Key Points
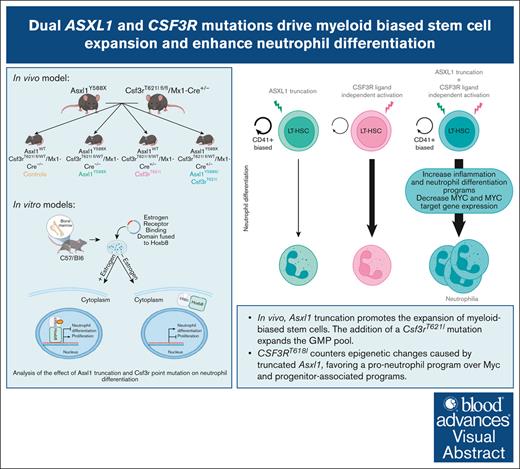
Asxl1 truncation promotes the expansion of myeloid-biased stem cells. Adding a Csf3rT621I mutation expands the granulocyte progenitor pool.
CSF3RT618I counters epigenetic changes caused by truncated Asxl1 favoring pro-neutrophil program over Myc and progenitor-associated programs.
Visual Abstract
Mutations in the epigenetic regulator Additional Sex Combs-Like 1 (ASXL1) are frequently observed in chronic neutrophilic leukemia (CNL). CNL is a myeloproliferative neoplasm (MPN) driven by activating mutations in the Colony Stimulating Factor 3 Receptor (CSF3R), which cause excessive neutrophil production. Despite the high rates of co-occurrence, the interplay between ASXL1 and CSF3R mutations in hematopoiesis and leukemia remains poorly understood. Here, we present a new mouse model with both Asxl1Y588X and Csf3rT621I mutations, which recapitulates features of human MPNs. Csf3r-mutant mice exhibit an age-associated depletion of hematopoietic stem cells, which is tempered by adding Asxl1Y588X. This combination of mutations causes an expansion of myeloid-biased long-term hematopoietic stem cells. As the mice age, they develop neutrophilia, but leukemia is rare, suggesting additional mutations may be required for transformation. Using models of myeloid differentiation, we find that Asxl1 truncation enhances CSF3RT618I-driven neutrophil differentiation, activating inflammatory pathways associated with mature myeloid cell production. Moreover, cells with both mutations have increased H3K4me1 at neutrophil-associated enhancers. Mutant ASXL1 is known to decrease the genome-wide abundance of the repressive histone mark H2AK119ub. Although we see the expected decrease in H2AK119ub in Asxl1-mutant cells, this effect is reversed when CSF3R is also mutated, suggesting a complex interplay between these mutations in regulating chromatin dynamics during hematopoiesis. Our findings highlight context-dependent effects of ASXL1 mutation in myeloid disorders and provide insights into the mechanisms underlying neutrophil differentiation in ASXL1 and CSF3R dual-mutant MPN.
Introduction
Additional Sex Combs-Like 1 (ASXL1) is an epigenetic modulator that regulates gene expression in hematopoietic development and is frequently mutated in hematologic disorders.1-8ASXL1 mutations are largely nonsense or frameshift mutations in the 12th exon.9 These mutations produce an unstable protein product10 and neomorphic interactions.9 Wild-type ASXL1 regulates several histone-modifying complexes. ASXL1 forms a deubiquitinase complex with BAP1 to remove the repressive histone mark H2A lysine 119 ubiquitination (H2AK119ub).11,12 Mutant ASXL1 stabilizes this complex to enhance the removal of H2AK119ub.13 ASXL1 also regulates the deposition of other histone marks, including H3K27me3 and H3K4me3.10,14 Dysregulation of the epigenome by mutant ASXL1 perturbs gene expression to promote the pathogenesis of myeloid malignancies.
ASXL1 mutations are common in myelodysplastic syndromes (15%-20%) and related dysplastic disorders.1,15 Murine models have demonstrated that Asxl1 mutation or deletion produces age-dependent myelodysplastic phenotypes.10,16-20 Despite a clear association with the underproduction of mature cells in dysplastic disorders, ASXL1 mutations can also occur in myeloproliferative neoplasms (MPNs), where too many mature cells are produced. Among MPNs, ASXL1 mutations are highly enriched in chronic neutrophilic leukemia (CNL; 30%-76%).21,22 CNL is characterized by neutrophil overproduction and mutations in the Colony Stimulating Factor 3 Receptor (CSF3R).7,21,22 CSF3R is essential for guiding granulocyte progenitors through differentiation and maturation, facilitating their development into neutrophils. The CSF3RT618I mutation causes ligand-independent receptor activation and robust signaling through the JAK/STAT pathway. Activated STAT transcription factors initiate a gene expression program that drives excessive neutrophil production.23-25 When both CSF3R and ASXL1 are mutated in CNL, patient outcomes are exceptionally poor.7 Despite clear prognostic significance, the mechanistic role of mutant ASXL1 in CNL pathogenesis remains unclear.
In this study, we observed an expansion of myeloid-biased stem cells in the bone marrow and neutrophilia in the blood of mice with both Asxl1 and Csf3r mutations. Using in vitro models, we found that the comutation of Asxl1 and Csf3r confers enhanced neutrophil differentiation through epigenetic mechanisms. Indeed, although mutant Asxl1 reduced H2AK119ub, the addition of a Csf3r mutation reversed this phenotype to allow chromatin compaction at stem-associated genes and increased priming of neutrophil enhancers.
Methods
Transgenic mouse models
Asxl1Y588X mice were generously provided by Yang et al18 and Maria Figueroa. The CSF3RT621I-inducible transgenic strain was generated by Ingenious Targeting Laboratory as described in the supplemental Methods. Mx-1 Cre (B6.Cg-Tg(Mx1-cre)1Cgn/J-003556) mice were obtained from JAX. Poly I:C (P1530, Sigma) was dissolved in phosphate-buffered saline and injected intraperitoneally at 12 mg/kg on days 1, 3, and 5 at 6 weeks of age to induce Cre-based recombination of the Csf3RT621I allele. Blood counts were measured through an automated blood counter (Heska, Element HT5). Experiments were conducted following the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of Oregon Health & Science University (Protocol #TR01_IP00000482). Mouse tissue harvesting, processing, and analysis are described in the supplemental Methods.
Cell culture
The HoxB8-ER construct was used to generate HoxB8-ER cells from the bone marrow of a C57BL/6J mouse.26 Cells were maintained in RPMI (11875119, Gibco) supplemented with 10% heat-inactivated fetal bovine serum (SH30088.03HI, HyClone), 2 mM GlutaMAX (35050061, Gibco), 100 U/mL penicillin/streptomycin (15140122, Gibco), 2% SCF-CHO conditioned media, and 0.6 μM estrogen (E-2758, Sigma). 32Dcl3 cells were maintained in RPMI with 10% fetal bovine serum, 2 mM GlutaMAX, 100 U/mL penicillin/streptomycin, and 0.001 μg/mL of mouse IL3 (213-13, Peprotech). Cell lines were tested monthly for mycoplasma contamination using MycoAlert Kit (LT07-118, Lonza). Detailed methods for the establishment of the cell lines are in the supplemental Methods.
