Key Points
T-ALL and subsets of B-ALL are functionally co-dependent on BCL2 and BCL-XL for survival.
Dual BCL2/BCLXL inhibitor AZD0466 demonstrates pre-clinical activity in T-ALL in vitro and in vivo.
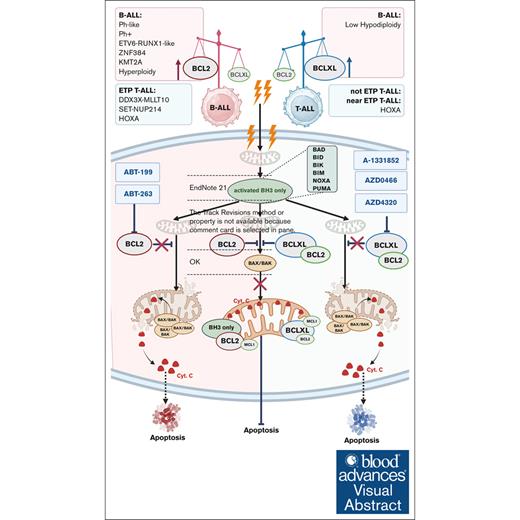
Visual Abstract
The upregulation of B-cell lymphoma 2 (BCL2) and B-cell lymphoma–extra large (BCL-XL), 2 proteins in the BCL2 family of proteins, leads to a disproportional expression of prodeath and prosurvival proteins in favor of leukemia survival, tumorigenesis, and chemoresistance. In different subsets of acute lymphoblastic leukemia (ALL), the proportion of these 2 proteins varies, and their potential as therapeutic targets needs detailed characterization. Here, we investigated BCL2 and BCL-XL, the genes that encode BCL2 and BCL-XL, and their expression differences between B-cell acute lymphoblastic leukemia (B-ALL) and T-cell ALL (T-ALL). We also evaluated the therapeutic potential of targeting these proteins with AZD0466, a novel drug-dendrimer conjugate of the BCL2/BCL-XL inhibitor AZD4320, and with BCL2 inhibitor venetoclax (ABT-199). Gene expression and activity analyses supported by the protein expression patterns in ALL cell lines and primary samples demonstrated increased levels of BCL2 expression in B-ALL, with high sensitivity to venetoclax or AZD4320. In contrast, strong BCL-XL expression and sensitivity to dual BCL2/BCL-XL inhibition was observed specifically in T-ALL samples. This observation was confirmed by BH3 profiling, demonstrating BCL2/BCL-XL codependence in T-ALL and BCL2 dependence in B-ALL. In a mouse model of T-ALL, AZD0466 but not venetoclax reduced leukemic burden and prolonged survival without significant toxicities. Our findings therefore suggest that the novel dual BCL2/BCL-XL inhibitor AZD0466 outperforms single BCL2 inhibition by venetoclax in T-ALL. These findings facilitate the translation of dual BCL2/BCL-XL inhibitors into ALL clinical trials, either alone or in combination with standard-of-care chemotherapy and immune therapies.
Introduction
The prognosis of adult patients with relapsed/refractory acute lymphocytic leukemia (ALL) remains poor because of genetic and molecular aberrations, with inferior response to salvage chemotherapy, and dismal overall survival.1-4 Although novel monoclonal antibodies (eg, blinatumomab and inotuzumab) and CD19-directed chimeric antigen receptor T-cell therapy significantly improve outcomes for patients with relapsed/refractory B-cell ALL (B-ALL), no targeted therapies exist for patients with relapsed/refractory T-cell ALL (T-ALL), severely limiting their therapeutic options.5-8
Cancer cells can evade apoptosis by sequestering proapoptotic proteins with B-cell lymphoma 2 (BCL2), B-cell lymphoma–extra large (BCL-XL), or myeloid cell leukemia-1 (MCL1), anti-apoptotic proteins from the BCL2 family.9 During normal T-cell development, the BCL-2/DN2 → BCL-XL/DN3 → BCL-2/DPCD3+ transition pattern is dependent on the JAK–STAT signaling pathway,10-12 suggesting that such dependence is an anti-apoptotic adaptation in the pathogenesis of ALL and thus a unique targetable vulnerability. The upregulation of BCL2 and BCL-XL, the key regulators of apoptosis, was reported in chronic lymphocytic leukemia and acute myeloid leukemia, and targeting BCL2 became a groundbreaking therapeutic strategy for these leukemias.13-16 However, in ALL, preclinical studies showed impeded efficacy of such inhibitors because of the heterogenous upregulation of BCL2, BCL-XL, and MCL1,17,18 indicating diverse dependencies on BCL2 family members across ALL subtypes.
BCL2 dependency was predominantly observed in early T-cell precursor (ETP) ALL and was characterized by sensitivity to selective BCL2 inhibitor venetoclax-based chemotherapy.19,20 In contrast, more mature subsets of T-ALL have exhibited increased dependency on BCL-XL, indicating that they could be targeted by the first-generation dual BCL2/BCL-XL inhibitor navitoclax.10 Upregulation of the JAK-STAT signaling pathway increased BCL-XL priming in Philadelphia chromosome (Ph)-like B-ALL cases harboring CRLF2 or JAK2 mutations,21,22 indicating the potential of dual BCL2/BCL-XL inhibitors to eradicate both B- and T-ALL progenitors.21,22 BCL2 and BCL-XL coinhibition was further demonstrated by increased sensitivity and mitochondrial priming to apoptosis in preclinical acute myeloid leukemia models18,23-25 and with potent response rates and acceptable tolerability of combined low doses of navitoclax, venetoclax, and chemotherapy for patients with relapsed/refractory ALL in the clinical trial NCT03181126.1 Additionally, correlative studies using BH3 profiling, which determines the initial BH3 domain dependencies and measures the dynamic response of mitochondria, demonstrated a common codependency of BCL2 and BCL-XL in B-ALL and a switch to BCL-XL dependency in T-ALL upon progression.1,20 The heterogeneous dependency on BCL2 family proteins among different subtypes of ALL indicates the need of cotargeting multiple anti-apoptotic family members to induce apoptosis.26 However, the association with BCL-XL inhibition and on-target thrombocytopenia27 was observed in clinical trials with navitoclax in chronic lymphocytic leukemia,28,29 limiting its clinical use.
AZD4320 is a second-generation dual BCL2/BCL-XL inhibitor with tumor regression potential in the broad spectrum of hematological malignancies with lower rates of thrombocytopenia than navitoclax.18 AZD0466 comprises AZD4320 chemically conjugated to a pegylated polylysine nanosize dendrimer with an optimized drug release rate from the dendrimer complex. AZD0466 is dosed on an intermittent schedule, mitigating thrombocytopenia risk.18,30 We therefore hypothesized that AZD0466, as a tolerable and effective dual BCL2/BCL-XL inhibitor, will lead to enhanced antileukemic activity in preclinical models of ALL.
Here, we report the preclinical efficacy of AZD4320 and AZD0466 in ALL models in vitro and in vivo, along with superior efficacy of AZD0466 in preclinical patient-derived xenograft (PDX) T-ALL models compared with in B-ALL models, and confirm a sparing platelet property.
Materials and methods
ALL cell lines, PDX models, and primary patient samples
We obtained the PF-382, SUPT1, and KOPT--K1 T-ALL; and RS4-11, NALM6, and SEMK2 B-ALL cell lines; and the BALL1 B-ALL, and MHH-CALL4; and MUTZ5 Ph-like ALL cell lines from the American Type Culture Collection and the German Collection of Microorganisms and Cell Cultures; and PALL2 from Japanese Collection of Research Bioresources Cell Bank. All cell lines were maintained in RPMI 1640 medium with 2 mM glutamine (Sigma-Aldrich) containing 10% to 15% fetal calf serum and 1% penicillin-streptomycin at The University of Texas MD Anderson Cancer Center.
Peripheral blood from patients with T-ALL and B-ALL was obtained per institutional review board–approved protocol (LAB02-652 and LAB02-652). Mononuclear cells were separated by Ficoll-Hypaque (Sigma-Aldrich) density gradient centrifugation. Primary samples were maintained in quality biological serum-free medium containing 20% fetal calf serum (Gemini Bio-Products) and 1% penicillin-streptomycin (Life Technologies Laboratories). All cells were grown at 37°C, 5% CO2.
