Key Points
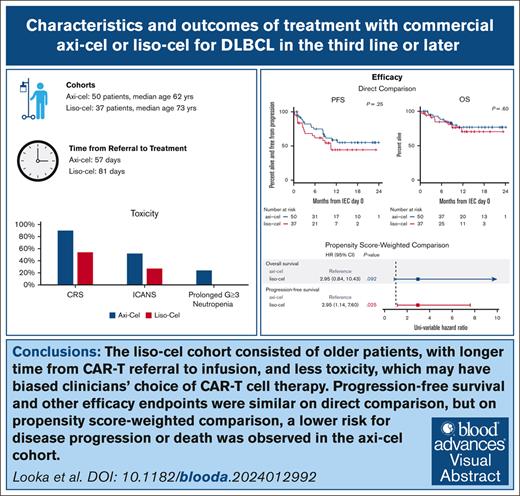
Axi-cel and liso-cel had similar outcomes, but when accounting for differences in risk factors, axi-cel was associated with superior PFS.
We observed longer time from apheresis to treatment with liso-cel and more frequent CRS, ICANS, and prolonged neutropenia with axi-cel.
Visual Abstract
Lisocabtagene maraleucel (liso-cel) and axicabtagene ciloleucel (axi-cel) are anti-CD19 chimeric antigen receptor (CAR) T-cell therapies approved for relapsed and refractory large B-cell lymphoma (LBCL); however, there is currently no published data on liso-cel outside of clinical trials nor any data comparing these therapies. In this retrospective analysis, we reviewed patients with LBCL receiving liso-cel or axi-cel at a single institution in the third-line setting. From June 2021 to September 2022, a total of 50 patients received axi-cel and 37 liso-cel. Baseline patient characteristics were similar, aside from older age in liso-cel recipients. The median time from leukapheresis to CAR T-cell infusion was significantly longer for liso-cel (41 days) than axi-cel (30 days). Complete response rates were not significantly different between axi-cel (72%) and liso-cel (62%). At a median follow-up of 11 months, progression-free survival (PFS) was not significantly different between axi-cel and liso-cel cohorts, with 12-month PFS of 59% and 44%, respectively. However, on a propensity score analysis, an inferior PFS was observed with liso-cel (hazard ratio, 2.95; 95% confidence interval , 1.14-7.60). The rates of cytokine release syndrome, immune effector cell-associated neurotoxicity syndrome, and prolonged neutropenia were higher with axi-cel than liso-cel. Overall, direct comparison of axi-cel and liso-cel cohorts shows similar key outcomes including response rate and PFS, but prolonged wait times for liso-cel may have resulted in biased selection of patients with more favorable characteristics for liso-cel. When accounting for these higher-risk characteristics, an inferior PFS is observed with liso-cel compared with axi-cel. These findings warrant further evaluation in a multicenter setting.
Introduction
Anti-CD19 chimeric antigen receptor (CAR) T-cell therapy has revolutionized the treatment and outcomes for relapsed and refractory (R/R) large B-cell lymphoma (LBCL). Originally approved by the US Food and Drug Administration for use in the third-line setting, 2 CAR T-cell products were recently approved for use in the second line for primary refractory or early relapsing LBCL, based on results of the randomized ZUMA-7 and TRANSFORM studies: axicabtagene ciloleucel (axi-cel; Yescarta) and lisocabtagene maraleucel (liso-cel; Breyanzi).1-4 In the third-line or later setting, complete response rates (CRRs) to axi-cel and liso-cel were 58% and 53%, and the median progression-free survival (PFS) was 5.8 and 6.8 months, respectively.1,2 The rates of high-grade cytokine release syndrome (CRS) were 13% for axi-cel and 2% for liso-cel, and the rates of high-grade immune effector cell-associated neurotoxicity syndrome (ICANS) were 28% vs 10%, respectively.1,2
Comparison of these products is limited by the lack of randomized data and limitations of crosstrial comparisons, particularly with differences in trial eligibility, manufacturing times, and frequency of in-specification products. Furthermore, no published data exist to date for liso-cel in the commercial, nontrial setting.