Flow cytometry
To assess myeloid differentiation, estrogen was removed from HoxB8 cell media for 48 hours and 32Dcl3 cells were cultured in media with granulocyte colony-stimulating factor (G-CSF; 250-05, Peprotech) and without IL3 for 3 days. Cells (2.5 × 105) were stained in 100 μL of staining buffer (554656, BD Biosciences) with antibodies outlined in supplemental Table 1. To assess mouse bone marrow cell differentiation, cells post-red blood cell lysis (3 × 106) were stained in 100 μL of staining buffer (554656, BD Biosciences) with antibodies found in supplemental Table 2. Cells were analyzed on an LSRFortessa flow cytometer (BD).
RNA sequencing
After 24 hours from estrogen removal, RNA was isolated using a RNeasy Plus Micro Kit (74004, Qiagen). BGI performed the library preparation and 50 bp paired-end sequencing. Detailed analytic methodology can be found in the supplemental Methods.
ATAC sequencing
HoxB8 cells were cultured in media without estrogen for 48 hours. Cells (5 × 104) were resuspended in cold PBS and tagmentation master mix (25 μL of 2× tagmentation buffer, 2.5 μL of TDE1 [20034197, Illumina], 0.5 μL of 1% digitonin [D141, Sigma]) was added. Samples were incubated at 37°C for 30 minutes. DNA was purified using Clean and Concentrator-5 Kit (D4003, Zymo). Transposed DNA was amplified and purified as described previously with adapted primers.27,28 Samples were quantified using Qubit dsDNA HS Assay Kit (Q32851, Invitrogen), qualified with the TapeStation HS D1000 kit (5067-5585 and 5067-5584, Agilent), pooled, and sequenced by OHSU Massively Parallel Sequencing Shared Resource with a NovaSeq (Illumina) using 75 bp paired-end sequencing. Reads were aligned to the mouse genome (mm10) using Burrow-Wheeler Aligner maximal exact matches (BWA-MEM).29 Duplicates were marked with Sambamba30; duplicates and mitochondrial reads were removed. Peaks were called using MACS2.31 Count-per-million–normalized bigWig coverage tracks were generated from filtered BAM files using bamCoverage from deepTools.32 Counts-per-million–normalized coverage tracks were averaged across all replicates for each sample group using WiggleTools33 and UCSC bedGraphToBigWig utility.83 Motif analysis was performed using HOMER.34 All packages and parameters for the processing and subsequent analysis can be found in the supplemental Methods.
CUT&Tag
Cleavage under targets and tagmentation (CUT&Tag) was performed with 1 × 105 cells cultured without estrogen for 48 hours using the CUTANA CUT&Tag kit (14-1102, EpiCypher). Antibodies included immunoglobulin G (IgG) rabbit (13-0042t, EpiCypher), H3K27me3 (13-0055t, EpiCypher), H3K4me1 (5326, Cell Signaling Technology), and H2AK119Ub (8240, Cell Signaling Technology). Samples were quantified using Qubit dsDNA HS Assay Kit, qualified with the TapeStation HS D1000 kit, pooled, and sequenced by OHSU Massively Parallel Sequencing Shared Resource with a NovaSeq (Illumina) using 75 bp paired-end sequencing. Reads were aligned to the mouse genome (GRCm38/mm10) using Bowtie2.35 Peaks were called using GoPeaks.36 High-confidence peaks were defined as those present in at least 2 replicates. Consensus bed files were formed by merging the high-confidence peaks from each sample using BEDTools.37 Additional packages and parameters for the processing and subsequent analysis can be found in the supplemental Methods.
Quantification and statistical analysis
Data are revealed as mean ± standard error of the mean. Prism software (version 10.1; Prism Software Corporation) was used to perform statistical analysis, which is described in the figure legends. All data were analyzed with paired t tests or analysis of variance followed by Holm-Sidak correction or Tukey multiple comparison test.
Results
Development of a new Csf3r-mutant myeloproliferative neoplasm mouse model
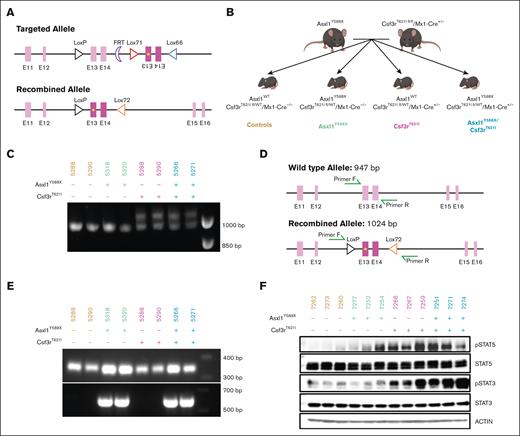
To understand the role of ASXL1 mutations in the development of Csf3r-mutant MPNs, we first generated a Csf3rT621I murine model (supplemental Figure 1A). Cytokine-independent growth assays in Ba/F3 cells revealed that Csf3rT621I had a similar transformation capacity as the homologous human CSF3RT618I mutation (supplemental Figure 1B). In this novel model, Cre recombination causes the deletion of a wild-type exon and inversion of the mutant exon to express Csf3rT621I from the endogenous locus (Figure 1A). These mice were crossed with Mx1-Cre and Asxl1Y588XTg knockin transgenic mice18 to evaluate the impact of mutant Asxl1 on disease biology. This yielded a cohort of 87 mice with the following 4 genotypes: Asxl1Y588X/Csf3rT621I/Cre+/−, Asxl1Y588X/Csf3rT621I/Cre−/−, Asxl1WT/Csf3rT621I/Cre+/−, and Asxl1WT/Csf3rT621I/Cre−/− (Figure 1B), referred to as the double mutant, Asxl1Y588X/Csf3rT621I, Asxl1Y588X, Csf3rT621I, and control, respectively. Both alleles were heterozygous. Polymerase chain reaction confirmed recombination of the Csf3rT621I allele after poly I:C injection and the presence of the Asxl1Y588X allele (Figure 1C-E).
Validation of a new mouse model of Csf3r and Asxl1 comutation. (A) Schematic of the recombination of the Csf3r-mutated allele. (B) Breeding scheme for the establishment of the Csf3r/Asxl1-mutated cohort with appropriate controls producing all following 4 groups: control, Asxl1Y588X, Csf3rT621I, and double mutant. (C) Polymerase chain reaction (PCR) validation of recombination of the Csf3rT621I allele. The recombined allele is 1024 bp long; the wild type one is 947 bp. (D) Primer design for the PCR validation of Csf3rT621I allele recombination in panel C. (E) PCR validation of the Asxl1Y588X allele insertion. The primers used are P1 and P2 from Yang et al18 giving a 350-bp amplicon for the wild-type allele and a 570-bp amplicon for the mutated allele. (F) Western blot revealing enhanced STAT3 and STAT5 phosphorylation in the bone marrow of mice with the Csf3rT621I allele.
