and Fisher exact test was used to compare death rates between groups.
Cytolytic deficiency types in HLH tested against K562.
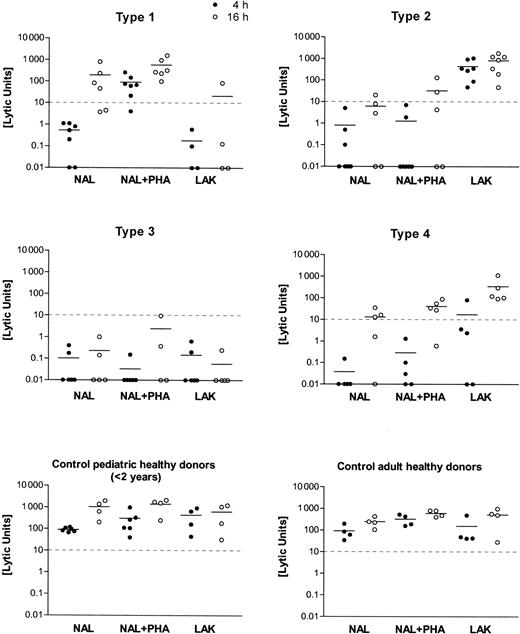
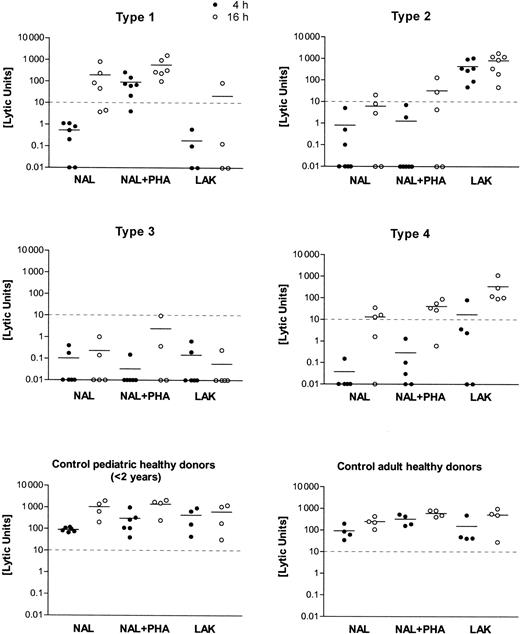
Distinct cytotoxic deficiency types 1, 2, 3, and 4 were identified by testing NAL and LAK from patients against K562 targets, with and without agglutinating mitogen (PHA) and at different target incubation times (4 hours, 16 hours). Killer function was calculated as lytic units (LU) from the regression analysis of 3-4 titrations of the initial effector/target cell ratio ranging between 100:1 and 30:1. Mean values of the results are shown as a horizontal bar in the scatter plots of 4-hour (filled circles) and 16-hour (open circles) incubation time in the assay. The cutoff level of 10 LU for the minimum cytolytic function by NK cells (4-hour incubation time, K562 targets) is given as a horizontal dashed line.
Cytolytic deficiency types in HLH tested against K562.
Distinct cytotoxic deficiency types 1, 2, 3, and 4 were identified by testing NAL and LAK from patients against K562 targets, with and without agglutinating mitogen (PHA) and at different target incubation times (4 hours, 16 hours). Killer function was calculated as lytic units (LU) from the regression analysis of 3-4 titrations of the initial effector/target cell ratio ranging between 100:1 and 30:1. Mean values of the results are shown as a horizontal bar in the scatter plots of 4-hour (filled circles) and 16-hour (open circles) incubation time in the assay. The cutoff level of 10 LU for the minimum cytolytic function by NK cells (4-hour incubation time, K562 targets) is given as a horizontal dashed line.
Elevation of IL-10, IFN-γ, sCD25, and sCD95-ligand in types 1-, 2-, 3-, and 4-deficient HLH.