Cell viability assays
Cells samples from cell lines, primary patient samples, and PDX models were seeded at 40 000 cells per well on a 96-well U-bottom plate; and 24 hours after treatment with AZD4320 (AstraZeneca), venetoclax (Selleckchem), A-1331852 (Selleckchem), or the MCL1 inhibitor AZD5991 (AstraZeneca) at 0 to 10 μM ranges, cells were evaluated using a Cell Titer-Glo (Promega) viability assay and annexin-V/DAPI(4’,6-diamidino-2-phenylindole)/beads apoptosis assay by flow cytometry. The triplicate data were normalized to the vehicle-only (dimethyl sulfoxide at 0.001%) control. A specific apoptosis rate was determined by the percentage of early apoptotic cells after exposure to inhibitors compared with dimethyl sulfoxide–treated controls using a previously published formula.31
The response of primary human ALL cells to navitoclax, AZD4320, and AZD5991, and PDX cells cocultured with mesenchymal stem cells at 15 000 cells per well in AIM-V media (Gibco) in a 384-well plate was evaluated in duplicate for concentrations range 0.01 to 1000 nM, for 96 hours,32 15 000 cells in 50 μL media. The live cell populations in each well were detected by Operetta CLS high-content analysis system (Perkin Elmer). The viable cell percentage was normalized by comparing the number of treated cells to that of live cells in the control wells. A 4-parameter sigmoidal dose-response curve was fitted based on the percentage of viable cells at the given drug concentrations compared with the controls without drugs. The area under the fitted dose-response curve was estimated using the trapezoidal rule over the concentration range tested in a log10 scale.
RNA-seq
The total RNA from PDX or primary patient samples were extracted using Qiagen RNeasy mini kit (Qiagen). The RNA-sequencing (RNA-seq) library was generated using an Illumina TrueSeq stranded total RNA library preparation kit (Illumina) and sequenced using the NovaSeq 6000 platform (Illumina; 2 × 100 base pair paired-end reads). STAR (version 2.6.0b)33 was used to align the gene expression under default parameters with the human genome (GRCh38/hg38). The total RNA-seq cohorts were further used to evaluate gene expression levels from RNA-seq data,34,35 to reconstruct the human B-ALL and T-ALL interactome via SJARACNe,36 to analyze NetBID activity in T-ALL and B-ALL subtypes, and to predict BCL2/BCL-XL activity across B-ALL and T-ALL subtypes using MetaVIPER activity analysis. More information on these methods can be found in the supplemental Methods.34-42
BH3 profiling
Immunoblotting
Selected ALL cell lines were lysed using radioimmunoprecipitation assay buffer with phosphatase and protease inhibition cocktails, and total protein was fractionated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to polyvinylidene fluoride membranes (Millipore Sigma), and probed with indicated antibodies. Primary antibodies to BCL2 (Agilent DAKO), BCL-XL, BH3-associated X protein (BAX) (Cell Signaling Technology), MCL1 (Santa Cruz), BCL2L11, BH3-inhibiting mitochondrial protein (BIM) (Abcam), and β-actin (Sigma-Aldrich) were used. The infrared fluorochrome-conjugated secondary antibodies (Odyssey IRDye 680 RD anti-mouse and Odyssey IRDye 800 CW goat anti-rabbit) were used and imaged according to Li-COR protocols.
Animal studies
All experimental animal procedures were approved by The University of Texas MD Anderson Cancer Center’s institutional animal care and use committee in compliance with relevant ethical regulations regarding animal research and studies. Diverse PDX ALL models were used in this study. NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice were xenografted via the tail vein with PDX-ALL cells.45-47
Mice were dosed variably with AZD0466 (103 mg/kg and 34 mg/kg weekly, IV); AZD5991 (60 mg/kg weekly, IV), venetoclax (100 mg/kg, per oral gavage, daily, for 15 days), or vincristine/dexamethasone/L-asparaginase (VXL) vincristine (0.15 mg/kg); dexamethasone (5 mg/kg, IV); L-asparagine (1000 IU/kg, ∼25 IU per mouse) alone via intraperitoneal injection or in combination with AZD0466 or with the corresponding vehicles. Weekly tumor burden measurement via flow cytometry, and platelet counts were monitored as outlined in the supplemental Methods.
Statistical analysis
Data (mean ± standard error of the mean) were analyzed with 1-way analysis of variance with pairwise comparison among treatment groups and log-rank (Mantel-Cox) test, as applicable. The Gehan-Breslow-Wilcoxon method was used to correct P values for pairwise survival comparisons. P values were not adjusted for potential multiple comparisons.
The institutional animal care and use committee–approved protocol 00000862 was used; principal investigator was S.K., The University of Texas MD Anderson Cancer Center, Houston, TX.
Results
Diverse subtypes of T-ALL and B-ALL show differential activity of BCL2 family genes
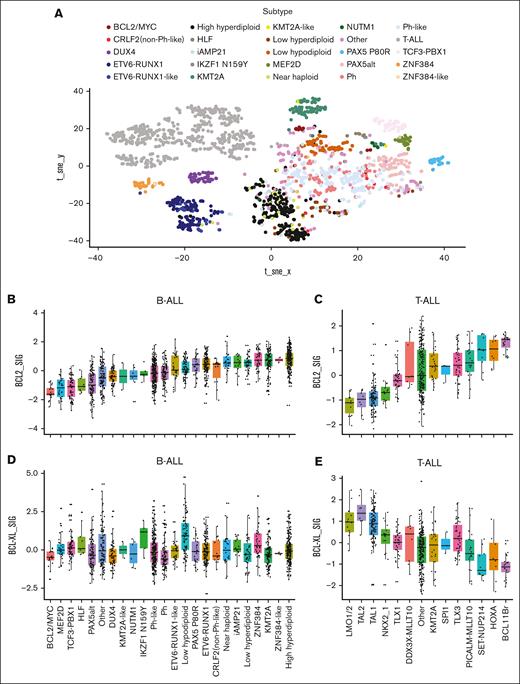
We first analyzed gene expression profiles of BCL2 and BCL-XL genes in B- and T-ALL from the Pan-ALL cohort, which includes 1976 (1418 B-ALL and 558 T-ALL)34 patients (Figure 1A), via hierarchical clustering, t-distributed stochastic neighbor embedding analysis, and predictive modeling using cases of known subtypes, as previously described.34 Next, in conjunction with cytogenetic data, the gene activity of BCL2 and BCL-XL in B-ALL and T-ALL samples across specific ALL subtypes was analyzed separately using NetBID.39 Among 23 different B-ALL subtypes, 5 subtypes (BCL2/MYC, MEF2D, TCF3-PBX1, HLF, and PAX5alt) were characterized by relatively low BCL2 gene activity levels; whereas the low-hyperdiploidy, ZNF384, KMT2A, ZNF384-like, and high-hyperdiploidy subtypes exhibited the highest levels of BCL2 activity (Figure 1B-C). Among the T-ALL subset, there was a distinct cohort including LMO1/2, TAL2, TAL1, NKX21, and TLX1, characterized by lower and elevated activity levels of BCL2 and BCL-XL, respectively. The remaining subtypes showed a trend of increasing BCL2 gene activity, accompanied by decreased BCL-XL activity (Figure 1D-E), whereas there was no trend of increase or decrease of MCL-1 activity across these B-ALL or T-ALL subtypes (supplemental Figure 1). Taken together, these data provide transcriptional evidence of distinct anti-apoptotic gene activity levels of T-ALLs and B-ALLs. Furthermore, increased BCL2 activity in B-ALLs was accompanied by stable BCL-XL activity, regardless of genetic background. In contrast, the activity of BCL2 in T-ALLs was negatively correlated with that of BCL-XL and trended toward elevated levels of activity in genetic subtypes, suggesting more mature stages of cell differentiation.
BCL2/BCL-XL gene expression analysis identifies diverse subtypes across ALLs linked to specific genetic aberrations. (A) Gene expression profile’s landscape of the BCL2/BCL-XL in the ALL subtypes illustrated in a 2-dimensional t-distributed stochastic neighbor embedding (tSNE) map. Gene expression profiles of BCL2 and BCL-XL genes in B- and T-ALL were analyzed using a bioinformatical approach and RNA-seq data from leukemic cells of 1976 (1418 B-ALL and 558 T-ALL) and 916 (780 B-ALL and 136 T-ALL) patients diagnosed with ALL. Gene expression profiles were evaluated and analyzed using hierarchical clustering, tSNE analysis, and predictive modeling using cases of known subtypes, as previously described.33 Gene expression signal is displayed in log2. (B) Association of clusters based on the t-SNE map with subtypes shown for comparison with the hierarchical clustering approach. Cohort metrics defining BCL2 activity profiles to genetic abnormalities consists of 23 different B-ALL subtypes clustered based on known chromosomal abnormalities and genetic mutations as follows: BCL2/Myc, Mef2D, Tcf3-Px1, Hlf, Pax5alt, other, Dux4, Kmt2a-like, Nutm1, Ikzf1 N159Y, Ph-like, Ph, ETV6-Tunx1–like, low hypodiploid, Pax5 P80R, ETC6Runx1, Clrf2 (non–Ph-like), near haploid, iAMP21, low hyperdiploidy, Znf384, Kmt2a, Znf384-like, and high hyperdiploidy. (C) Association of the clusters based on the t-SNE map with the subtypes shown for comparison with the hierarchical clustering approach. Cohort metrics defining BCL2 activity profiles to genetic abnormalities consists of 14 different T-ALL subtypes clustered based on known chromosomal abnormalities and genetic mutations, as follows: LMO1/2, TAL1, TAL2, TLX1, TLX3, HOXA, NKX2-1, DDX3-MLLT10, PICALM-MLLT10, KMT2A, and Spi1. (D) BCL-XL activity across different B-ALL subtypes. (E) BCL-XL activity across different T-ALL subtypes.