Validation of a new mouse model of Csf3r and Asxl1 comutation. (A) Schematic of the recombination of the Csf3r-mutated allele. (B) Breeding scheme for the establishment of the Csf3r/Asxl1-mutated cohort with appropriate controls producing all following 4 groups: control, Asxl1Y588X, Csf3rT621I, and double mutant. (C) Polymerase chain reaction (PCR) validation of recombination of the Csf3rT621I allele. The recombined allele is 1024 bp long; the wild type one is 947 bp. (D) Primer design for the PCR validation of Csf3rT621I allele recombination in panel C. (E) PCR validation of the Asxl1Y588X allele insertion. The primers used are P1 and P2 from Yang et al18 giving a 350-bp amplicon for the wild-type allele and a 570-bp amplicon for the mutated allele. (F) Western blot revealing enhanced STAT3 and STAT5 phosphorylation in the bone marrow of mice with the Csf3rT621I allele.
To confirm that the Csf3rT621 allele was functional, we assessed the activation of the downstream signaling pathways (Figure 1F). Consistent with the activation of JAK/STAT signaling by CSF3R, immunoblot analysis of bone marrow hematopoietic cells revealed increased STAT3/5 phosphorylation in mice expressing Csf3rT621I. Interestingly, phosphorylation of STAT3 is further augmented in mice with both mutations.
Asxl1 truncation promotes the expansion of myeloid-biased stem cells
Because CSF3R-mutant MPNs are generally found in an aging population (median diagnosis at 66.5 years),38 we conducted a detailed analysis of how these mutations affect hematopoiesis in a cohort of aging mice. Both single- and double-mutant mice had increased marrow cellularity (Figure 2A). We observed an increased megakaryocyte proportion in the Asxl1Y588X-mutant groups and larger megakaryocytes in Csf3rT621I single- and double-mutant mice (supplemental Figure 2).
Asxl1 truncation promotes the expansion of myeloid-biased stem cells. (A) Representative hematoxylin and eosin (H&E)–stained tibia cross-sections of mice 1 year post-Cre induction (n = 3 per group). (B) Schematic of serial transplant of all 4 genotypes and timeline of bone marrow characterization by flow cytometry. (C) Flow cytometry analysis of the long-term hematopoietic stem, LT-HSC CD41+/CD41− ratio, and GMP progenitor cells from mice bone marrow at 4 months, 1 year after Cre induction and after both serial transplants (n = 3-4 per group). (D) Quantification of colony-forming unit assays at 1 year post-Cre induction and after both serial transplants using 1 × 104 cells per well of whole bone marrow cells (mean ± standard error of the mean [SEM]; n = 3 per group in triplicate). Significance was evaluated with a 2-way analysis of variance (ANOVA) with Tukey multiple comparison test. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.
Asxl1 truncation promotes the expansion of myeloid-biased stem cells. (A) Representative hematoxylin and eosin (H&E)–stained tibia cross-sections of mice 1 year post-Cre induction (n = 3 per group). (B) Schematic of serial transplant of all 4 genotypes and timeline of bone marrow characterization by flow cytometry. (C) Flow cytometry analysis of the long-term hematopoietic stem, LT-HSC CD41+/CD41− ratio, and GMP progenitor cells from mice bone marrow at 4 months, 1 year after Cre induction and after both serial transplants (n = 3-4 per group). (D) Quantification of colony-forming unit assays at 1 year post-Cre induction and after both serial transplants using 1 × 104 cells per well of whole bone marrow cells (mean ± standard error of the mean [SEM]; n = 3 per group in triplicate). Significance was evaluated with a 2-way analysis of variance (ANOVA) with Tukey multiple comparison test. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.
To assess the impact of these mutations on hematopoiesis, we used flow cytometry to analyze bone marrow at 4 months and 1 year post-Cre induction (supplemental Figure 3A).39,40 After 1 year, the bone marrow was transplanted into lethally irradiated recipients, analyzed, and retransplanted (Figure 2B-C). No hematopoietic population changes were observed at 4 months, but by 1 year, the long-term hematopoietic stem cell (LT-HSC) compartment expanded in Asxl1-truncated mice, a phenotype that persisted and intensified posttransplant. In contrast, Csf3r-mutant mice had fewer LT-HSCs after transplant. Double-mutant mice had LT-HSC expansion at 1 year and after the first transplant, but this was not sustained after the secondary transplant. Notably, these expanded LT-HSCs were largely CD41+, associated with myeloid bias, megakaryocyte/platelet development, and quiescence41 (Figure 2C; supplemental Figure 3B). The population of myeloid-biased CD150High LT-HSCs (also linked to LT-HSC myeloid bias42) increased in Asxl1Y588X-mutant mice at 1 year. Megakaryocyte-biased cKITHigh LT-HSCs43 increased in both single and double Asxl1 mutants after the first transplant (supplemental Figure 3B).
To better understand this LT-HSC phenotype, we performed serial colony-forming unit replating assays on isolated bone marrow cells at 1 year post-induction and after each transplant. The results revealed an increase in the self-renewal capacity of the LT-HSCs with Asxl1Y588X mutation, which was further augmented in the double-mutant mice after the first transplant. Consistent with the number of LT-HSCs in the bone marrow, this enhanced self-renewal capacity was only sustained after the second transplant in the Asxl1 single-mutant mice (Figure 2D).
Analysis of progenitor populations revealed increased numbers of granulocyte/monocyte progenitors (GMPs) in the Csf3r-mutant mice. This granulocytic bias is consistent with the known roles of CSF3R and G-CSF in granulopoiesis.44 The Csf3r-mutant mice did not sustain high GMPs after the transplant, but the double-mutant mice sustained elevated GMP numbers even after transplantation (Figure 2C).