After the US Food and Drug Administration approval of liso-cel for LBCL in the third line, to build institutional experience and help guide product choice, all CAR T-cell–eligible patients with R/R LBCL treated at Dana-Farber Cancer Institute (DFCI) were given liso-cel by default from June 2021 to May 2022. Axi-cel was used if an apheresis slot could not be obtained for liso-cel in a reasonable time. Before and after this 1-year period, axi-cel was the default choice for these patients. We subsequently performed a retrospective analysis of outcomes using axi-cel and liso-cel products during this time period.
Methods
Patients and treatment
We included all patients with R/R LBCL treated at DFCI between June 2021 and September 2022, who received commercial axi-cel or liso-cel as a third-line or later therapy per DFCI institutional guidelines. A total of 87 patients received CAR T-cell therapy, including 50 patients with axi-cel and 37 patients with liso-cel. Nonconforming liso-cel products were given to 10 patients through an expanded access program; permission was granted by Bristol Myers Squibb to include these patients in the study. All patients received CAR T-cell therapy in the hospital. Postinfusion observation and toxicity management were performed per DFCI institutional guidelines. No patients received prophylactic steroids or anakinra.
From June 2021 to May 2022, liso-cel was ordered by default, and from June 2022 to September 2022, axi-cel was ordered by default. If a slot for T-cell apheresis was not available within an acceptable time frame per clinician judgment and a time arranged for T-cell apheresis for the alternative product earlier, the alternative product was chosen.
This study was approved by the DFCI's Institutional Review Board. This retrospective study was determined to pose no more than minimal risk. Individual patient consent was not required.
Assessments and end points
Baseline patient characteristics, including basic demographics, disease characteristics, and prior therapy, were collected. To estimate tumor burden before treatment initiation, the sum of the products of diameters (SPD) was measured for each patient using the last positon emission tomography/computed tomography (PET/CT) scan before CAR T-cell treatment. The SPD was determined using the International Working Group criteria.5
The primary outcome of interest was PFS, defined as the time from infusion to progression of disease or death from any cause. Additional efficacy end points of interest included the best objective response rate (ORR), best CRR, overall survival (OS), and duration of response (DOR). OS was defined as the time from infusion to death from any cause. DOR was defined as the time from first response, defined as either complete response (CR) or partial response (PR), to progression of disease. Response assessments were performed at 1 month after infusion and throughout follow-up using Lugano criteria, as determined by treating clinicians.6 Primary safety end points included assessments of CRS and ICANS. CRS was graded according to the modified Lee criteria and the American Society for Transplantation and Cellular Therapy (ASTCT) consensus grading for CRS.7,8 ICANS was graded according to the Common Terminology Criteria for Adverse Events version 5 and the ASTCT ICANS consensus grading for adults.8 Time-to-treatment metrics were also recorded. Date of intention to treat (ITT) was defined as the time of CAR T-cell service notification, and the time from ITT to T-cell leukapheresis, time from leukapheresis to CAR T-cell infusion, and the overall time from ITT to infusion were evaluated. Additional end points of interest included length of hospital stay, intensive care unit (ICU) admission rates, readmission within 30 days of infusion, cardiac events, cytopenias, and infections after infusion.
Statistical analysis
Survival analyses for estimated PFS, OS, and DOR were performed using Kaplan-Meier estimates, and log-rank tests were used to assess differences in survival metrics between cohorts. Continuous variable differences with product use were assessed with Wilcoxon rank-sum tests. Categorical variables were assessed with Fisher exact tests. Cox proportional hazards models were used to assess covariates for time-to-event analyses, and binary outcomes were assessed with logistic regressions. Statistical significance was set at P value <.05 and binomial 95% confidence intervals (CIs) were used. All analyses were performed using R version 4.2.0 with packages survival v3.7.0 and MatchIt v4.5.5.