Plasma concentrations of IL-10, IFN-γ, and the soluble receptors sCD25, sCD95-L were determined in patients with active HLH. Numbers of patients with type 1, type 2, type 3, and type 4 cytotoxic deficiency are given below the x-axis. Median values are indicated by a single bar within the column scatter. Values below or within the dashed horizontal line indicate the individual range of values determined in plasma of healthy controls (normal values, 0-10 pg/mL for IL-10 [n = 92]; 0-10 pg/mL IFN-γ [n = 60]; 200-1000 U/mL for sCD25 [n = 120]; < 50 pg/mL for sCD95-ligand [n = 39]).
Elevation of IL-10, IFN-γ, sCD25, and sCD95-ligand in types 1-, 2-, 3-, and 4-deficient HLH.
Plasma concentrations of IL-10, IFN-γ, and the soluble receptors sCD25, sCD95-L were determined in patients with active HLH. Numbers of patients with type 1, type 2, type 3, and type 4 cytotoxic deficiency are given below the x-axis. Median values are indicated by a single bar within the column scatter. Values below or within the dashed horizontal line indicate the individual range of values determined in plasma of healthy controls (normal values, 0-10 pg/mL for IL-10 [n = 92]; 0-10 pg/mL IFN-γ [n = 60]; 200-1000 U/mL for sCD25 [n = 120]; < 50 pg/mL for sCD95-ligand [n = 39]).
Results
Cytotoxic defects in HLH
In vitro cytolytic function of lymphocytes from 65 patients with HLH differed markedly from those of the control subjects tested. HLH patients were classified according to 1 of 4 subgroups with respect to their lymphocyte cytotoxic functions. Individual results on the lytic units calculated from the respective titration curves are shown in Figure 1. Four to 7 NAL preparations from each type of cytotoxic effector defects are shown. An equal number of healthy donors (adults or children younger than 2), covering a less variable range of activity than the patients, is shown in Figure 1.
To exclude the effect by variable NK cell numbers, the relative amounts of CD56+ NK cells and CD8+ cytotoxic T lymphocytes (CTLs) were determined and are displayed as mean ± SD in Table 1. The values are no different from those of controls except for type 3–deficient patients, who had fewer CD56+ NK cells.
As displayed in Figure 1 and Table 1, type 1–deficient NK cells lacked lytic function against K562 lysis at 4 hours. NK function was reconstituted by PHA but not by high-dose IL-2 in a LAK protocol. Lysis at 16 hours was normal. The number of CD56+ NK cells was 12.3 ± 6.5 and was in a normal range. The number of CD8+CTLs was 35.6 ± 20.5 and, thus, slightly increased when compared with normal values. In type 2–deficient patients, all assay conditions gave low killing activity, but LAK treatment caused strong activation NK cell lysis. CD56+ cells were 10.5% ± 6.8% in the lymphocyte fraction and were thus diminished compared with values in the healthy controls. CD8+ CTL percentages were also higher than in the controls (33.8% ± 15.4%). In the type 3–deficient patients, the cellular cytotoxicity was entirely negative, and there were unequivocally fewer CD56+ NK cells than in the healthy controls (8.7% ± 5.3%). CD8+ CTL percentages were 26.5% ± 10.6%. Type 4–deficient patients displayed a negative 4-hour cytolytic function, including LAK. Cellular cytotoxicity normalized at 16 hours. CD56+ NK cells were in the normal range for children (13% ± 7.9%). CD8+lymphocytes had a mean of 29% ± 12.6%, similar to the controls. When the cytolytic effectors of pediatric and adult healthy controls were compared, we found more CD56+ NK cells (18.9% ± 5.2% vs 13.4% ± 4.4%) but fewer CD8+cytotoxic lymphocytes (31.4% ± 3.5% vs 26.7% ± 6.0%) in adults.