Mice with Csf3r and Asxl1 mutations develop neutrophilia with aging or inflammation but have a low incidence of leukemic transformation
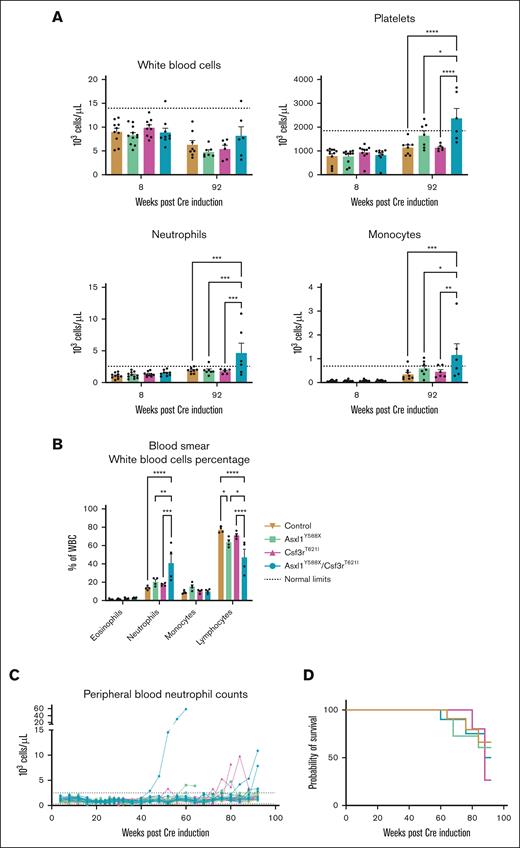
To determine whether mice with Asxl1 and Csf3r mutations would develop hematologic abnormalities, we followed the peripheral blood counts every 4 weeks for ∼2 years using an automated cell blood count. Contrary to previous results,18 the Asxl1Y588X-only mice did not develop myeloid malignancies in our facility. Consistent with previous studies,18 platelet counts were significantly higher in Asxl1Y588X and double-mutant mice. In female mice with both Csf3r and Asxl1 mutations, we found a significant increase in neutrophils and monocytes at 92 weeks (Figure 3A). At 54 weeks, we also assessed peripheral blood smears from 4 mice per genotype, revealing a significant increase in the proportion of neutrophils at the expense of lymphocytes in double-mutant mice (Figure 3B). Serum analysis from these mice did not reveal any changes in inflammatory cytokine production (supplemental Figure 4A). Throughout this experiment, only 1 female double-mutant mouse developed leukemia. This mouse started to develop the disease at week 40, with neutrophil numbers doubling every month (Figure 3C; supplemental Figure 4B). Two other double-mutant mice had increased neutrophil counts after 80 weeks. A few Csf3rT621I-only mice also had transiently high neutrophil counts (Figure 3C). All other mice died of old age or were euthanized because of infections or uterine prolapse, which can occur in elderly mice. Mutation status did not significantly alter the overall survival rate in our cohort (Figure 3D). This analysis was also performed in male mice, where we observed the expected differences in platelet counts but no neutrophilia (supplemental Figure 4C-E). Further analysis of the sex bias in this model is an interesting avenue for future investigation. These studies demonstrated that double-mutant mice exhibit signs of neutrophilia, but leukemic transformation is rare.
Csf3r mutation induces an aberrant neutrophil phenotype. (A) Comparison of peripheral blood cell counts between 8 weeks and 92 weeks post-Cre induction of female mice (mean ± SEM; n = 9-12 per group). Significance was evaluated with a mixed-effect analysis with Sidak multiple comparison test. (B) Quantification of white blood cells in peripheral blood smears of mice 1 year after Cre induction (mean ± SEM; n = 2 per group in duplicate). Significance was evaluated with a 2-way ANOVA with Tukey multiple comparison test. (C) Peripheral blood neutrophil counts of female mice in 92 weeks. Dotted lines represent normal count limits as described by the Charles River Laboratories for C57BL/6 mice in their North American Colonies for mice aged 8 to 10 weeks. (D) Survival of female mice from each genotype is illustrated in a Kaplan-Meier plot. No significance was found by the Mantel-Cox test. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.
Csf3r mutation induces an aberrant neutrophil phenotype. (A) Comparison of peripheral blood cell counts between 8 weeks and 92 weeks post-Cre induction of female mice (mean ± SEM; n = 9-12 per group). Significance was evaluated with a mixed-effect analysis with Sidak multiple comparison test. (B) Quantification of white blood cells in peripheral blood smears of mice 1 year after Cre induction (mean ± SEM; n = 2 per group in duplicate). Significance was evaluated with a 2-way ANOVA with Tukey multiple comparison test. (C) Peripheral blood neutrophil counts of female mice in 92 weeks. Dotted lines represent normal count limits as described by the Charles River Laboratories for C57BL/6 mice in their North American Colonies for mice aged 8 to 10 weeks. (D) Survival of female mice from each genotype is illustrated in a Kaplan-Meier plot. No significance was found by the Mantel-Cox test. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.
To further understand the role of these mutations in neutrophil production, we performed an inflammatory challenge with daily low-dose injections of lipopolysaccharide (6 μg/d) for 8 weeks (supplemental Figure 4F). This revealed an increased number of neutrophils at the expense of lymphocytes in the peripheral blood of the double-mutant mice (supplemental Figure 4G). Marrow characterization after inflammatory challenge revealed a higher ratio of myeloid-biased CD41+ LT-HSCs in the double-mutant mice (supplemental Figure 4H).
Collectively, these results suggest that mice with both Asxl1 and Csf3r mutations exhibit an age-dependent increase in myeloid-biased stem cells and GMPs in the bone marrow and peripheral neutrophilia. Furthermore, the Asxl1 mutation protects Csf3r-mutant mice from depletion of GMPs after transplantation. Together, these findings explain the high overlap between ASXL1 and CSF3R mutations in human MPNs.
Asxl1 mutations enhance CSF3R-driven differentiation
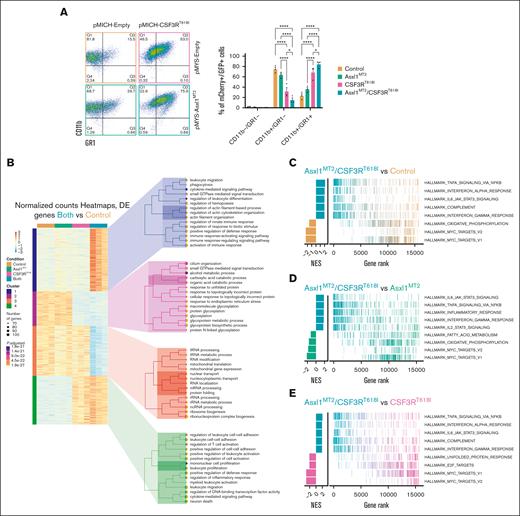
We next set out to understand the elevated neutrophil levels in mice with both Asxl1 and Csf3r mutations. Mutations in ASXL1 are classically associated with myelodysplasia, a failure to differentiate appropriately.1,15 How the effects of mutant ASXL1 manifest in the context of a strong prodifferentiation driver remains unclear. To understand the contribution of mutant ASXL1 to CSF3R-driven neutrophil production, we harnessed an in vitro model of myeloid differentiation (HoxB8). These cells express the HoxB8-ER fusion protein, which localizes to the nucleus in the presence of estrogen, thereby inducing a myeloid-progenitor gene expression program to maintain a progenitor-like state.26 On estrogen removal, the cells differentiate toward myeloid lineages. As they differentiate, they gain CD11b expression followed by GR1 expression. We introduced CSF3RT618I and/or an Asxl1-truncating mutation “MT2”45 using mCherry or GFP-expressing retroviral vectors. CSF3RT618I enhanced differentiation 48 hours after estrogen removal. Co-expression of the Asxl1MT2-truncating mutation augmented CSF3RT618I-induced differentiation, as evidenced by increased number of CD11b+/GR1+ cells (Figure 4A).