To account for differences in selection between axi-cel and liso-cel cohorts, propensity score matching was performed, and weights were used in multivariable models. Variables used in the propensity score weighting were age, International Prognostic Index before lymphodepletion, whether bridging therapy was performed, number of prior lines of therapy, lactate dehydrogenase (LDH) before lymphodepletion, Eastern Cooperative Oncology Group performance status, the SPD, day 0 C-reactive protein (CRP), and day 0 interleukin-6 (IL-6).
Results
Patients
Table 1 displays baseline patient characteristics for each product cohort. Of the 87 patients included in this study, 50 received axi-cel, and 37 received liso-cel, all in the third line or later. During the default liso-cel period (June 2021 to May 2022), 30 patients received liso-cel, and 36 received axi-cel; and for the period in which axi-cel was chosen by default, 14 received axi-cel, and 7 liso-cel (supplemental Figure 1). Of the 37 liso-cel recipients, 10 received nonconforming products on an expanded access protocol. All axi-cel patients received in-specification products.
Patient characteristics
| Characteristic . | Total (N = 87) . | Axi-cel (n = 50; 57%) . | Liso-cel (n = 37; 43%) . | P value∗ . |
|---|---|---|---|---|
| Age, median (range), y | 66 (35-84) | 62 (35-82) | 73 (55-84) | <.001 |
| ≤65 | 43 (49) | 35 (70) | 8 (22) | <.001 |
| >65 | 44 (51) | 15 (30) | 29 (78) | |
| Female sex | 33 (38) | 20 (40) | 13 (35) | .66 |
| Pathologic diagnosis | ||||
| De novo DLBCL | 52 (60) | 27 (54) | 25 (68) | .44 |
| tFL | 18 (21) | 13 (26) | 5 (14) | |
| HGBL | 8 (9) | 4 (8) | 4 (11) | |
| tMZL | 3 (3) | 1 (2) | 2 (5) | |
| tCLL | 2 (2) | 2 (4) | - | |
| Other† | 4 (5) | 3 (6) | 1 (3) | |
| DHL | 12 (14) | 7 (14) | 5 (14) | >.99 |
| THL | 5 (6) | 3 (6) | 2 (5) | >.99 |
| Stage (at the time of diagnosis) | ||||
| I | 2 (2) | 1 (2) | 1 (3) | .92 |
| II | 5 (6) | 3 (6) | 2 (5) | |
| III | 14 (16) | 7 (14) | 7 (19) | |
| IV | 62 (71) | 37 (74) | 25 (68) | |
| ECOG PS (before apheresis) | ||||
| 0 | 16 (18) | 10 (20) | 6 (16) | .96 |
| 1 | 43 (49) | 24 (48) | 19 (51) | |
| 2 | 25 (29) | 14 (28) | 11 (30) | |
| 3 | 2 (2) | 1 (2) | 1 (3) | |
| IPI (before lymphodepletion) | ||||
| 0-2 | 15 (17) | 10 (20) | 5 (14) | .57 |
| 3-5 | 68 (78) | 37 (74) | 31 (84) | |
| Preinfusion lines of therapy, median (range) | 2 (2 - 3) | 2 (2 - 3) | 2 (2 - 3) | >.99 |
| 2 | 52 (60) | 30 (60) | 22 (59) | >.99 |
| 3 | 35 (40) | 20 (40) | 15 (41) | |
| Prior autologous transplant | 17 (20) | 12 (24) | 5 (14) | .28 |
| Prior allogeneic transplant | 2 (2) | 1 (2) | 1 (3) | >.99 |
| Bridging therapy | 53 (61) | 27 (54) | 26 (70) | .18 |
| Chemotherapy | 31 (36) | 13 (26) | 18 (49) | .041 |
| Radiation | 14 (16) | 7 (14) | 7 (19) | .57 |
| Steroid | 15 (17) | 9 (18) | 6 (16) | >.99 |
| Tumor bulk, median (range), cm | 3.7 (1.0-44.0) | 3.6 (1.0-44.0) | 4.5 (1.0-17.5) | .53 |
| ≤5 | 40 (46) | 25 (50) | 15 (41) | .59 |
| >5-10 | 15 (17) | 8 (16) | 7 (19) | |