BCL2/BCL-XL gene expression analysis identifies diverse subtypes across ALLs linked to specific genetic aberrations. (A) Gene expression profile’s landscape of the BCL2/BCL-XL in the ALL subtypes illustrated in a 2-dimensional t-distributed stochastic neighbor embedding (tSNE) map. Gene expression profiles of BCL2 and BCL-XL genes in B- and T-ALL were analyzed using a bioinformatical approach and RNA-seq data from leukemic cells of 1976 (1418 B-ALL and 558 T-ALL) and 916 (780 B-ALL and 136 T-ALL) patients diagnosed with ALL. Gene expression profiles were evaluated and analyzed using hierarchical clustering, tSNE analysis, and predictive modeling using cases of known subtypes, as previously described.33 Gene expression signal is displayed in log2. (B) Association of clusters based on the t-SNE map with subtypes shown for comparison with the hierarchical clustering approach. Cohort metrics defining BCL2 activity profiles to genetic abnormalities consists of 23 different B-ALL subtypes clustered based on known chromosomal abnormalities and genetic mutations as follows: BCL2/Myc, Mef2D, Tcf3-Px1, Hlf, Pax5alt, other, Dux4, Kmt2a-like, Nutm1, Ikzf1 N159Y, Ph-like, Ph, ETV6-Tunx1–like, low hypodiploid, Pax5 P80R, ETC6Runx1, Clrf2 (non–Ph-like), near haploid, iAMP21, low hyperdiploidy, Znf384, Kmt2a, Znf384-like, and high hyperdiploidy. (C) Association of the clusters based on the t-SNE map with the subtypes shown for comparison with the hierarchical clustering approach. Cohort metrics defining BCL2 activity profiles to genetic abnormalities consists of 14 different T-ALL subtypes clustered based on known chromosomal abnormalities and genetic mutations, as follows: LMO1/2, TAL1, TAL2, TLX1, TLX3, HOXA, NKX2-1, DDX3-MLLT10, PICALM-MLLT10, KMT2A, and Spi1. (D) BCL-XL activity across different B-ALL subtypes. (E) BCL-XL activity across different T-ALL subtypes.
Functional validation of BCL2/BCL-XL codependence in T-ALL and BCL2 dependence in B-ALL
To further validate BCL2 and BCL-XL expression in ALL, we directly compared the level of each of these 2 proteins in 15 B-ALL and 13 T-ALL cell lines from DepMap. As shown in Figure 2A, there was significantly higher BCL2 protein level in B-ALL cell lines than those in T-ALL, whereas the protein level of BCL-XL showed the opposite trend between B- and T-ALL. We validated protein expression levels by immunoblotting in selected B- or T-ALL cell lines (Figure 2B). Compared with T-ALL cell lines, B-ALLs showed moderate or elevated BCL2 expression with lower levels of BCL-XL, except for the Ph-like B-ALL MUTZ5. T-ALL cell lines showed the opposite trend.
BCL2/BCL-XL codependence in ALL cell lines in vitro. (A) Protein expression by reverse phase protein array (RPPA) for anti-apoptotic BCL2 and BCL-XL (BCL2/BCL-XL) in 15 B-ALL and 13 T-ALL cell lines. The violin plots show the median expression, maximum (Q3 + 1.5 × interquartile range [IQR]), and minimum value (Q1 + 1.5 × IQR). Data were analyzed with 2-sided t test. (B) The protein expression levels of anti-apoptotic family members (BCL2, BCL-XL, and MCL1) and prodeath activators (BIM and BAX) determined by Western blot analysis. Whole-cell lysates from a panel of B- and T-ALL cell lines were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and probed for the indicated antibodies. The cropped images (boxed) of cell lines reflecting specific ALL subsets generated from the single immunoblot across probed proteins. (C-D) BH3 profiling functional assay to measure apoptotic priming. Dependencies were measured in a panel of B- and T-ALL cell lines. All calculations were performed using GraphPad prism statistical software version 9.
BCL2/BCL-XL codependence in ALL cell lines in vitro. (A) Protein expression by reverse phase protein array (RPPA) for anti-apoptotic BCL2 and BCL-XL (BCL2/BCL-XL) in 15 B-ALL and 13 T-ALL cell lines. The violin plots show the median expression, maximum (Q3 + 1.5 × interquartile range [IQR]), and minimum value (Q1 + 1.5 × IQR). Data were analyzed with 2-sided t test. (B) The protein expression levels of anti-apoptotic family members (BCL2, BCL-XL, and MCL1) and prodeath activators (BIM and BAX) determined by Western blot analysis. Whole-cell lysates from a panel of B- and T-ALL cell lines were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and probed for the indicated antibodies. The cropped images (boxed) of cell lines reflecting specific ALL subsets generated from the single immunoblot across probed proteins. (C-D) BH3 profiling functional assay to measure apoptotic priming. Dependencies were measured in a panel of B- and T-ALL cell lines. All calculations were performed using GraphPad prism statistical software version 9.
Next, we analyzed the interplay of BCL2 and BCL-XL with other BH3 family members, namely MCL1, BIM, and BAX. Although MCL1 protein expression in B-ALL was rather weak, both MCL1 and BCL-XL expression in Ph-like B-ALL and T-ALL was elevated (Figure 2B). Except for the NALM6 B-ALL cell line, which lacked the BAX protein, no significant differences were found in expression of the proapoptotic proteins BIM and BAX across different ALL types (Figure 2B). BH3 profiling revealed ALL cells were in a primed state for specific anti-apoptotic proteins. The prodeath activation of BAX/BAK through BIM and BID peptides that bind all anti-apoptotic family members was observed, indicating that both B- and T-ALL cells are capable of cytochrome-c release (Figure 2C-D; supplemental Figure 2). The Bad peptide that inhibits BCL2-, BCL-XL–, and BCL-W–induced cytochrome-c release in the B-ALL cell lines SEMK2 and B-ALL1 (supplemental Figure 2A,C). Such release was present to a lesser extent in NALM6 and was not present in any T-ALL cell lines (Figure 2D; supplemental Figure 2B-C). Although selective BCL2 inhibition with venetoclax caused cytochrome-c release in B- and T-ALL cells (except in the T-ALL KOPTK1 cell line), the selective BCL-XL peptidic inhibitor Harakiri (HRK) caused cytochrome-c release only in T-ALL cells (Figure 2D; supplemental Figure 2B-C). Therefore, these results imply codependency of BCL2 and BCL-XL in T-ALL, and a BCL2-driven pattern of response in B-ALL.