Assignment of cytotoxic defects to the 65 patients analyzed here demonstrated that type 1–deficient (n = 21) and type 3–deficient (n = 31) patients formed the major groups (Table2). In our population Turkish patients have a higher incidence of consanguinity than non-Turkish persons. Hence, we distinguished Turkish from non-Turkish white patients. Accordingly, the relative amount of type 3–deficient patients was higher among Turkish patients (10 of 16; Table 2) than in non-Turkish whites (18 of 43; Table 2). Four Turkish type 3–deficient patients had the homozygous Trp374 stop mutation in the perforin coding exon 3.17
Plasma cytokines, ligands, and enzymes
The distribution of the plasma cytokine levels (IL-10 and IFN-γ and the elevations of the alpha chain of the IL-2 receptor and the CD95-ligand are similar in the 4 groups of patients, who differ by their cytotoxicity deficiency patterns, despite the fact that type 3–deficient patients appear to have the highest IL-10 levels.
When we calculated Spearman rank correlation coefficients (SCC), we found that in types 1-, 2-, and 4-deficient patients, neither soluble receptors, such as sCD25 and sCD95-L, nor IFN-γ and IL-10 correlated with the elevated liver enzymes GOT and GPT. In addition, the nonspecific marker, LDH, indicating tissue damage and hemolysis, was not correlated with any of these inflammatory markers. However, in the type 3–deficient patients, IFN-γ correlated with elevated GPT and GOT, as indicated by higher SCCs (0.66 [GPT] and 0.74 [GOT]), respectively. Again, neither sCD95-ligand (sCD178) nor sCD25—each of which was a marker indicating the extent of histiocytic infiltration in tissues and organs—was correlated with elevated liver enzymes as in bacterial sepsis.20 Type 3–deficient patients had a remarkable correlation of counterregulatory cytokines IFN-γ with IL-10 (SSC = 0.62), in that 22 of 31 (71%) patients had high IFN-γ and IL-10 levels, simultaneously. The correlation of IFN-γ with IL-10 was also seen in types 1-, 2-, and 4-deficient patients (SCC = 0.51) but was not very strong because only 13 of 34 (38%) patients had simultaneously elevated IL-10 and IFN-γ values. The relevance of IL-10 plasma concentrations is further stressed by high SCC with sCD25 (SCC = 0.64 in type 3; SCC = 0.53 in types 1, 2, 4) and with sCD95-L (sCD178) (SCC = 0.60 in type 3; SCC = 0.51 in types 1, 2, 4). This ligand may be produced by T cells and by dendritic cells.
Patients with HLH were either treated symptomatically, which included immunoglobulin and steroids if the disease was associated with certain infections and appeared to be less severe, or were treated according to the HLH-94 protocol with high-dose steroids, cyclosporin A, and etoposide.18 Patients with clinically severe and unequivocal familiar disease, along with patients with poor response or relapse, were candidates for allogeneic bone marrow transplantation.
RNase protection assay
To understand the selective and overall deficiency in NK and cellular cytotoxicity in the presence of phenotypically normal effector populations, we analyzed transcripts involved in the induction of apoptosis by perforin-mediated killer activity. Exogenous IL-2 plays an important role in stimulating and amplifying cellular cytotoxicity, but type 3– and type 1–deficient patients retain negative status following IL-2 incubation. Thus, short-term IL-2–activated cell lines were subjected to transcriptional analysis of genes involved in target-cell apoptosis.