| >10 | 7 (8) | 3 (6) | 4 (11) | |
| LDH before lymphodepletion, median (range), U/L | 226 (138-1603) | 218 (138-1603) | 235 (139-919) | .63 |
| SPD, median (range) | 20.0 (0-321) | 15.0 (0-272) | 23.1 (1.4-321) | .39 |
| SUVmax, median (range) | 16.2 (3.5-51.6) | 17.6 (3.5-40.9) | 13.7 (6.0-51.6) | .98 |
| Comorbidities | ||||
| BMI >30 | 23 (26) | 14 (28) | 9 (24) | .81 |
| Diabetes | 10 (11) | 3 (6) | 7 (19) | .09 |
| Statin use | 26 (30) | 12 (24) | 14 (38) | .24 |
| LVEF <50% | 5 (6) | 3 (6) | 2 (5) | >.99 |
| GFR <50 mL/min | 8 (9) | 3 (6) | 5 (14) | .28 |
| Characteristic . | Total (N = 87) . | Axi-cel (n = 50; 57%) . | Liso-cel (n = 37; 43%) . | P value∗ . |
|---|---|---|---|---|
| Age, median (range), y | 66 (35-84) | 62 (35-82) | 73 (55-84) | <.001 |
| ≤65 | 43 (49) | 35 (70) | 8 (22) | <.001 |
| >65 | 44 (51) | 15 (30) | 29 (78) | |
| Female sex | 33 (38) | 20 (40) | 13 (35) | .66 |
| Pathologic diagnosis | ||||
| De novo DLBCL | 52 (60) | 27 (54) | 25 (68) | .44 |
| tFL | 18 (21) | 13 (26) | 5 (14) | |
| HGBL | 8 (9) | 4 (8) | 4 (11) | |
| tMZL | 3 (3) | 1 (2) | 2 (5) | |
| tCLL | 2 (2) | 2 (4) | - | |
| Other† | 4 (5) | 3 (6) | 1 (3) | |
| DHL | 12 (14) | 7 (14) | 5 (14) | >.99 |
| THL | 5 (6) | 3 (6) | 2 (5) | >.99 |
| Stage (at the time of diagnosis) | ||||
| I | 2 (2) | 1 (2) | 1 (3) | .92 |
| II | 5 (6) | 3 (6) | 2 (5) | |
| III | 14 (16) | 7 (14) | 7 (19) | |
| IV | 62 (71) | 37 (74) | 25 (68) | |
| ECOG PS (before apheresis) | ||||
| 0 | 16 (18) | 10 (20) | 6 (16) | .96 |
| 1 | 43 (49) | 24 (48) | 19 (51) | |
| 2 | 25 (29) | 14 (28) | 11 (30) | |
| 3 | 2 (2) | 1 (2) | 1 (3) | |
| IPI (before lymphodepletion) | ||||
| 0-2 | 15 (17) | 10 (20) | 5 (14) | .57 |
| 3-5 | 68 (78) | 37 (74) | 31 (84) | |
| Preinfusion lines of therapy, median (range) | 2 (2 - 3) | 2 (2 - 3) | 2 (2 - 3) | >.99 |
| 2 | 52 (60) | 30 (60) | 22 (59) | >.99 |
| 3 | 35 (40) | 20 (40) | 15 (41) | |
| Prior autologous transplant | 17 (20) | 12 (24) | 5 (14) | .28 |
| Prior allogeneic transplant | 2 (2) | 1 (2) | 1 (3) | >.99 |
| Bridging therapy | 53 (61) | 27 (54) | 26 (70) | .18 |
| Chemotherapy | 31 (36) | 13 (26) | 18 (49) | .041 |
| Radiation | 14 (16) | 7 (14) | 7 (19) | .57 |
| Steroid | 15 (17) | 9 (18) | 6 (16) | >.99 |
| Tumor bulk, median (range), cm | 3.7 (1.0-44.0) | 3.6 (1.0-44.0) | 4.5 (1.0-17.5) | .53 |
| ≤5 | 40 (46) | 25 (50) | 15 (41) | .59 |
| >5-10 | 15 (17) | 8 (16) | 7 (19) | |
| >10 | 7 (8) | 3 (6) | 4 (11) | |
| LDH before lymphodepletion, median (range), U/L | 226 (138-1603) | 218 (138-1603) | 235 (139-919) | .63 |
| SPD, median (range) | 20.0 (0-321) | 15.0 (0-272) | 23.1 (1.4-321) | .39 |
| SUVmax, median (range) | 16.2 (3.5-51.6) | 17.6 (3.5-40.9) | 13.7 (6.0-51.6) | .98 |
| Comorbidities | ||||
| BMI >30 | 23 (26) | 14 (28) | 9 (24) | .81 |
| Diabetes | 10 (11) | 3 (6) | 7 (19) | .09 |
| Statin use | 26 (30) | 12 (24) | 14 (38) | .24 |
| LVEF <50% | 5 (6) | 3 (6) | 2 (5) | >.99 |
| GFR <50 mL/min | 8 (9) | 3 (6) | 5 (14) | .28 |