Comparison of leukemia sensitivity with BH3 mimetics in B-ALL vs T-ALL
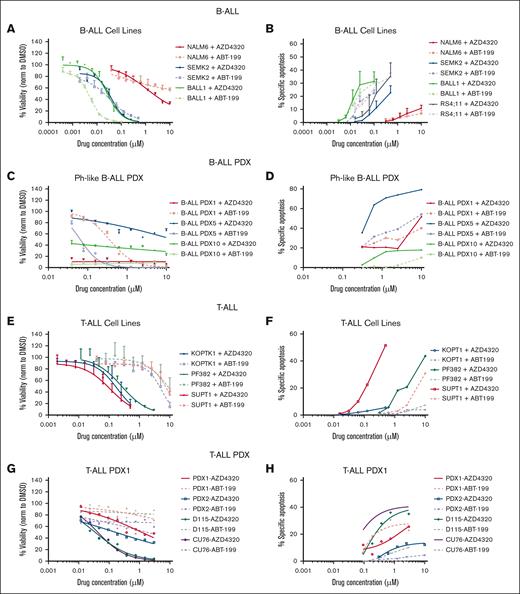
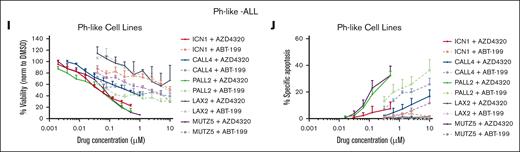
Next, we examined BCL2/BCL-XL dependence of ALLs, evaluating their sensitivity to venetoclax and AZD4320 in a subset of B-ALL and T-ALL cell lines and PDX models. Consistent with the results of BH3 profiling, B-ALL cells responded similarly to both inhibitors, indicating prevalent BCL2 priming (Figure 3A,C; supplemental Table 1). In contrast, in T-ALL cell lines, AZD4320 reduced viability at concentrations nearly 10-fold lower than those of venetoclax, suggesting the codependence of T-ALL survival on BCL2 and BCL-XL (Figure 3E,G; supplemental Table 1). Next, we evaluated the apoptosis-inducing potential of BCL2 and BCL2/BCL-XL inhibitors. Only a moderate level of apoptosis was induced in B-ALL cell lines and PDX samples (Figure 3B,D). However, a higher level of dose-dependent apoptosis was induced upon AZD4320 treatment in most T-ALL samples (Figure 3F,H), and similar efficacy was observed in Ph-like cell lines, indicating BCL2/BCL-XL codependence (Figure 3I-J). Furthermore, we performed viability and apoptosis assessment in T-ALL cell lines to compare the dual inhibitor AZD4320 with the selective BCL-XL inhibitor (A-1331852) and the BCL-2 inhibitor (ABT-199). AZD4320 reduced viability at concentrations nearly 10-fold and 4-fold to 5-fold lower than those of venetoclax and A-1331852 in PF382 and SUPT1 cells, respectively, suggesting the codependence of T-ALL survival on BCL-XL and BCL2 (supplemental Figure 3A). Next, we evaluated the apoptosis-inducing potential of BCL2, BCL-XL, and BCL2/BCL-XL inhibitors. Consistent with viability reduction, higher apoptosis was induced by A-1331852 and AZD4320 (1-10 nM) than ABT-199 in T-ALL cells (supplemental Figure 3B). In addition, the drug potency was determined by comparing the 50% infective dose and area under drug response curve (AUC) for ABT199, A-1331852, and AZD4320 in T-ALL (supplemental Table 2). The 50% infective dose results and AUC indicated higher sensitivity to dual inhibitor AZD4320 than single inhibitors ABT-199 or A-1331852 (supplemental Table 2). Taken together, these results provide cell-specific BCL2 dependency in B-ALL, and BCL2/BCL-XL codependency in T-ALL, and confirm their specific sensitivity to venetoclax and AZD4320, respectively.
In vitro efficacy of BCL2 and BCL2/BCL-XL inhibition in panel of B- and T-ALL cell lines and PDX models. Cells were tested for the inhibitory effect of the BCL2/BCL-XL dual inhibitor AZD4320 compared with that of the selective BCL2 inhibitor venetoclax. Cell viability and apoptosis were evaluated, followed by 24 hours incubation with indicated drug concentrations and measured by CellTiter-Glo and annexin-V assays. Results were summarized and presented separately for (A-B) B-ALL cell lines, (C-D) PDX B-ALL models, (E-F) T-ALL cell lines, (G-H) PDX T-ALL models, and (I-J) Ph-like B-ALL cell lines. All calculations were performed using GraphPad prism statistical software version 9.
In vitro efficacy of BCL2 and BCL2/BCL-XL inhibition in panel of B- and T-ALL cell lines and PDX models. Cells were tested for the inhibitory effect of the BCL2/BCL-XL dual inhibitor AZD4320 compared with that of the selective BCL2 inhibitor venetoclax. Cell viability and apoptosis were evaluated, followed by 24 hours incubation with indicated drug concentrations and measured by CellTiter-Glo and annexin-V assays. Results were summarized and presented separately for (A-B) B-ALL cell lines, (C-D) PDX B-ALL models, (E-F) T-ALL cell lines, (G-H) PDX T-ALL models, and (I-J) Ph-like B-ALL cell lines. All calculations were performed using GraphPad prism statistical software version 9.
T-ALL exhibits unique pattern of sensitivity to BCL2/BCL-XL dual inhibition
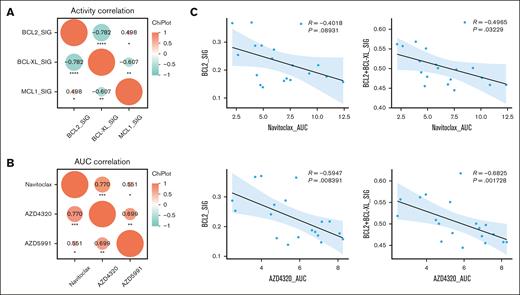
Focusing on T-ALL, we sought to examine the relationship between BCL2 family gene activity and leukemia sensitivity to BCL2/BCL-XL dual inhibitors, using 19 primary samples from adults and children with T-ALL (Figure 4A). A strong negative correlation between BCL2 and BCL-XL (P = −0.782) or BCL-XL and MCL1 (P = −0.607) and a positive correlation between BCL2 and MCL1 (P = .498) activity were observed (Figure 4A; Table 1). Then, we evaluated the in vitro responses to navitoclax and AZD4320 in the same cohort (Figure 4B) and found a significant correlation for sensitivities between different drugs such as navitoclax with AZD4320 (P = .77) and navitoclax with AZD5991 (P = .551). A similar correlation was observed for AZD4320 and AZD5991 (P = .699), indicating a codependency of BCL2/BCL-XL and MCL1 in these T-ALL samples. Correlative analyses of both BCL2 and BCL1L1 gene activity to selected inhibitors indicated that a BCL2-dependent or -associated efficacy of navitoclax (AUC) further improved the cooperative activity of both BCL2 and BCL-XL, indicating a codependency on BCL2 and BCL-XL in the subset of patients with T-ALL (r = −0.4965; P = .03229; Figure 4C, upper panel; Table 1). A similar trend was observed in response to AZD4320, resulting in significant dependency on BCL2 (r = −5947; P = .008391) and codependency of BCL2 and BCL-XL (r = −0.6825; P = .001728; Figure 4C, lower panel; Table 1). To confirm our bioinformatic studies, we conducted a comparative in vitro efficacy analysis that validated the outcompeting efficacy of BCL2 inhibition with venetoclax or with AZD4320 over MCL1 inhibition in B-ALL cell lines and the highest sensitivity to dual BCL2/BCL-XL inhibition in T-ALL cell lines, whereas responses to venetoclax and AZD5991 were less profound (supplemental Figure 4).
Prosurvival protein dependency in primary T-ALL in vitro. (A) The correlation of drug sensitivity expressed as area under drug response curve (AUC) for navitoclax, AZD4320, and AZD5991 in select samples from patient with ALL. (B-C) Correlative studies on gene activity and drug sensitivity for select gene/inhibitor pairs. The statistical differences between treatments conditions were evaluated using unpaired Student t tests; P and R values are indicated on individual figure panels. All calculations were performed using GraphPad prism statistical software version 9.
Prosurvival protein dependency in primary T-ALL in vitro. (A) The correlation of drug sensitivity expressed as area under drug response curve (AUC) for navitoclax, AZD4320, and AZD5991 in select samples from patient with ALL. (B-C) Correlative studies on gene activity and drug sensitivity for select gene/inhibitor pairs. The statistical differences between treatments conditions were evaluated using unpaired Student t tests; P and R values are indicated on individual figure panels. All calculations were performed using GraphPad prism statistical software version 9.