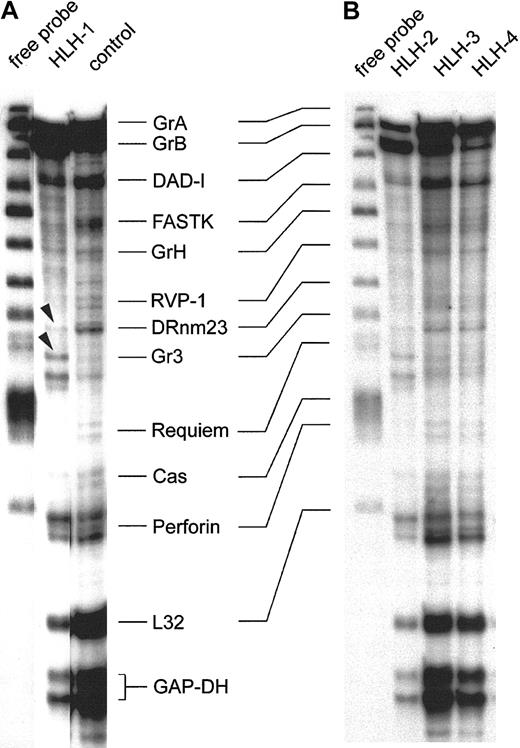
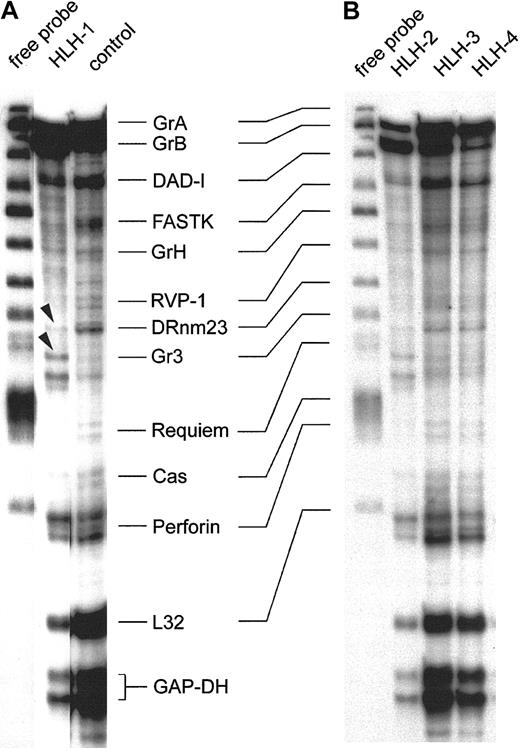
Figure 3 shows the representative expression patterns of genes involved in the induction of target-cell apoptosis by cytotoxic NK and T cells from 4 type 3–deficient patients and 1 healthy control by RNase protection analysis. These results are consistent with those obtained with 8 other preparations from patients with HLH and 4 others from healthy donors. The first lane of panels A and B in Figure 3 shows the free probe of the genes tested, derived from 2 separate experiments. Note the precisely corresponding hybridization bands in the 2 gels. The patterns of the protected transcripts of the RNA isolates, which display a characteristically retarded migration pattern of individual bands, are shown in the other lanes. Hybridization signals for housekeeping RNA species are GAPDH and L32, and these were similarly strong in all isolates. Further comparisons revealed that granzyme A, granzyme B, DAD-1, and perforin are detectable in all isolates, including the RNA of the healthy controls (Figure 3A-B). Some minor alterations were found with FastK, which gave a weaker signal in the HLH isolates, especially in HLH-1 and HLH-2. Granzyme H–specific bands are weak throughout. RVP-1, Cas, and Requiem are almost negative. The proapoptotic gene DRnm23appears to be an exception because it is almost undetectable in HLH but is positive in the control cell mRNA (Figure 3A). Moreover, the granzyme 3–specific bands are unique in HLH-1 (arrowheads), similar in HLH-2, and faint in the other HLH lines and in the control.
RNase protection assay of transcripts in IL-2–activated and –propagated lymphocytes derived from patients with type 3 deficiency in cellular cytotoxicity.
RNA isolates of IL-2–propagated lymphocytes (LAK + 7 day IL-2 culture) from 4 patients with type 3 cytotoxic deficiency (HLH-1, HLH-2, HLH-3, HLH-4) were subjected to the RiboQuant RNase protection assay. Probes for defined transcripts are given in panels A and B (first lane). Horizontal lines link shifted mRNA bands (A) and the free probe (first lane, B). Control transcripts (L32 and GAPDH) are positive in healthy controls and in type 3–deficient patients. Arrowheads mark minor differences in the relative density of the granzyme 3–specific bands in HLH-1 and HLH-2.