BMI, body mass index; DHL, double-hit lymphoma; DLBCL, diffuse LBCL; ECOG PS, Eastern Cooperative Oncology Group performance status; GFR, glomerular filtration rate; HGBL, high-grade B-cell lymphoma; IPI, International Prognostic Index; LVEF, left ventricular ejection fraction; SUVmax, maximum standardized uptake value; tCLL, transformed chronic lymphocytic leukemia; tFL, transformed follicular lymphoma; THL, triple-hit lymphoma; tMZL, transformed marginal zone lymphoma.
Continuous variables compared using Wilcoxon rank-sum test and categorical variables using Fisher exact test.
Other diagnoses include T-cell/histiocyte rich B-cell lymphoma (1), primary mediastinal B-cell lymphoma (1), transformed Waldenström macroglobulinemia (1), and Burkitt lymphoma (1).
Baseline characteristics of the axi-cel and liso-cel cohorts were largely similar, with the exception of median age (axi-cel, 62 years vs liso-cel, 73 years; P < .001). Measures of tumor burden were not significantly different between the cohorts, with median LDH of 218 and 235 U/L in axi-cel and liso-cel, respectively (P = .63). The median SPD was 15.0 cm in the axi-cel and 23.1 cm in the liso-cel cohorts (P = .39).
A higher proportion of patients receiving liso-cel underwent bridging therapy, although this was not statistically significant (70% vs 54%; P = .18). However, the use of chemotherapy as bridging therapy was significantly higher in the liso-cel cohort than the axi-cel cohort (49% vs 26%; P = .041). The most frequent systemic bridging regimens were rituximab + polatuzumab vedotin (R-Pola; n = 17), R-Pola with bendamustine (n = 4), and rituximab, gemcitabine, and oxaliplatin (n = 6).
The most common histologic diagnosis in both cohorts was diffuse LBCL, not otherwise specified (60%), followed by transformed follicular lymphoma (21%), with no statistically significant difference between the cohorts. Double- or triple-hit cytogenetics were seen in 20% and 19% of the axi-cel and liso-cel cohorts, respectively. All patients had received either 2 (60%) or 3 prior lines of therapy (40%), with similar prior lines of therapy in axi-cel and liso-cel cohorts.
Select baseline comorbidity data including body mass index, diabetes, cardiac ejection fraction <50%, and estimated glomerular filtration rate were collected, and there were no significant differences noted between the cohorts.
Time to treatment
The time from CAR T-cell service referral (ITT) to CAR T-cell infusion was significantly longer with liso-cel, at a median of 81 days (interquartile range [IQR], 67-91) for liso-cel and 57 days (IQR, 53-68) for axi-cel (P < .001). The median time from ITT to leukapheresis was 35 days (IQR, 29-42) and 28 days (IQR, 22-35) for liso-cel and axi-cel, respectively (P < .001). The median time from leukapheresis to CAR T-cell infusion was 41 days (IQR, 37-49) for liso-cel and 30 days (IQR, 27-33) for axi-cel (P < .001). None of the patients required a second apheresis for manufacturing failure.