Asxl1 mutation enhances CSF3R-driven differentiation. (A) Representative flow cytometric plots of CD11b and GR1 48 hours after estrogen withdrawal in HoxB8 cells transduced with plasmids containing Asxl1MT2-GFP and/or CSF3RT618I-mCherry and/or their corresponding empty controls producing all following 4 groups: control, Asxl1MT2, CSF3RT618I, and double mutant or Asxl1MT2/CSF3RT618I. Quantification of transduced mCherry/GFP–positive cells (mean ± SEM; n = 3 per group). Significance was evaluated with a 2-way ANOVA with a Tukey multiple comparison test. (B) RNA sequencing was performed 24 hours after estrogen withdrawal with all 4 groups (n = 3 per group). Heat maps represent the normalized, row-scaled (gene-based z-score scaled) counts of the most significantly differentially expressed genes (Padj < .05) between the control and double-mutant cells, and genes are clustered by similar expression patterns. Each cluster has a corresponding Gene Ontology (GO) tree of the top 15 most significantly enriched GO terms and their associated number of gene hits and adjusted P values. (C) Gene Set Enrichment Analysis (GSEA) between Asxl1MT2/CSF3RT618I and control cells. (D) GSEA between Asxl1MT2/CSF3RT618I and Asxl1MT2 cells. (E) GSEA between Asxl1MT2/CSF3RT618I and CSF3RT618I cells. Gene ranks from GSEA are represented as line charts for the top 5 most significant hallmark gene sets from the Molecular Signatures Database (MSigDB). ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001. NES, normalized enrichment score; Padj, adjusted P value.
Asxl1 mutation enhances CSF3R-driven differentiation. (A) Representative flow cytometric plots of CD11b and GR1 48 hours after estrogen withdrawal in HoxB8 cells transduced with plasmids containing Asxl1MT2-GFP and/or CSF3RT618I-mCherry and/or their corresponding empty controls producing all following 4 groups: control, Asxl1MT2, CSF3RT618I, and double mutant or Asxl1MT2/CSF3RT618I. Quantification of transduced mCherry/GFP–positive cells (mean ± SEM; n = 3 per group). Significance was evaluated with a 2-way ANOVA with a Tukey multiple comparison test. (B) RNA sequencing was performed 24 hours after estrogen withdrawal with all 4 groups (n = 3 per group). Heat maps represent the normalized, row-scaled (gene-based z-score scaled) counts of the most significantly differentially expressed genes (Padj < .05) between the control and double-mutant cells, and genes are clustered by similar expression patterns. Each cluster has a corresponding Gene Ontology (GO) tree of the top 15 most significantly enriched GO terms and their associated number of gene hits and adjusted P values. (C) Gene Set Enrichment Analysis (GSEA) between Asxl1MT2/CSF3RT618I and control cells. (D) GSEA between Asxl1MT2/CSF3RT618I and Asxl1MT2 cells. (E) GSEA between Asxl1MT2/CSF3RT618I and CSF3RT618I cells. Gene ranks from GSEA are represented as line charts for the top 5 most significant hallmark gene sets from the Molecular Signatures Database (MSigDB). ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001. NES, normalized enrichment score; Padj, adjusted P value.
To uncover the molecular basis of enhanced differentiation of cells with both mutations, we performed RNA sequencing 24 hours after estrogen removal. Relative to control and single-mutant conditions, cells with both mutations had increased expression of genes with Gene Ontology (GO) terms related to immune cell activation, leukocyte migration, actin organization, and leukocyte adhesion (Figure 4B). This is consistent with the observation that cells with both mutations exhibit enhanced differentiation. Interestingly, in CSF3RT618I-expressing cells, GO terms linked to metabolic processes were enriched (supplemental Figure 5A). Using Gene Set Enrichment Analysis we identified enriched IL6/JAK/STAT3 and TNFα-related gene expression programs in cells with both mutations (Figure 4C-E). These signaling programs are associated with neutrophil differentiation46,47 and activation.48,49 These transcriptomic data support the hypothesis that mutant ASXL1 enhances the ability of CSF3R to induce gene expression programs that promote the expansion and differentiation of neutrophils.
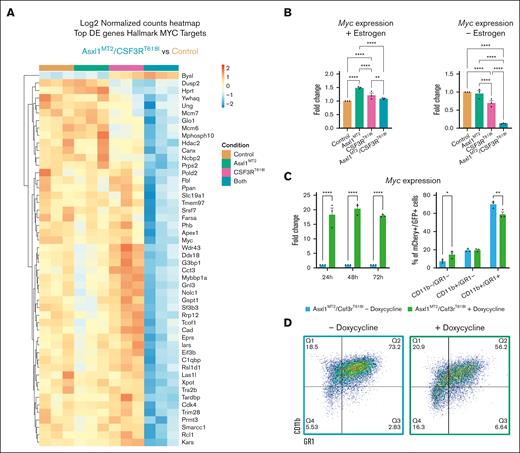
In double-mutant cells, the hallmark MYC targets 1 and 2 were notably downregulated (Figure 4C-E), consistent with MYC’s higher expression in hematopoietic progenitors.50 Individual MYC target genes also exhibit reduced expression in cells harboring both mutations (Figure 5A). Furthermore, Myc expression decreased in the absence of estrogen (Figure 5B), and forced Myc expression inhibited neutrophil differentiation (Figure 5C-D). Myc downregulation enhances differentiation in CSF3RT618I cells. It has minimal impact on double mutants, potentially due to their already low baseline Myc expression (supplemental Figure 5B). These findings establish Myc repression as a mechanism by which mutant ASXL1 promotes CSF3RT618I-driven myeloid differentiation.
MYC overexpression reduces myeloid differentiation of Asxl1- and CSF3R-mutant cells. (A) Heat maps representing the normalized, row-scaled counts of the top 50 most significantly differentially expressed genes (Padj < .05) between the control and double-mutant cells from the gene subset, Hallmark MYC targets. (B) Quantitative PCR (qPCR) for Myc before and after estrogen withdrawal (mean ± SEM; n = 3 per group). Significance was evaluated with a 2-way ANOVA with Sidak multiple comparison test. (C) qPCR for Myc expression in transduced double-mutant cells with a Tet-on MYC plasmid (mean ± SEM; n = 3 per group). Significance was evaluated with a 2-way ANOVA with Sidak multiple comparison test. (D) Representative flow cytometry plots of CD11b and GR1 at 48 hours post estrogen withdrawal of the double-mutant cells with a Tet-on MYC plasmid. These cells were treated with or without doxycycline 24 hours before estrogen withdrawal. Quantification of CSF3R-mCherry/Asxl1-GFP–positive cells reveals significant repression of CD11b+/GR1+ (mean ± SEM; n = 3 per group). Significance was evaluated with a 2-way ANOVA with Sidak multiple comparison test. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.
MYC overexpression reduces myeloid differentiation of Asxl1- and CSF3R-mutant cells. (A) Heat maps representing the normalized, row-scaled counts of the top 50 most significantly differentially expressed genes (Padj < .05) between the control and double-mutant cells from the gene subset, Hallmark MYC targets. (B) Quantitative PCR (qPCR) for Myc before and after estrogen withdrawal (mean ± SEM; n = 3 per group). Significance was evaluated with a 2-way ANOVA with Sidak multiple comparison test. (C) qPCR for Myc expression in transduced double-mutant cells with a Tet-on MYC plasmid (mean ± SEM; n = 3 per group). Significance was evaluated with a 2-way ANOVA with Sidak multiple comparison test. (D) Representative flow cytometry plots of CD11b and GR1 at 48 hours post estrogen withdrawal of the double-mutant cells with a Tet-on MYC plasmid. These cells were treated with or without doxycycline 24 hours before estrogen withdrawal. Quantification of CSF3R-mCherry/Asxl1-GFP–positive cells reveals significant repression of CD11b+/GR1+ (mean ± SEM; n = 3 per group). Significance was evaluated with a 2-way ANOVA with Sidak multiple comparison test. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.