RNase protection assay of transcripts in IL-2–activated and –propagated lymphocytes derived from patients with type 3 deficiency in cellular cytotoxicity.
RNA isolates of IL-2–propagated lymphocytes (LAK + 7 day IL-2 culture) from 4 patients with type 3 cytotoxic deficiency (HLH-1, HLH-2, HLH-3, HLH-4) were subjected to the RiboQuant RNase protection assay. Probes for defined transcripts are given in panels A and B (first lane). Horizontal lines link shifted mRNA bands (A) and the free probe (first lane, B). Control transcripts (L32 and GAPDH) are positive in healthy controls and in type 3–deficient patients. Arrowheads mark minor differences in the relative density of the granzyme 3–specific bands in HLH-1 and HLH-2.
In conclusion, HLH and controls express a similar pattern of transcripts, with the exception of DRnm23, FastK, and granzyme 3. RNA species coding for effector proteins involved in target-cell apoptosis are especially abundant.
Results of Figure 3 were compared with 8 other isolates from HLH patients. Two more isolates were similar to HLH-1 and HLH-2, and 6 residual isolates resembled the pattern displayed by HLH-3 and HLH-4. Moreover, the slight differences in the intensities of individual hybridization signals were unrelated to phenotypical variations in the IL-2 activated cells (data not shown).
Discussion
The current study describes 4 distinct defects in cellular cytotoxicity in patients with hemophagocytic lymphohistiocytosis. In the defined types 1–, 2–, and 4–deficient patient cells, negative cytolytic function against K562 target cells could be reconstituted by either mitogen, by high-dose IL-2 activation, or by prolonged E/T incubation times, respectively (Figure 1). Most patients belonged to the so-called type 3 group, whose killer-deficient lymphocytes could not be reconstituted by any of the experimental variations described above.
A role of cytokines is unlikely because a totally defective and restricted cytotoxic dysfunction persisted in states of clinical remission (data not shown). Generally, however, NK function is highly sensitive to oxidative stress and is also found to be impaired in leukemic patients.21-23 The contribution to NK inhibition by macrophages and histiocytes has been also demonstrated.24
Natural killer effectors constitute a large and heterogeneous family of cytolytic cells that share properties with lymphoid and myeloid cells. Their killing effector pathway is, however, unique by binding perforin monomers to the phosphorylcholine moiety of the target cell, polymerizing 10 to 20 monomers in the presence of calcium and at neutral pH, and forming a functional pore.25,26Subsequently, a family of serine proteases named granzymes is activated and initiates target-cell apoptosis.27 In the neonate, NK effectors appear to play an important role in protecting the host against virus infections until specific immunity matures and MHC-restricted killer cells are generated.28,29 Thus the deficiency of NK effector cell function may play an important role in immune regulatory events, including the control of dendritic cells,30 eventually conditioning for the manifestation of hemophagocytic lymphohistiocytosis.