Characteristics of primary patient samples used in gene activity studies
| ID . | Alterations . | Final subtype . | RNA-seq sample type . | BCL2_SIG . | BCL2L1_SIG . | MCL1_SIG . | Navitoclax_AUC . | AZD4320_AUC . | AZD5991_AUC . | AZD3202_AUC . |
|---|---|---|---|---|---|---|---|---|---|---|
| SJALL068378_X1 | STIL::TAL1; TAL1 overexpression | TAL1 | PDX | 0.161123343 | 0.297878018 | 0.283187653 | 6.92 | 4.796 | 8.385 | 4.976 |
| SJALL068371_X1 | STIL::TAL1; TAL1 overexpression | TAL1 | PDX | 0.164212402 | 0.280235192 | 0.362725949 | 7.515 | 5.932 | 7.751 | 9.09 |
| SJALL068426_X1 | STIL::TAL1; TAL1 overexpression | TAL1 | PDX | 0.169629934 | 0.301991468 | 0.251066081 | 7.154 | 6.963 | 9.756 | 6.458 |
| SJALL068412_X1 | TLX3 overexpression | TLX3 | PDX | 0.177082138 | 0.281105117 | 0.326443938 | 10.86 | 8.089 | 10.435 | 7.343 |
| SJALL068439_X1 | LYL1::TRB | LYL1 | PDX | 0.187850451 | 0.31320111 | 0.339500387 | 9.259 | 6.645 | 9.815 | 8.367 |
| SJALL068383_X1 | TAL1 overexpression | TAL1 | PDX | 0.146731322 | 0.308581267 | 0.295253413 | 4.733 | 7.121 | 7.016 | 6.543 |
| SJALL068399_X1 | STIL::TAL1; TAL1 overexpression | TAL1 | PDX | 0.181634202 | 0.308624573 | 0.24844915 | 4.402 | 6.807 | 8.945 | 6.404 |
| SJALL068395_X1 | STIL::TAL1; TAL1 overexpression | TAL1 | PDX | 0.157522101 | 0.300441164 | 0.410820809 | 12.395 | 8.268 | 10.951 | 10.951 |
| SJALL068400_X1 | LYL1::TRB (but LYL1 expression is low) | LYL1 | PDX | 0.217354708 | 0.258394302 | 0.754426451 | 10.094 | 7.568 | ||
| SJALL068408_X1 | TLX3 overexpression | TLX3 | PDX | 0.216314445 | 0.281007657 | 0.361744227 | 7.705 | 7.16 | 9.137 | 8.241 |
| SJTALL055667_R2 | TLX3::14q32.2; TLX3 overexpression | TLX3 | Primary | 0.370879302 | 0.197211276 | 0.478839168 | 3.911 | 3.982 | 6.864 | 4.933 |
| SJTALL055667_R1 | TLX3::14q32.2; TLX3 overexpression | TLX3 | Primary | 0.367791552 | 0.194304436 | 0.559975966 | 2.126 | 3.67 | 6.883 | 7.173 |
| ID . | Alterations . | Final subtype . | RNA-seq sample type . | BCL2_SIG . | BCL2L1_SIG . | MCL1_SIG . | Navitoclax_AUC . | AZD4320_AUC . | AZD5991_AUC . | AZD3202_AUC . |
|---|---|---|---|---|---|---|---|---|---|---|
| SJALL068378_X1 | STIL::TAL1; TAL1 overexpression | TAL1 | PDX | 0.161123343 | 0.297878018 | 0.283187653 | 6.92 | 4.796 | 8.385 | 4.976 |
| SJALL068371_X1 | STIL::TAL1; TAL1 overexpression | TAL1 | PDX | 0.164212402 | 0.280235192 | 0.362725949 | 7.515 | 5.932 | 7.751 | 9.09 |
| SJALL068426_X1 | STIL::TAL1; TAL1 overexpression | TAL1 | PDX | 0.169629934 | 0.301991468 | 0.251066081 | 7.154 | 6.963 | 9.756 | 6.458 |
| SJALL068412_X1 | TLX3 overexpression | TLX3 | PDX | 0.177082138 | 0.281105117 | 0.326443938 | 10.86 | 8.089 | 10.435 | 7.343 |
| SJALL068439_X1 | LYL1::TRB | LYL1 | PDX | 0.187850451 | 0.31320111 | 0.339500387 | 9.259 | 6.645 | 9.815 | 8.367 |
| SJALL068383_X1 | TAL1 overexpression | TAL1 | PDX | 0.146731322 | 0.308581267 | 0.295253413 | 4.733 | 7.121 | 7.016 | 6.543 |
| SJALL068399_X1 | STIL::TAL1; TAL1 overexpression | TAL1 | PDX | 0.181634202 | 0.308624573 | 0.24844915 | 4.402 | 6.807 | 8.945 | 6.404 |
| SJALL068395_X1 | STIL::TAL1; TAL1 overexpression | TAL1 | PDX | 0.157522101 | 0.300441164 | 0.410820809 | 12.395 | 8.268 | 10.951 | 10.951 |
| SJALL068400_X1 | LYL1::TRB (but LYL1 expression is low) | LYL1 | PDX | 0.217354708 | 0.258394302 | 0.754426451 | 10.094 | 7.568 | ||
| SJALL068408_X1 | TLX3 overexpression | TLX3 | PDX | 0.216314445 | 0.281007657 | 0.361744227 | 7.705 | 7.16 | 9.137 | 8.241 |
| SJTALL055667_R2 | TLX3::14q32.2; TLX3 overexpression | TLX3 | Primary | 0.370879302 | 0.197211276 | 0.478839168 | 3.911 | 3.982 | 6.864 | 4.933 |
| SJTALL055667_R1 | TLX3::14q32.2; TLX3 overexpression | TLX3 | Primary | 0.367791552 | 0.194304436 | 0.559975966 | 2.126 | 3.67 | 6.883 | 7.173 |
Collectively, these data provide a comprehensive pharmacoinformatical and bioinformatical proof of universal BCL2/BCL-XL dependency in samples from patients with primary ALL and their sensitivity to AZD4320 and other BCL2/BCL-XL inhibitors in ALL.
Novel dual BCL2/BCL-XL inhibitor AZD0466 prolongs survival in PDX T-ALL mouse models
The in vivo efficacy of venetoclax, AZD0466, and AZD5991 targeting BCL2, BCL-XL, and MCL1 were examined in different cohorts of PDX T-ALL models (Figure 5).45 The peripheral blood leukemic burden, circulating platelet counts, and body weights were monitored weekly. Although AZD0466 at both dose levels (34 mg/kg and 103 mg/kg) slightly reduced circulating fraction of leukemic cells (Figure 5B), AZD0466 significantly eradicated leukemia cells in the bone marrow in the T-ALL CU76 model (Figure 5C). Venetoclax-, AZD0466-, and vehicle-treated groups did not reduce platelet counts (Figure 5D). No significant changes in body weight were seen in mice treated with AZD0466 (Figure 5E), indicating the safety of the compound at these doses.
The novel dual BCL2/BCL-XL inhibitor AZD0466 outperformed single BCL2 inhibition in T-ALL PDX models. (A) Schematic design of study. NSG mice were first sublethally irradiated (250 cGy) and inoculated with human PDX T-ALL cells (1 × 106 cells per 200 μL phosphate-buffered saline) 24 hours later via tail vein injection. The engraftment status of the human cells (leukemic burden) in the recipient mice was monitored weekly from the peripheral blood (PB) by flow cytometry. Once engraftment was established, mice (n = 10/group) were randomized to receive treatment with the BCL2/BCL-XL inhibitor AZD0466, the MCL1 inhibitor AZD5991, and the BCL2 inhibitor venetoclax, along with the respective vehicles at the indicated doses and treatment schedules. (B) Tumor burden development in 2 aggressive PDX T-ALL models measured as weekly detection of circulating human CD45+ cells in the PB and expressed as percent of normalized human to sum of human and murine CD45+ cells in mice undergoing treatment with vehicle, AZD0466, AZD5991, and venetoclax; mean ± standard deviation (SD), n = 10 mice per treatment arm. The statistical differences between 2 separate treatments were evaluated by 2-way analysis of variance, ∗P = .013; ∗∗P = .0064. (C) Leukemic burden in the bone marrow after 4 weeks of the treatment (number of mice analyzed, n = 3; mean ± SD indicated in the figure). P values were determined between 2 groups using an unpaired Student t test, ∗P = .013; ∗∗P = .0064; ∗∗∗P = .0009; ∗∗∗∗P < .0001. (D) Platelet counts upon selected treatment regimens. The statistical differences between 2 separate treatments were evaluated by an unpaired Student t test. (E) Body weight monitoring during treatment period. The statistical differences between 2 separate treatments were evaluated by an unpaired Student t test, ∗P = .015. (F) Kaplan-Meier survival curves of mice transplanted with T-ALL and the PDX models PDX1 and CU76 treated with anti-apoptotic inhibitors. Treatment was initiated once the level of circulating leukemia cells in the PB reached 0.5% to 1%; the timeframe of treatment is labeled by the yellow windows on the graphs. The differences in survival between the 2 groups were compared and determined by Gehan-Breslow-Wilcoxon log-rank test (n = 4 mice per group). ∗∗∗P = .001; ∗∗∗∗P < .0001. All calculations were performed using GraphPad prism statistical software version 9. ns, not significant; PBL, peripheral blood lymphocyte.