Collectively, these data suggest that mutant ASXL1 can augment the production of mature cells in the presence of a strong progranulocytic driver mutation. This analysis highlights how context can alter the manifestation of ASXL1-truncating mutations.
Asxl1 mutations change the epigenetic landscape to favor CSF3R-driven differentiation
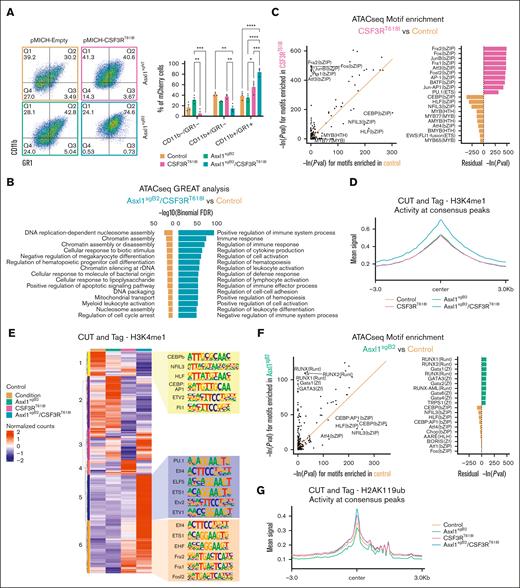
To validate these phenotypes in a model expressing mutant Asxl1 in the endogenous locus, we modified the HoxB8 cell line with CRISPR-Cas9 and isolated an Asxl1 homozygous-mutant clone, Asxl1sgB2 (2919_2933del; R805fsX2; supplemental Figure 6A). We generated the following 4 groups: control (nontargeting sgRNA and empty vector), Asxl1sgB2, CSF3RT618I, and Asxl1sgB2/CSF3RT618I. Consistent with our finding in an exogenously expressed Asxl1 mutant, 48 hours after estrogen removal, the endogenous Asxl1 mutation enhanced CSF3RT618I-driven differentiation (Figure 6A). In the presence of mutant CSF3R, a proportion of these cells became Ly6G positive, a marker of more mature murine neutrophils51 (supplemental Figure 6B). These data demonstrated that an endogenous truncating mutation in the Asxl1 allele enhances CSF3R-driven differentiation. This effect was recapitulated in a second myeloid cell line, 32Dcl3, which we also engineered with an Asxl1 mutation in the endogenous locus (2910_2942del; R805fs5X; supplemental Figure 6C-D).
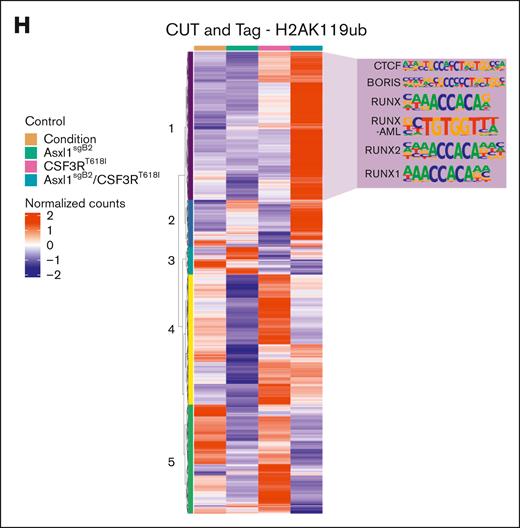
Asxl1 truncation changes the epigenetic landscape to favor CSF3R-driven differentiation. (A) Representative flow cytometry plots of CD11b and GR1 at 48 hours after estrogen withdrawal in HoxB8 CRISPR-Cas9 modified for Asxl1 truncation and transduced with CSF3RT618I or their corresponding control plasmid producing the following 4 groups: control, Asxl1sgB2, CSF3RT618I, and double mutant or Asxl1sgB2/CSF3RT618I. Quantification of the percentage of CSF3R-mCherry–positive cells (mean ± SEM; n = 3 per group). Significance was evaluated with a 2-way ANOVA with a Tukey multiple comparison test. (B) ATAC sequencing was performed 48 hours after estrogen withdrawal with all 4 groups (n = 3 per group). GREAT analysis using differential peaks between Asxl1sgB2/CSF3RT618I and control groups reveal enrichment of immune system activation and differentiation in the double-mutant mice. (C) HOMER motif enrichment results from the ATAC sequencing differential peaks between CSF3RT618I and control. The orange trend line in the scatterplot is used to compare natural log-transformed motif P values between the sample groups. Residual values in the bar plot represent the difference between the trend line and transformed motif P values. (D) CUT&Tag was performed 48 hours after estrogen withdrawal with all 4 groups (n = 3 per group) with H3K4me1 antibody. Profile plot of mean H3K4me1 activity at regions spanning its consensus peak centers ± 3 kb. (E) Heat map displaying row-scaled (region-based z-score scaled), normalized read counts for each sample condition (averaged across replicates) in regions where H3K4me1 is uniquely active. The top 6 significant enriched motifs from HOMER are revealed for region clusters 1, 5, and 6. (F) HOMER motif enrichment results from the ATAC sequencing differential peaks between Asxl1sgB2 and control. The orange trend line in the scatterplot is used to compare natural log-transformed motif P values between the sample groups. Residual values in the bar plot represent the difference between the trend line and transformed motif P values. (G) CUT&Tag was performed 48 hours after estrogen withdrawal with all 4 groups (n = 3 per group) with H2AK119ub antibody. Profile plot of mean H2AK119ub activity at regions spanning its consensus peak centers ± 3 kb. (H) Heat map displaying row-scaled (region-based z-score scaled) normalized read counts for each sample condition (averaged across replicates) in regions where H2AK119ub is uniquely active. Top 6 significant enriched motifs from HOMER are revealed for region cluster 1. CTCF and BORIS are not specific to cluster 1 as they are top 1 and 2 in all but 1 cluster. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.