In type 1–deficient patients, the negative NK function against K562 target cells can be reconstituted by agglutination with PHA, indicating that E/T adhesion interaction is affected in these patients. A multiplicity of surface receptors has been described that coordinate target-cell recognition and initiation of the lytic pathways and belong not only to the adhesive molecules but also to receptors recognizing major histocompatibility complex antigens.31-35 Possibly the type 1–deficiency pattern indicates that the rapid lytic machinery requires surface recognition, which is different from that active in long-term incubation experiments. Adhesion receptor expression is not only crucial for cellular cytolysis,36 it is modulated by cytokines, by age,37 and by interaction with defined chemokines to bind and to polarize NK cell/target-cell interaction.38
In contrast to the impaired E/T binding, individual patients (type 2) lacked NK function unless effector lymphocytes were in vitro activated by a LAK protocol using IL-2 (Figure 1). IL-2 exhibits multiple effects on NK activity and is not only necessary for activation but also for differentiation of NK cells. At least in vitro, IL-2 may substitute for the bone marrow stroma-derived IL-1539 and may stimulate NK cell differentiation in mice40 and humans.41,42 In addition, IL-2 causes up-regulation of the killer effector machinery through the extracellular signal–regulated protein kinase (ERK-1) and mitogen-activated protein kinase (MAPK) within minutes.43 The stimulation of perforin expression and the apoptosis-inducing granzyme family machinery by IL-2 occurs directly,44,45 or through the interaction of additionally stimulating surface receptors such as CD38,46 and by antigenic stimulation if expressed in CD8 or NK T cells.47,48 The potential reconstitution of NK function by either mitogen (PHA in type 1–deficient effectors) or by the activation protocol using IL-2 (type 2–deficient effector cells) may suggest that differentiation defects of either surface receptors attributed to the inhibiting classes of receptors49 and adhesion receptors such as CD56- and cytokine-guided differentiation processes50 are involved.
In type 3–deficient patients, the involvement of cytolytic effector molecules appears to be likely. Indeed, 4 patients had a homozygous null mutation of perforin. In most type 3–deficient patients, however, cytotoxic effector molecules leading to target-cell apoptosis appear to be normal. These include various granzymes (grA, grB, gr3, grH; Figure3) and perforin (Figure 3).48 Indeed granzymes and perforin are necessary to enter the cytoplasmic department of the target cell, to translocate to the nucleus, and to induce nuclear fragmentation.51 Patients with type 3 deficiency have the worst prognosis, even with specific therapy (Tables 3, 4).
In contrast to type 3 deficiency, a granzyme B deficiency may be likely to explain the type 4–cytotoxicity defect. NK cells of type 4 need extensive time to perform cellular cytolysis (approximately 16 hours), which is characteristic of granzyme A–mediated target-cell apoptosis.51-55 Lack of granzyme B function can be also explained by blockade through proteinase inhibitor 9,56 by α1-plasma protease inhibitor (α1-PI), by antithrombin III (ATIII), and by certain viral inhibitors such as CrmA (cytokine response modifier).27 Moreover, granzyme B processing that depends on exopeptidase I (synonym to cathepsin C57 in mice) may be limiting.
Thus the different and reproducible patterns in cytolytic deficiencies constitute a characteristic to further dissect hemophagocytic lymphohistiocytosis, even though all the patients suffer from highly elevated concentrations of inflammatory cytokines (Figure 2). Correlation analyses were performed to identify the sources of elevated cytokines and receptors in HLH. The elevation of antagonistic cytokines such as IFN-γ and IL-10 in most type 3–deficient patients, including MIP-1α (data not shown), may indicate uncontrolled activation of T-cell subpopulations58,59 escaping immune surveillance by antigen presentation on the level of dendritic cell function,60-64 or it may be caused by IL-12, IL-18, or IL-15 hyperstimulation of certain NK cells.41
The high plasma concentrations of the CD95-ligand (Figure2)65 were thought to indicate liver cell damage caused by the release of IL-1866 and other inflammatory cytokines. These mediators (IL-18, CD95-L) are characteristic of a severe sepsislike disease in humans20 and endotoxin-induced liver injury in mice.67,68 Moreover, liver disease associated with viral infections is also related to IL-18 and sCD95-L.69 Indeed, IL-18 appears to be a member of HLH-associated hypercytokinemia, exceeding a cutoff level of 1500 pg/mL in the plasma of 48 patients with active HLH (data not shown). Because acute infections are often negative in HLH, it has been proposed that organ-infiltrating histiocytes expressing CD95-L and CD25 cause the activation of liver enzymes.70 71 Spearman correlation coefficients, however, revealed that only IFN-γ appears to be related to the elevated liver enzymes GOT and GPT in type 3–deficient patients. In addition, because IFN-γ is related to IL-10, sCD25, and sCD95-L, there is indirect evidence that all mediators play a role in elevated liver enzymes in type 3–deficient patients but not so clearly in types 1–, 2–, and 4–deficient patients.