To account for possible improvements in apheresis slot availability and turnaround time, we also analyzed the time from ITT to CAR T cell with liso-cel, comparing an early cohort (June 2021 to February 2022) and a later cohort (March 2022 to September 2022), but did not observe a difference in ITT to apheresis, apheresis to CAR T-cell infusion, or overall ITT to CAR T-cell infusion between the 2 time intervals.
Responses and survival
The median follow-up in the axi-cel and liso-cel cohorts was 12 months (range, 10-18) and 10 months (range, 9-14), respectively. The best ORR among patients who received axi-cel was 92%, with a CR in 72% (95% CI, 58-84; Table 2). The ORR in the liso-cel cohort was 84%, with a CR in 62% (95% CI, 45-78). For both axi-cel and liso-cel, 3 patients in each cohort (6% and 8%, respectively) had an initial PR at 1 month and went on to have a CR at a later time point without additional therapy.
Responses to CAR T-cell therapy and survival
| . | Total (N = 87) . | Axi-cel (n = 50) . | Liso-cel (n = 37) . |
|---|---|---|---|
| Median follow-up (95% CI), mo | 11 (10-14) | 12 (10-18) | 10 (9-14) |
| Best response | |||
| CR | 68% (57-77) | 72% (58-84) | 62% (45-78) |
| PR | 21% (13-31) | 20% (10-34) | 22% (10-38) |
| 1-mo response | |||
| CR | 54% (43-65) | 62% (47-75) | 43% (27-61) |
| PR | 28% (19-38) | 26% (15-40) | 30% (16-47) |
| PFS, median (95% CI) | NR (9 to NR) | NR (9 to NR) | 11 (6 to NR) |
| 12-mo PFS | 53%, (42-66) | 59% (45-76) | 44% (29-68) |
| DOR, median (95% CI) | NR (11 to NR) | NR (11 to NR) | NR (9 to NR) |
| 12-mo DOR | 61% (49-76) | 62% (48-81) | 59% (41-86) |
| OS, median (95% CI) | NR (NR to NR) | NR (NR to NR) | NR (NR to NR) |
| 12-mo OS | 74% (64-86) | 77% (65-92) | 71% (55-91) |
| . | Total (N = 87) . | Axi-cel (n = 50) . | Liso-cel (n = 37) . |
|---|---|---|---|
| Median follow-up (95% CI), mo | 11 (10-14) | 12 (10-18) | 10 (9-14) |
| Best response | |||
| CR | 68% (57-77) | 72% (58-84) | 62% (45-78) |
| PR | 21% (13-31) | 20% (10-34) | 22% (10-38) |
| 1-mo response | |||
| CR | 54% (43-65) | 62% (47-75) | 43% (27-61) |
| PR | 28% (19-38) | 26% (15-40) | 30% (16-47) |
| PFS, median (95% CI) | NR (9 to NR) | NR (9 to NR) | 11 (6 to NR) |
| 12-mo PFS | 53%, (42-66) | 59% (45-76) | 44% (29-68) |
| DOR, median (95% CI) | NR (11 to NR) | NR (11 to NR) | NR (9 to NR) |
| 12-mo DOR | 61% (49-76) | 62% (48-81) | 59% (41-86) |
| OS, median (95% CI) | NR (NR to NR) | NR (NR to NR) | NR (NR to NR) |
| 12-mo OS | 74% (64-86) | 77% (65-92) | 71% (55-91) |
Values are presented as rate (95% CI) unless otherwise stated.
NR, not reached.