Asxl1 truncation changes the epigenetic landscape to favor CSF3R-driven differentiation. (A) Representative flow cytometry plots of CD11b and GR1 at 48 hours after estrogen withdrawal in HoxB8 CRISPR-Cas9 modified for Asxl1 truncation and transduced with CSF3RT618I or their corresponding control plasmid producing the following 4 groups: control, Asxl1sgB2, CSF3RT618I, and double mutant or Asxl1sgB2/CSF3RT618I. Quantification of the percentage of CSF3R-mCherry–positive cells (mean ± SEM; n = 3 per group). Significance was evaluated with a 2-way ANOVA with a Tukey multiple comparison test. (B) ATAC sequencing was performed 48 hours after estrogen withdrawal with all 4 groups (n = 3 per group). GREAT analysis using differential peaks between Asxl1sgB2/CSF3RT618I and control groups reveal enrichment of immune system activation and differentiation in the double-mutant mice. (C) HOMER motif enrichment results from the ATAC sequencing differential peaks between CSF3RT618I and control. The orange trend line in the scatterplot is used to compare natural log-transformed motif P values between the sample groups. Residual values in the bar plot represent the difference between the trend line and transformed motif P values. (D) CUT&Tag was performed 48 hours after estrogen withdrawal with all 4 groups (n = 3 per group) with H3K4me1 antibody. Profile plot of mean H3K4me1 activity at regions spanning its consensus peak centers ± 3 kb. (E) Heat map displaying row-scaled (region-based z-score scaled), normalized read counts for each sample condition (averaged across replicates) in regions where H3K4me1 is uniquely active. The top 6 significant enriched motifs from HOMER are revealed for region clusters 1, 5, and 6. (F) HOMER motif enrichment results from the ATAC sequencing differential peaks between Asxl1sgB2 and control. The orange trend line in the scatterplot is used to compare natural log-transformed motif P values between the sample groups. Residual values in the bar plot represent the difference between the trend line and transformed motif P values. (G) CUT&Tag was performed 48 hours after estrogen withdrawal with all 4 groups (n = 3 per group) with H2AK119ub antibody. Profile plot of mean H2AK119ub activity at regions spanning its consensus peak centers ± 3 kb. (H) Heat map displaying row-scaled (region-based z-score scaled) normalized read counts for each sample condition (averaged across replicates) in regions where H2AK119ub is uniquely active. Top 6 significant enriched motifs from HOMER are revealed for region cluster 1. CTCF and BORIS are not specific to cluster 1 as they are top 1 and 2 in all but 1 cluster. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.
To understand changes in the chromatin accessibility driving differentiation-associated gene expression, we performed assay for transposase-accessible chromatin (ATAC) sequencing on differentiating HoxB8 cells 48 hours after estrogen withdrawal. High-confidence differential peaks were identified between the groups. We used GREAT52,53 (Genomic Regions Enrichment of Annotations Tool) to identify genes associated with each peak, followed by GO term enrichment. GO terms associated with immune regulation and activation (a feature of mature myeloid cells) are enriched in the double mutant relative to all other groups (Figure 6B; supplemental Figure 7A). This is consistent with increased differentiation-associated gene expression when Asxl1 is truncated in CSF3RT618I-expressing cells.
We next evaluated transcription factor (TF) motif enrichment in these accessible regions and found that AP1 and ETS family motifs were enriched in CSF3RT618I-upregulated peaks, including JUNB and PU.1, which are transcription factors with a central role in neutrophil differentiation (Figure 6C; supplemental Figure 7B).54-56 This is consistent with activation of the MAPK pathway by the CSF3RT618I mutation, which acts upstream of AP1 TFs.57
Because it has been found that AP1 motifs are enriched at H3K4me1-marked enhancers in activated neutrophils,58 we performed CUT&Tag. CUT&Tag was performed in triplicate 48 hours after estrogen withdrawal with an antibody against H3K4me1, a mark of primed and activated enhancers.59,60 Double-mutant cells exhibited a global increase in H3K4me1, indicating that this group has more primed and activated regulatory regions (Figure 6D). Hierarchical clustering revealed 6 clusters with distinct H3K4me1 patterns across the groups (Figure 6E; supplemental Table 1). In clusters 5 and 6, the H3K4me1 signal is the highest in cells with both mutations. These peaks were enriched for AP1 and ETS complex family motifs, thus regulatory regions linked to neutrophil differentiation are more primed and activated.
Next, we assessed the contribution of H2AK119ub, an ASXL1-associated histone mark, to altered differentiation. H2AK119ub is associated with chromatin compaction and gene repression.61 Truncated ASXL1 strengthens its interaction with BAP1, which enhances removal of H2AK119ub.62 As expected, global H2AK119ub signal was decreased in Asxl1sgB2 cells.
Surprisingly, H2AK119ub levels increased globally in both the double mutant and CSF3RT618I conditions, indicating that mutant CSF3R either overrides or reverses the ASXL1-mutant phenotype (Figure 6G). Hierarchical clustering of the regions with H2AK119ub signal revealed 5 clusters (Figure 6H). Cluster 1 had the highest H2AK119ub signal in the double mutant and strong enrichment for RUNX family motifs (Figure 6H; supplemental Table 1). RUNX transcription factors positively regulate hematopoietic stem cell number.63 In this cluster, we found that Myc has increased H2AK119ub and decreased chromatin accessibility in the double mutant compared with all other groups (supplemental Figure 7C). High H2AK119ub levels may be a consequence of altered cell state in the double-mutant cells, inducing chromatin compaction to close off stem and progenitor pathways and support differentiation.64 Of note, 48 hours after inducing differentiation, these regions are marked for repression but are not yet closed, as assessed using paired ATAC sequencing data (supplemental Figure 7D).
Taken together, these studies revealed that cells with both ASXL1 and CSF3R mutations have increased activating H3K4me1-marked regulatory elements linked to neutrophil differentiation. Simultaneously, chromatin compaction occurs at stem and progenitor-associated genes, such as Myc, in cells with both mutations. These parallel mechanisms help explain the increased ability of double-mutant cells to support neutrophil differentiation.
Discussion
ASXL1-truncating mutations are classically associated with myelodysplastic disorders and impaired myeloid differentiation. However, they are prevalent in CNL, a myeloproliferative neoplasm characterized by excessive mature neutrophil production. This study aims to elucidate the effect of ASXL1 truncation on CSF3R-driven myeloproliferative neoplasm biology.
To address this, we developed a novel mouse model harboring both Asxl1Y588X and Csf3rT621I mutations. The Csf3rT621I transgenic mouse represents the first model with a ligand-independent Csf3r mutation (Figure 1). Csf3rT621I-mutant mice displayed an age-associated decrease in LT-HSCs and other multipotent progenitor cells (Figure 2C; supplemental Figure 3B). CSF3R and its ligand G-CSF play important roles in stem cell mobilization.65 Constitutive activation of mutant CSF3R23 could enhance stem and progenitor cell mobilization or drive higher flux down the neutrophil lineage, causing the depletion of the stem and progenitor populations over time. These data suggest that additional mutations may be required to maintain the stem and progenitor pool in MPNs.
The Asxl1Y588X mutation, when combined with the Csf3rT621I mutation, expands the LT-HSCs stem cell compartment, which may support leukemogenesis. In mice with both mutations and Asxl1-only mutation, the expanded LT-HSC compartment has a CD41+ bias (Figure 2C), indicating myeloid bias and enrichment for megakaryocyte/platelet programs.41,66 This expansion of CD41+ LT-HSCs also explains the higher levels of megakaryocytes and platelets in both Asxl1Y588X- and double-mutant mice. In the HoxB8 model, we discovered an enrichment of open chromatin–containing RUNX family motifs in cells with Asxl1 truncation. Others have linked RUNX to megakaryocyte production,63,67,68 providing a potential explanation for increased megakaryocyte production in mice with mutant Asxl1. Loss or repression of RUNX1 has furthermore been linked to Lin- Sca-1+ c-Kit+ expansion, which could contribute to the expanded stem/progenitor populations observed in the double-mutant mice.69 These findings provide a potential explanation for why such a high percentage of CSF3R-mutated leukemias also harbor an ASXL1 mutation.