Following IL-2 activation, 12 isolates of type 3–deficient patients were analyzed and found to express abundant RNA of granzymes A, B, and H along with perforin, all of which were important for the cytotoxic effector function. Message coding for the death-inhibitory protein DAD-1 (defender against apoptotic cell death) was also strong but not different from that of controls. Relatively faint bands were found for the apoptosis-promoting DRnm23 in HLH but not in the control. This molecule plays a role in inhibition of differentiation and antiapoptosis of myeloid cells72,73 and also in adhesion processes.74 By contrast, Fast-K (Fas-activated serine/threonine kinase), acting as a promotor of apoptosis following dephosphorylation,75 Cas (cellular apoptosis susceptibility), which is involved in the microtubular response of apoptosis,76 and Requiem are weakly expressed in all HLH isolates and controls (Figure 3 and data not shown).
The second difference gave the granzyme 3–specific probe,77 giving rise to a double-band hybridization signal in HLH-1 and HLH-2 (arrowheads). It is faint in the other 2 HLH lines and in the control. In summary, the 4 (Figure 3) plus the 8 additional IL-2–activated cell lines from patients express high levels of transcripts necessary to induce target-cell apoptosis, but they lack transcripts that might cause autologous cell death. With the exception of granzyme 3 and DRnm23, results were identical in all HLH isolates and were not different from those for the controls.
We speculate that the resistance of apoptosis is a prerequisite for cellular cytotoxic effector function, and it may be worthwhile to follow this hypothesis as a mechanism to explain cytotoxic dysfunction in HLH patients with type 3 deficiency. More detailed studies along these lines will help to identify susceptibility genes for the manifestation of HLH and to clarify the physiological impact of defective cellular cytolysis and uncontrolled phagocytosis by an as yet ill-defined cellular element of the antigen-presenting cell repertoires.
We thank a number of colleagues for providing blood from patients, clinical data, and outcome analysis: Drs Behrens, Krauss-Etschmann, and Müller-Weihrich, Munich; Bekassy, Lund; Bucsky, Lübeck; Blütters-Sawatzki, Giessen; Egeler, Rotterdam; Feldges, St Gallen; Göbel and Körholz, Düsseldorf; Glöckel, Bamberg; Gromek and Schwamborn, Cologne; Gross and Koszielnak, Stuttgart; Helmig and Peters, Regensburg; Jabali, Budweis; Jacobi, Celle; Jones, Salzburg; Jorch, Bielefeld; Kaulfersch, Klagenfurt; Klingebiel and Handgretinger, Tübingen; Kremens, Stollmann-Gibbels, Havers, and Schweigerer, Essen; Lassay, Aachen; Leuthold and Göbel, Siegen; Martinez, Nanduri, and Webb, London; Navajas, Baracaldo Spain; Pongratz, Augsburg; Rath, Münster; Redenbacher, Nuremberg; Schroeder, Aarhus; Schulz, Ulm; Selle, Heidelberg; Sörensen, Frankfurt; Storm-Mathiesen, Oslo; Strauss, Hannover; Urban, Graz; Wiesel, Datteln. We thank Shinsaku Imashuku (Kyoto, Japan) for his valuable contribution in discussing HLH, as well as Jan Inge Henter (Stockholm, Sweden) for promoting NK data analysis within the HLH study group. We give special thanks to Manuel Staib, Philipp Wollmann, and Andreas Maehler for their involvement in data processing and documentation.
Dedicated to Michael Georgieff on the occasion of his 50th birthday.