The novel dual BCL2/BCL-XL inhibitor AZD0466 outperformed single BCL2 inhibition in T-ALL PDX models. (A) Schematic design of study. NSG mice were first sublethally irradiated (250 cGy) and inoculated with human PDX T-ALL cells (1 × 106 cells per 200 μL phosphate-buffered saline) 24 hours later via tail vein injection. The engraftment status of the human cells (leukemic burden) in the recipient mice was monitored weekly from the peripheral blood (PB) by flow cytometry. Once engraftment was established, mice (n = 10/group) were randomized to receive treatment with the BCL2/BCL-XL inhibitor AZD0466, the MCL1 inhibitor AZD5991, and the BCL2 inhibitor venetoclax, along with the respective vehicles at the indicated doses and treatment schedules. (B) Tumor burden development in 2 aggressive PDX T-ALL models measured as weekly detection of circulating human CD45+ cells in the PB and expressed as percent of normalized human to sum of human and murine CD45+ cells in mice undergoing treatment with vehicle, AZD0466, AZD5991, and venetoclax; mean ± standard deviation (SD), n = 10 mice per treatment arm. The statistical differences between 2 separate treatments were evaluated by 2-way analysis of variance, ∗P = .013; ∗∗P = .0064. (C) Leukemic burden in the bone marrow after 4 weeks of the treatment (number of mice analyzed, n = 3; mean ± SD indicated in the figure). P values were determined between 2 groups using an unpaired Student t test, ∗P = .013; ∗∗P = .0064; ∗∗∗P = .0009; ∗∗∗∗P < .0001. (D) Platelet counts upon selected treatment regimens. The statistical differences between 2 separate treatments were evaluated by an unpaired Student t test. (E) Body weight monitoring during treatment period. The statistical differences between 2 separate treatments were evaluated by an unpaired Student t test, ∗P = .015. (F) Kaplan-Meier survival curves of mice transplanted with T-ALL and the PDX models PDX1 and CU76 treated with anti-apoptotic inhibitors. Treatment was initiated once the level of circulating leukemia cells in the PB reached 0.5% to 1%; the timeframe of treatment is labeled by the yellow windows on the graphs. The differences in survival between the 2 groups were compared and determined by Gehan-Breslow-Wilcoxon log-rank test (n = 4 mice per group). ∗∗∗P = .001; ∗∗∗∗P < .0001. All calculations were performed using GraphPad prism statistical software version 9. ns, not significant; PBL, peripheral blood lymphocyte.
In both the PDX1 and CU76 T-ALL PDX models, AZD0466 outperformed single BCL2 inhibition with venetoclax or MCL1 inhibition with AZD5991 and led to significant survival extension at higher doses (P < .0001 and P < .001, respectively; Figure 5F). These findings indicate that AZD0466 demonstrates antileukemia potential in aggressive PDX T-ALL models, without causing significant toxicities.
Dual BCL2/BCL-XL inhibitor AZD0466 combined with chemotherapy prolongs survival in T-ALL PDX mouse model
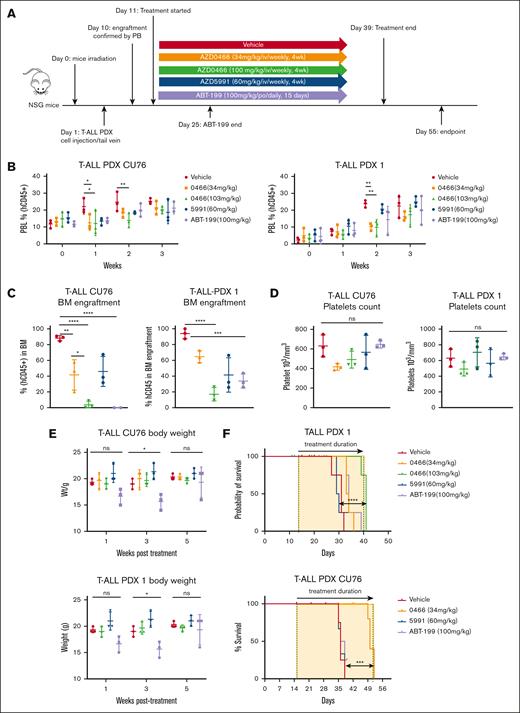
Next, we determined the combinatorial efficacy of AZD0466 with low-intensity chemotherapy VXL in the aggressive PDX T-ALL model D115 (Figure 6A).45 The combination of AZD0466 (34 mg/kg) with VXL caused a significant (P < .0001) reduction of leukemic burden in the bone marrow compared with AZD0466 or VXL alone (Figure 6B). A moderate reduction in platelet count was observed in mice treated with the VXL-based regimen (Figure 6C). After 5 weeks of treatment, stable weight loss without early death was observed only in cohorts treated with the VXL-based regimen but not with AZD0466 (Figure 6D). Furthermore, a low dose of AZD0466 alone and in combination with tolerable VXL doses led to a significant antileukemia effect and survival extension in the PDX T-ALL model (P < .0001; Figure 6E).
The novel dual BCL2/BCL-XL inhibitor AZD0466 outperformed single BCL2 inhibition in a PDX T-ALL model. (A) Schematic study design. Recipient mice were irradiated at 250 cGy 24 hours before injection of cells. All mice were injected with 1 × 106 cells via the tail vein. Mice with established baseline levels of leukemic burden with 0.5% to 5% of circulating leukemia cells in the peripheral blood were randomized into 6 groups. Treatment was started with vehicle, AZD0466 inhibitor (34 mg/kg; administered IV weekly for 4 weeks), low-dose VXL therapy (intraperitoneally once weekly for 4 weeks; dexamethasone [5 mg/kg], vincristine [0.15 mg/kg]; and L-asparagine [1000 IU/kg, ∼25 IU per mouse]) were used. (B) Bone marrow engraftment in mice inoculated with the PDX T-ALL model D115 evaluated 4 weeks after treatment initiation. Mean ± SD indicated in the figure (number of mice analyzed n = 3 per treatment arm). The statistical differences between 2 separate treatments were evaluated by an unpaired Student t test, ∗∗∗∗P < .0001. (C) Reduction of platelets upon AZD0466 shows no statistically significant reduction as monotherapy or combination therapy with VXL. The statistical differences between 2 separate treatments were evaluated by an unpaired Student t test, ∗P = 0.02. (D) Body weight monitoring over time. Average body weight of PDX T-ALL D115 mice were derived from the experiments illustrated in above panel A. Mice were weighed as individuals 24 hours before weekly treatments. The statistical differences between 2 separate treatments were evaluated by an unpaired Student t test; week 3: vehicle vs combo, ∗∗P = 0.002, vehicle vs VXL, ns = 0.07, 0466 vs combo, ∗∗∗P = 0.0006; week 5: vehicle vs combo, ∗∗∗P = 0.0003, vehicle vs VXL, ∗∗∗P = 0.0004, 0466 vs combo, ∗∗P = 0.005. (E) Kaplan-Meier survival curves of mice bearing T PDX -ALL model D115, treated with vehicle, AZD0466, VXL, or combination. Percentages of survival of mice from study cohorts in panel A are shown. The differences in survival between the 2 groups were compared by Gehan-Breslow-Wilcoxon test, (n = 3 mice per group): ∗P = .01, ∗∗P = .0062, ∗∗∗∗P < .0001. All calculations were performed using GraphPad prism statistical software version 9. BM, bone marrow; ns, not significant.
The novel dual BCL2/BCL-XL inhibitor AZD0466 outperformed single BCL2 inhibition in a PDX T-ALL model. (A) Schematic study design. Recipient mice were irradiated at 250 cGy 24 hours before injection of cells. All mice were injected with 1 × 106 cells via the tail vein. Mice with established baseline levels of leukemic burden with 0.5% to 5% of circulating leukemia cells in the peripheral blood were randomized into 6 groups. Treatment was started with vehicle, AZD0466 inhibitor (34 mg/kg; administered IV weekly for 4 weeks), low-dose VXL therapy (intraperitoneally once weekly for 4 weeks; dexamethasone [5 mg/kg], vincristine [0.15 mg/kg]; and L-asparagine [1000 IU/kg, ∼25 IU per mouse]) were used. (B) Bone marrow engraftment in mice inoculated with the PDX T-ALL model D115 evaluated 4 weeks after treatment initiation. Mean ± SD indicated in the figure (number of mice analyzed n = 3 per treatment arm). The statistical differences between 2 separate treatments were evaluated by an unpaired Student t test, ∗∗∗∗P < .0001. (C) Reduction of platelets upon AZD0466 shows no statistically significant reduction as monotherapy or combination therapy with VXL. The statistical differences between 2 separate treatments were evaluated by an unpaired Student t test, ∗P = 0.02. (D) Body weight monitoring over time. Average body weight of PDX T-ALL D115 mice were derived from the experiments illustrated in above panel A. Mice were weighed as individuals 24 hours before weekly treatments. The statistical differences between 2 separate treatments were evaluated by an unpaired Student t test; week 3: vehicle vs combo, ∗∗P = 0.002, vehicle vs VXL, ns = 0.07, 0466 vs combo, ∗∗∗P = 0.0006; week 5: vehicle vs combo, ∗∗∗P = 0.0003, vehicle vs VXL, ∗∗∗P = 0.0004, 0466 vs combo, ∗∗P = 0.005. (E) Kaplan-Meier survival curves of mice bearing T PDX -ALL model D115, treated with vehicle, AZD0466, VXL, or combination. Percentages of survival of mice from study cohorts in panel A are shown. The differences in survival between the 2 groups were compared by Gehan-Breslow-Wilcoxon test, (n = 3 mice per group): ∗P = .01, ∗∗P = .0062, ∗∗∗∗P < .0001. All calculations were performed using GraphPad prism statistical software version 9. BM, bone marrow; ns, not significant.