On direct comparison, there were no significant differences in PFS (P = .25), DOR (P = .79), or OS (P = .60) between the axi-cel and liso-cel cohorts (Figure 1). The median PFS was not reached (95% CI, 9 months to not reached [NR]) in the axi-cel cohort, and it was 11 months (95% CI, 6 to NR) in the liso-cel cohort. PFS at 12 months in the axi-cel cohort was 59% (95% CI, 45-76), compared with 44% (95% CI, 29-68) in the liso-cel cohort. On post hoc analysis, with 37 liso-cel and 50 axi-cel patients, this study had 74% power to detect a 20% difference in the proportion of patients alive and progression free at 12 months, using a 1-sided 0.1 Fisher exact test.
Survival outcomes. Kaplan-Meier curves illustrating time-to-event for the overall cohort and comparing axi-cel and liso-cel cohorts. (A) DOR curves for patients who achieved a CR or PR at first restaging after therapy. (B) PFS curves. (C) OS curves. IEC, immune effector cell.
Survival outcomes. Kaplan-Meier curves illustrating time-to-event for the overall cohort and comparing axi-cel and liso-cel cohorts. (A) DOR curves for patients who achieved a CR or PR at first restaging after therapy. (B) PFS curves. (C) OS curves. IEC, immune effector cell.
The median DOR was not reached for either cohort; 12-month DOR was 62% (95% CI, 49-76) for axi-cel and 59% (95% CI 41-86) for liso-cel. The median OS was not reached in either cohort; 12-month OS was 77% (95% CI, 65-92) in the axi-cel cohort and 71% (95% CI, 55-91) in the liso-cel cohort.
Given the significantly higher age in the liso-cel cohort and possible biases in choice of therapy due to the limited availability of apheresis slots, we performed propensity score weighting to compare outcomes between the axi-cel and liso-cel cohorts, accounting for possible confounding variables. On propensity score-weighted comparison, a lower rate of overall response was seen with liso-cel than with axi-cel (odds ratio [OR], 0.16; 95% CI, 0.02-0.67; Figure 2). CRRs were not significantly different (OR, 0.60; 95% CI, 0.24-1.52). An inferior PFS was observed in the liso-cel cohort (hazard ratio [HR] for progression/death, 2.95; 95% CI, 1.14-7.60; P = .025). There was a nonsignificant higher risk for death in the liso-cel cohort as well (HR, 2.95; 95% CI, 0.84-10.43; P = .09).
Weighted comparison of clinical outcomes. (A-B) Propensity score-weighted comparison of outcomes between axi-cel and liso-cel cohorts, including ORs for ORR and CRR (A), as well as HRs for OS and PFS (B).
Weighted comparison of clinical outcomes. (A-B) Propensity score-weighted comparison of outcomes between axi-cel and liso-cel cohorts, including ORs for ORR and CRR (A), as well as HRs for OS and PFS (B).
Safety
Rates of any-grade CRS were significantly higher in the axi-cel cohort than the liso-cel cohort, at 90% and 54%, respectively (P < .001). However, rates of grade 3+ CRS were similar between the cohorts (6% vs 5% for axi-cel vs liso-cel, respectively, P = .64). The median time to onset of CRS was 3 days in the axi-cel cohort and 2 days in the liso-cel cohort. The median duration of CRS was longer with axi-cel (5 vs 4 days; P = .025). Tocilizumab was administered to 80% of patients in the axi-cel cohort, which was significantly higher than in the liso-cel cohort (32%; P = .016). Dexamethasone was administered for CRS to 72% of patients in the axi-cel cohort compared with only 27% of patients in the liso-cel cohort (P = .02). Notably, these patients did not receive prophylactic steroids or other anti-inflammatory medications for CRS/ICANS prevention.
Rates of any-grade ICANS were significantly higher in the axi-cel cohort than the liso-cel cohort, at 52% and 27%, respectively (P = .028). The rate of grade 3+ ICANS was 20% in the axi-cel cohort and 11% in liso-cel cohort (P > .99). The median time to onset of ICANS (7 vs 6 days) and the duration of ICANS (5 vs 4 days) were similar between the cohorts. Dexamethasone was given for ICANS in 38% of axi-cel recipients and 19% of liso-cel recipients (P = .11).