Prepublished online as Blood First Edition Paper, June 14, 2002; DOI 10.1182/blood-2001-12-0260.
Supported in part by the International Histiocyte Society.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
E. Marion Schneider, Sektion Experimentelle Anaesthesiologie, Universitaetsklinikum Ulm, Steinhoevelstr 9, 89075 Ulm, Germany; e-mail: marion.schneider@medizin.uni-ulm.de.

![Fig. 2. Elevation of IL-10, IFN-γ, sCD25, and sCD95-ligand in types 1-, 2-, 3-, and 4-deficient HLH. / Plasma concentrations of IL-10, IFN-γ, and the soluble receptors sCD25, sCD95-L were determined in patients with active HLH. Numbers of patients with type 1, type 2, type 3, and type 4 cytotoxic deficiency are given below the x-axis. Median values are indicated by a single bar within the column scatter. Values below or within the dashed horizontal line indicate the individual range of values determined in plasma of healthy controls (normal values, 0-10 pg/mL for IL-10 [n = 92]; 0-10 pg/mL IFN-γ [n = 60]; 200-1000 U/mL for sCD25 [n = 120]; < 50 pg/mL for sCD95-ligand [n = 39]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/8/10.1182_blood-2001-12-0260/4/m_h82023254002.jpeg?Expires=1769144077&Signature=CsnPZJJ-i1cyPNGFIFVQ24wDlGX1RxgKyEKUqkRPdRU5hBUM-c9yxW1EXMPoSRzw4dyPtadncszGHptaFDyx4k-uAbMkTIlfv0DnxfaMklIaA-Td-ur2K9wP89tWYZr99KzgQ-G9T2W9tgXqi~rdob85s4irRMXVC~0UR7eug4e7xMamOX2iOn~CPibcNWa3H6DWURkjsefHINcdg0l3WJ~Cxakf6LRmtc5JOlZNMZYvb0x~QFZUyT0Q77xqUxjznZjEmE117kVH-fUshvsb6p2iRT2EO-yEr3Q5YCh-LL3VoYyy3Zd3ekW1qsMEd9CGB8m05aYmlgwCZDPfJo5pag__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 2. Elevation of IL-10, IFN-γ, sCD25, and sCD95-ligand in types 1-, 2-, 3-, and 4-deficient HLH. / Plasma concentrations of IL-10, IFN-γ, and the soluble receptors sCD25, sCD95-L were determined in patients with active HLH. Numbers of patients with type 1, type 2, type 3, and type 4 cytotoxic deficiency are given below the x-axis. Median values are indicated by a single bar within the column scatter. Values below or within the dashed horizontal line indicate the individual range of values determined in plasma of healthy controls (normal values, 0-10 pg/mL for IL-10 [n = 92]; 0-10 pg/mL IFN-γ [n = 60]; 200-1000 U/mL for sCD25 [n = 120]; < 50 pg/mL for sCD95-ligand [n = 39]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/8/10.1182_blood-2001-12-0260/4/m_h82023254002.jpeg?Expires=1769305375&Signature=1Wsha0WdLTT4ifOgIuyupcuYcyTae4jzTFWQqobAY9E5PdW77gB7RRKgo7HyuRBIWSPyhKinkDgd9UZ~ZA0M0E0HZzhTgYtZbAVISHYNW2iJ9256qNZPAzUZFHO2rJrXDBFlY1UWY3PKLI-wZDXrb52wY5Ie1pLPXh5AGTT9BQ-s-EbJ9tivwHNNkfSV~AIzsFWsPG9NdoZzMrAGbMpdGv9~e3dw3eeB9Zj~fGrREEJuX-14i6wwPAXIRYaEs2~tWPQcmfU8uRSdgVzv6cEOzr3whkdHB4UrYDWH9fjKQI8dueWK33ml3zyg-Ooec-kEkWjltpB69Dj4nTX7YajU6g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)