Furthermore, given the high expression of BCL2 in Ph-like ALL, we examined the efficacy of BCL2/BCL-XL inhibition in Ph-like PDX B-ALL models (supplemental Figure 5A). Weekly leukemic burden levels indicated the reduction of circulating leukemia cells after 2 weeks of treatment in mice treated with higher doses of AZD0466 and after 3 weeks of treatment across all treatment regimens (supplemental Figure 5B). Although neither thrombocytopenia (supplemental Figure 5C) nor significant weight loss was observed upon AZD0466 treatment (supplemental Figure 5D), AZD0466 failed to extend survival. Only slightly prolonged survival was observed in the venetoclax arm compared with the vehicle arm (supplemental Figure 5E).
Discussion
Our study aimed to characterize the prosurvival dependencies on BCL2 family proteins in adult T-ALL and B-ALL cells and to evaluate the efficacy of the selective BCL2 inhibitor venetoclax (ABT199) and of the dual BCL2/BCL-XL inhibitor AZD4320. Our findings indicate dual BCL2/BCL-XL dependency in mature T-ALL cells and suggest cotargeting BCL2/BCL-XL as an effective way to control T-ALL disease burden in vitro and in vivo. We also found that the dual BCL2/BCL-XL inhibitor AZD0466 has additive efficacy with low-intensity standard chemotherapy VXL.
T-ALL is a rare, aggressive hematologic malignancy, commonly driven by NOTCH gene mutations and with limited targeted options, especially at the time of relapse. Among T-ALL subtypes, ETP-ALL is particularly associated with dismal therapy response and a high risk of relapse, for which durable treatment response is seldom achieved.48-50
Venetoclax is an established BCL2 inhibitor with apoptosis-inducing properties in a wide range of hematologic malignancies. However, the diversity of responses in T-ALL samples treated with venetoclax required further investigation. Although more differentiated T-ALL cases seem to be dependent on BCL-XL,19 BCL2 dependence was reported previously in ETP T-ALL using BH3 profiling.20
In our comprehensive analysis of BCL2 family protein expression, BH3 profiling and apoptotic response to selective or dual-specificity BH3 mimetics in vitro, gene expression and gene activity analyses indicated a consistent trend of decreased BCL2 activity consistent with the maturation process of T-ALL cells. Furthermore, in line with recently reported dependency in different subsets of T-ALL using BH3 profiling,20 our data show that ETP T-ALLs have stronger dependence on BCL2 among all analyzed T-ALL subtypes. Similar to earlier reports,10,20,51,52 we confirmed that BCL-XL activity peaks in more mature T-ALL subsets.
The analysis of protein expression patterns in ALL cell lines revealed increased levels of BCL2 in B-ALL and of BCL-XL in Ph-like B-ALL and T-ALL cell lines. BH3 profiling is an important tool to determine prior BH3 domain dependencies20 and thus predict responses to BH3-mimetic antitumor inhibitors. In our BH3 profiling analysis of ALL cell lines, we demonstrated BCL2 and BCL-XL codependency in Ph-like B-ALL and T-ALL cell lines with major BCL2 dependency in B-ALL cell lines. In contrast, T-ALL cell lines were most sensitive to AZD4320, with BCL-XL–selective inhibitor A-1331852 inducing higher apoptotic rates than ABT199. These results in conjunction with BH3 profiling support the utility of dual coinhibition in T-ALL.
As reported here, BCL2/BCL-XL is highly expressed in T-ALL cell lines and primary samples, supporting the codependency of BCL2 and BCL-XL as an anti-apoptotic driver; clinically, however, cotargeting BCL2 and BCL-XL with navitoclax was associated with thrombocytopenia,53 warranting safer treatments with a similar target profile. Targeting BCL2/BCL-XL with AZD4320 has been shown to drive cell death through the mitochondrial apoptotic pathway in BCL2/BCL-XL–dependent leukemic cell lines.18 Preclinical activity of AZD0466 has been demonstrated in several hematological and solid tumor models, enabling the progression of AZD0466 into clinical development.30 Thus, we used in vivo administration of AZD0466, in which AZD4320 is packaged within nanosize dendrimers capable of slowing drug release, increasing tumor-specific delivery and mitigating thrombocytopenia risk.18,30 Our preclinical studies in PDX T-ALL models showed a moderate response to venetoclax but a significantly prolonged survival, and decreased leukemic burden with AZD0466, with no significant weight loss or thrombocytopenia. Clinical data are required to understand the potential of AZD0466 or platelet-sparing BCL-XL PROTAC such as DT221654 to affect platelet aggregation reported as on-target toxicity in preclinical studies with navitoclax.55
In B- and T-ALL, the JAK-STAT signaling pathway is positively correlated with increased BCL2 family function.56 Importantly, this pathway plays an important role in the switch between BCL2 and BCL-XL dependencies in normal developing T cells. However, recently the coexpression of BCL2 and BCL-XL was linked with higher therapeutic resistance in different types of ALL.10,57,58 To facilitate clinical translation, the combination of AZD0466 with other therapy regimens requires further evaluation. We demonstrated that low-dose AZD0466 therapy increased the efficacy of the standard-of-care chemotherapy agents commonly used in ALL treatment. Further exploratory studies using AZD0466 in different T-ALL subtypes, with either low-intensity chemotherapy, targeted inhibitors of other oncogenic molecular lesions, or immune therapies, might lead to potential therapeutic advancement in T-ALL. Considering the results of the gene expression profile analysis, ETP-ALL might especially benefit from dual BCL2/BCL-XL combination. Our findings suggest that AZD0466 outperforms single BCL2 and MCL1 inhibition in T-ALL. Thus, future in vivo studies focusing on efficacy of AZD0466 alone or in combination with chemotherapeutics or selected small molecule inhibitors are warranted.
A comparison of pharmacological response to AZD4320 in vitro or AZD0466 treatments in vivo in PDX Ph-like ALL models demonstrated lower therapeutic efficacy than that observed in T-ALL PDX. In fact, in Ph-like ALL models venetoclax was more efficacious than AZD0466. Although the BH3-mimetic combination was demonstrated to be a potent killer in primary Ph-like B-ALL cases in vitro,43,59 similar studies in a PDX Ph-like ALL model43,59 showed no major antileukemic effects by navitoclax. Given the known upregulation of MCL1 upon BCL2 blockade, studies on cotargeting MCL1 together with BCL2/BCL-XL using AZD0466, and studies aimed at understanding resistance mechanisms are warranted in this poor-risk B-ALL subset.
In our large genomic analysis of BCL2 and BCL-XL gene activity using NetBID algorithm, we have identified for the first time, to our knowledge, several genomic subsets within B- or T-ALL characterized by low or high functional activity of these genes. Although informative, our drug response data were generated in a relatively small cohort of T-ALL, limiting our ability to dissect specific genomic subsets. Future larger analyses using BH3 profiling and BH3 mimetic responses are needed to characterize responses and selective vulnerabilities in both, B-ALL, or T-ALL. Yet, our data convincingly indicate codependency on BCL2 and BCL-XL in the T-ALL subset, providing strong preclinical rationale for the translation of AZD0466 and other dual BCL2/BCL-XL inhibitors into ALL clinical trials, alone or in combination with standard chemotherapy.