Despite changes in stem and progenitor populations and increased neutrophil production, few mice developed overt leukemia. Mice have a short life span and, therefore, have limited time for leukemia development, whereas the median age of diagnosis for CNL in humans is ∼66 years.38 This extended time frame in humans allows for the accumulation of additional mutations that may be crucial for disease development. In addition to ASXL1 and CSF3R mutations, mutations in other epigenetic regulators (eg, SETBP1 and EZH2) and spliceosome proteins (eg, SRSF2 and U2AF1)22 are common. Thus, additional mutations may be necessary for leukemia to manifest in this model.
In vitro, Asxl1 truncation enhanced CSF3RT618I-driven neutrophil differentiation by downregulating Myc programs while upregulating inflammatory signatures associated with myeloid maturation (Figures 4 and 5). These 2 mutations jointly influence the epigenetic landscape and activate neutrophil differentiation pathways while suppressing other hematopoietic programs. Cells with both mutations had enhanced priming of neutrophil-linked gene enhancers.
Asxl1 truncation decreased H2AK119ub, as anticipated, but a CSF3R mutation reversed this effect (Figure 6G-H). Restoration of H2AK119ub levels in the presence of mutant CSF3R could be due to the repression of genes associated with non-neutrophil fates, as CSF3R forces cells to commit to the neutrophil lineage. Cells with both mutations have RUNX1 motif enrichment in regions with increased H2AK119ub expression. RUNX1 is highly expressed in myeloid progenitors, and its expression declines as cells mature.64 Increased H2AK119ub at RUNX1 targets may indicate the silencing of progenitor-associated chromatin regions during differentiation. The Myc locus also had increased H2AK119ub expression, decreased chromatin accessibility, and reduced expression during differentiation. Thus, mutant ASXL1 supports myeloid differentiation in the presence of a strong pro-neutrophil driver. Collectively, our results emphasize that mutation combinations can have distinct effects at different stages of myeloid development.
In this study, we investigated the role of mutant ASXL1 in CSF3R-mutant MPNs. Asxl1Y588X counteracted the decline in stem and progenitor populations caused by Csf3rT621I and promoted the expansion of myeloid-biased LT-HSCs in aging mice. Although mice with both mutations develop neutrophilia, overt leukemia rarely develops. In vitro models revealed that Asxl1 truncation enhances CSF3RT618I-driven neutrophil differentiation by enhancing pro-neutrophil programs at the expense of other hematopoietic lineages. This study highlights that the effects of ASXL1 mutations in myeloid malignancies are highly context-specific.
Acknowledgments
The authors thank the members of the Maxson and Braun groups for their advice, helpful discussion, and assistance. The authors thank the following Oregon Health & Science University core facilities for their assistance: flow cytometry, histopathology, advanced light microscopy, massive parallel sequencing, ExaCloud cluster computational resources, and advanced computing centers.
Funding was provided by a National Heart Lung and Blood Institute grant (R01 #5R01HL157147 to J.E.M). J.E.M. is supported by a Leukemia & Lymphoma Society Scholar award. T.P.B. is supported by an Edward P. Evans Foundation award. S.T. is supported by a Medical Research Foundation Early Clinical Investigator award. S.C.T. is supported by a grant from the National Cancer Institute (R01 CA247943-04S1). A.P. is supported by an American Association of Hematology Minority Hematology Graduate award. K.E.N. is supported by an HHMI Gilliam Fellowship. E.M.P. is a scholar of the Leukemia and Lymphoma Society.
Authorship
Contribution: L.D. and J.E.M. were responsible for conception and design; L.D., A.J.B., H.B., S.B.S., A.P., S.T., S.C.T., and H.L.C. performed in vivo experiments; L.D., A.J.B., A.C.F., and T.P.B. performed in vitro experiments; T.T.N. and T.P.B. performed computational analyses; K.E.N. and E.M.P. provided flow cytometry resources and assistance with data analysis; L.D., A.J.B., T.T.N., K.E.N., E.M.P., and J.E.M. analyzed and interpreted the data; L.D. and J.E.M. wrote the manuscript; and all authors reviewed and revised the manuscript.
Conflict-of-interest disclosure: J.E.M. receives research funding from Blueprint Medicines and Kura Oncology. T.P.B. receives research support and consulting fees from Blueprint Medicines, research support from AstraZeneca, and consulting fees from Novartis. The other authors declare no competing financial interests.
Correspondence: Julia E. Maxson, Hematology & Medical Oncology, Oregon Health & Science University, Mail code KR-HEM, 3181 S.W. Sam Jackson Park Rd, Portland, OR 97239; email: maxsonj@ohsu.edu.
References
Author notes
The Gene Expression Omnibus accession numbers for all sequencing data reported in this article are GSE266087, GSE266090, and GSE266091.
Other data generated in this study are available within the article and its supplementary data files.
The full-text version of this article contains a data supplement.

![Asxl1 truncation promotes the expansion of myeloid-biased stem cells. (A) Representative hematoxylin and eosin (H&E)–stained tibia cross-sections of mice 1 year post-Cre induction (n = 3 per group). (B) Schematic of serial transplant of all 4 genotypes and timeline of bone marrow characterization by flow cytometry. (C) Flow cytometry analysis of the long-term hematopoietic stem, LT-HSC CD41+/CD41− ratio, and GMP progenitor cells from mice bone marrow at 4 months, 1 year after Cre induction and after both serial transplants (n = 3-4 per group). (D) Quantification of colony-forming unit assays at 1 year post-Cre induction and after both serial transplants using 1 × 104 cells per well of whole bone marrow cells (mean ± standard error of the mean [SEM]; n = 3 per group in triplicate). Significance was evaluated with a 2-way analysis of variance (ANOVA) with Tukey multiple comparison test. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/7/10.1182_bloodadvances.2024014362/2/m_blooda_adv-2024-014362-gr2.jpeg?Expires=1767718400&Signature=da2Y6J6OGx3rCIam25jQM~iZI6rZ7tEpm5M5xdy744iHFL5SFxLkGe1qUWPBd~f5nEyoSe77EF0dF0Q-L3DIlAhf2KtdmoGgwdJH5RKpIE-YeAJriScYpL5cATE5DxY5LBPmZR4XUX5oGSkv5VZt~zomOHr3O097ELwv64l4otTS2e3wgAIhB5SB3UUd56J3dNu5uI-hlbEH7Y5nfP~l9qStvw7e15enLMlJmhEuNbPabEdykxuDoSfYcu3QazmSVXsV6SZzEma8RCVjSOyLjCWXth6VQHEbkhIX3BAtNyh1uLrO-wBRK5NTKm3l2hqlKM5raORti8eUfgMU-~ySpQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)