Acknowledgments
The authors thank the patients and parents who participated in the MD Anderson Cancer Center and St. Jude and Children's Oncology Group (COG) clinical trials included in this study, and the clinicians and research staff at MD Anderson Cancer Center and St. Jude Children’s Research Hospital and COG institutions. The authors thank Patrick Zweidler McKay from the Pediatricsrics Research department at the University of Texas MD Anderson Cancer Center for providing D115 T-ALL PDX cells, Adolfo Ferrando (Columbia University Medical Center) for providing PDX CU76, and Marcus Muschen (University of California San Francisco) for providing us with PDX B-ALL models including ICN1 and LAX. Mesenchymal stem cells were gifted by Dario Campana from the National University of Singapore. The authors thank Julia E. Wells, a postdoctoral fellow from the Leukemia Department, the University of Texas MD Anderson Cancer Center, for her contributions to in vitro studies. The authors thank Ashli Nguyen-Villarreal and Bryan Tutt of the Research Medical Library, The University of Texas MD Anderson Cancer Center, for editing this manuscript.
The studies were supported by a grant from AstraZeneca (S.K., S.G., Q.Z., and M.K.). This work was supported, in part, by grants from the Cancer Prevention and Research Institute of Texas (CPRIT) and the National Cancer Institute (NCI) at National Institutes of Health (NIH) R01CA231364, Leukemia SPORE P50 CA100632, and CPRIT RP150006 (M.K.). This work was supported by NIH grants CA21765, CA98543, CA114766, CA98413, CA180886, and CA180899 (J.Y.) and a Laboratory Incentive Fund from the Division of Pediatrics, MD Anderson Cancer Center (FY2019 Laboratory Incentive Program; S.K.). This research was performed in the Flow Cytometry & Cellular Imaging Core Facility, which is supported, in part, by the NIH through MD Anderson Cancer Center support grant CA016672.
Authorship
Contribution: S.K., M.K., and J.J.Y. were responsible for conceptualization, methodology, investigation; S.K., M.K., and J.J.Y. provided resources; S.K., M.K., J.J.Y., Y.L., X.Y., S.Y., and N.B. were responsible for formal analysis; S.K., N.B., S.G., S.B., C.L.A., J.C., N.J., Y.L., X.Y., Z.L., Y.H., J.Y., and Q.Z. performed research design and were responsible for interpretation of data curation; S.K. and M.K. prepared the original manuscript draft; S.K., N.B., J.Y., and M.K. reviewed and edited the manuscript; S.K., J.J.Y., and M.K. supervised the study; S.K., J.J.Y., and M.K. were responsible for project administration; and all authors have read and agreed to the published version of the manuscript.
Conflict-of-interest disclosure: S.B., C.L.A., and J.C. are current or former AstraZeneca employees and shareholders. The remaining authors declare no competing financial interests.
Correspondence: Marina Konopleva, Albert Einstein College of Medicine and Montefiore Medical Center, 1300 Morris Park Ave, Bronx, NY 10461; email: marina.konopleva@einsteinmed.edu; and Jun J. Yang, St. Jude Children's Research Hospital, 262 Danny Thomas Pl, Memphis, TN 38105-3678; email: jun.yang@stjude.org.
References
Author notes
S.K., Y.L., and N.B. are joint first authors.
Data are available through public repositories (eg, European Genome-Phenome Archive) with the permission of St. Jude Children’s Research Hospital.
The full-text version of this article contains a data supplement.

![BCL2/BCL-XL codependence in ALL cell lines in vitro. (A) Protein expression by reverse phase protein array (RPPA) for anti-apoptotic BCL2 and BCL-XL (BCL2/BCL-XL) in 15 B-ALL and 13 T-ALL cell lines. The violin plots show the median expression, maximum (Q3 + 1.5 × interquartile range [IQR]), and minimum value (Q1 + 1.5 × IQR). Data were analyzed with 2-sided t test. (B) The protein expression levels of anti-apoptotic family members (BCL2, BCL-XL, and MCL1) and prodeath activators (BIM and BAX) determined by Western blot analysis. Whole-cell lysates from a panel of B- and T-ALL cell lines were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and probed for the indicated antibodies. The cropped images (boxed) of cell lines reflecting specific ALL subsets generated from the single immunoblot across probed proteins. (C-D) BH3 profiling functional assay to measure apoptotic priming. Dependencies were measured in a panel of B- and T-ALL cell lines. All calculations were performed using GraphPad prism statistical software version 9.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/3/10.1182_bloodadvances.2024013423/5/m_blooda_adv-2024-013423-gr2.jpeg?Expires=1767771464&Signature=VbVMybvBH9QvCxLgu7B5otK1ihQ7Pp4j3E7gLcRALS-oXOn3dqpG8bndaCVE9I8A5Y75TITL2aY4oP1JnpDhvTzJT2lnW2cULJbpPQWIEycScVXv0Fl9E86u7Ng4M2D7iX3oLadPEI6lQ20NE6KpE~z4rqdLh~eUNaIQjfdRvfteRu12p0STPpuUnV8TXpjM1weT-dA5wZTT6cZ28MD065vuB~urg-JUPfumXt2HK4S2RMnmdqYAW4g6bnaeVexgvJ~9zGJd8Holto13bO-7wqNro7zcU8~DpWyHul6~rAtHIUsNWDf9PH39Xb3tSzz-YtKz~fjqfPUb9vWty~bHtg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![The novel dual BCL2/BCL-XL inhibitor AZD0466 outperformed single BCL2 inhibition in a PDX T-ALL model. (A) Schematic study design. Recipient mice were irradiated at 250 cGy 24 hours before injection of cells. All mice were injected with 1 × 106 cells via the tail vein. Mice with established baseline levels of leukemic burden with 0.5% to 5% of circulating leukemia cells in the peripheral blood were randomized into 6 groups. Treatment was started with vehicle, AZD0466 inhibitor (34 mg/kg; administered IV weekly for 4 weeks), low-dose VXL therapy (intraperitoneally once weekly for 4 weeks; dexamethasone [5 mg/kg], vincristine [0.15 mg/kg]; and L-asparagine [1000 IU/kg, ∼25 IU per mouse]) were used. (B) Bone marrow engraftment in mice inoculated with the PDX T-ALL model D115 evaluated 4 weeks after treatment initiation. Mean ± SD indicated in the figure (number of mice analyzed n = 3 per treatment arm). The statistical differences between 2 separate treatments were evaluated by an unpaired Student t test, ∗∗∗∗P < .0001. (C) Reduction of platelets upon AZD0466 shows no statistically significant reduction as monotherapy or combination therapy with VXL. The statistical differences between 2 separate treatments were evaluated by an unpaired Student t test, ∗P = 0.02. (D) Body weight monitoring over time. Average body weight of PDX T-ALL D115 mice were derived from the experiments illustrated in above panel A. Mice were weighed as individuals 24 hours before weekly treatments. The statistical differences between 2 separate treatments were evaluated by an unpaired Student t test; week 3: vehicle vs combo, ∗∗P = 0.002, vehicle vs VXL, ns = 0.07, 0466 vs combo, ∗∗∗P = 0.0006; week 5: vehicle vs combo, ∗∗∗P = 0.0003, vehicle vs VXL, ∗∗∗P = 0.0004, 0466 vs combo, ∗∗P = 0.005. (E) Kaplan-Meier survival curves of mice bearing T PDX -ALL model D115, treated with vehicle, AZD0466, VXL, or combination. Percentages of survival of mice from study cohorts in panel A are shown. The differences in survival between the 2 groups were compared by Gehan-Breslow-Wilcoxon test, (n = 3 mice per group): ∗P = .01, ∗∗P = .0062, ∗∗∗∗P < .0001. All calculations were performed using GraphPad prism statistical software version 9. BM, bone marrow; ns, not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/3/10.1182_bloodadvances.2024013423/5/m_blooda_adv-2024-013423-gr6.jpeg?Expires=1767771464&Signature=KmCpAWhbs3cHSeLqw0PPhcuuOOjkSnSUdcCdnkjy4hMvyOwn2~bJ60DJLPD4ZMBK5F3ilmuxQPWK0r8pgqgkwEsdSl~8BncFBkDNpcQtT1wW1p7ClsAQIyXyUqM~kXDFzcv87ozg9Guj3cU-P0OkZM8OKMiq7Ebv~5~g5WAZvOKPfvFgC2xEkChj3sAMkU2npXpFtfazLiaM28WCPcsGsgN14--~l46Uyy06emcjcJwk0E2~9xfro985YaMDlt1t0qLqPR5yC4DLyU5rwm1E2n72Q~PfH8TOlyTY1sqv0VWirDKrqY9Mf9co9fa2kiYTqlB~nWlcq-4J-rv9iHXBeA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)