Patients in the axi-cel cohort had a longer median length of hospitalization than the liso-cel cohort (15 vs 10 days, respectively; P = .0017). There was no difference in rates of ICU admission (8% for each cohort) or duration of ICU admission (10 vs 6 days for axi-cel and liso-cel, respectively). There were similar rates of readmission within the first 30 days of CAR T-cell infusion (12% vs 8% for axi-cel vs liso-cel, respectively).
Grade ≥3 neutropenia at day 30 or later was more common in the axi-cel cohort (24% vs 0%; P = .003). This remained significantly higher when patients with preexisting grade ≥3 cytopenias at the time of lymphodepletion were excluded (18% vs 0%; P = .019). Rates of infection were numerically higher after axi-cel but not statistically so, and most of this was related to viral infections (26% vs 14% for axi-cel and liso-cel, respectively; P = .19). Rates of bacterial and fungal infections were similar between the cohorts.
During follow-up, there were 9 deaths among those treated with axi-cel (18%) and 8 deaths among those treated with liso-cel (22%). Most deaths were related to disease progression (7 of 9 in the axi-cel cohort and 6 of 8 in the liso-cel cohort). There were no deaths related to CRS or ICANS in either arm. There was 1 infection-related death after liso-cel due to COVID-19 and 1 additional death after liso-cel due to end-stage chronic obstructive pulmonary disease. There was 1 infection-related death after axi-cel due to aspiration pneumonia.
Biomarkers and association with response, survival, and toxicities
Notable biomarkers at baseline and peak levels after CAR T-cell infusion are summarized in supplemental Table 1. Baseline biomarkers at the day of CAR T-cell infusion and subsequent trends were available for >95% of patients, with the exception of peak ferritin, which was available for 94% of patients.
There were no significant differences in day 0 CRP, IL-6, ferritin, or absolute lymphocyte count between axi-cel and liso-cel cohorts. The axi-cel cohort had higher peak CRP (119 vs 75; P = .01) and higher peak IL-6 (median, 691 vs 82; P < .001) than the liso-cel cohort.
Biomarkers associated with inferior PFS included higher prelymphodepletion LDH, along with higher day 0 CRP, IL-6, LDH, and ferritin, peak ferritin, and peak absolute lymphocyte count (supplemental Figure 2). Higher SPD was associated with inferior PFS (HR, 1.5 for each 50-cm increase; 95% CI, 1.17-1.94).
Postinfusion toxicities were also associated with multiple preinfusion and postinfusion biomarkers and clinical features (supplemental Figure 3). Biomarkers that were associated with increased odds of any-grade ICANS included IL-6, CRP, and ferritin at day 0, glomerular filtration rate <50 mL/min before therapy, and peak CRP and IL-6 (OR, 20.97, 8.78, 1.10, 12.07, 3.63, and 1.04, respectively).
The only factor found to be associated with increased odds of prolonged neutropenia (present at day 30 or later) in all patients was any-grade ICANS (OR, 4.29). Increased risk of prolonged anemia was associated with prolonged thrombocytopenia, increased IL-6, CRP, LDH and ferritin at day 0, and peak ferritin (OR, 21.87, 17.00, 7.51, 1.26, 1.05, and 1.02, respectively). Factors associated with increased odds of prolonged thrombocytopenia included prolonged anemia, any-grade ICANS, CRP and ferritin at day 0, and peak ferritin (OR, 21.87, 5.90, 4.42, 1.13, and 1.07, respectively).
Discussion
In this real-world study, we evaluated distinct cohorts of patients who received either axi-cel or liso-cel for R/R LBCL. Efficacy based on response rate, PFS, and OS were similar between the 2 cohorts on direct comparison. However, on propensity score-weighted comparison to account for differences in risk factors, an inferior ORR and PFS were observed with liso-cel. Higher rates of ICANS and CRS were observed in the axi-cel cohort, although rates of grade ≥3 toxicity were similar. These differences, in particular the difference in PFS between axi-cel and liso-cel on weighted comparison, warrant further evaluation in a multicenter context.
The default choice of CAR T-cell therapy varied between axi-cel and liso-cel based on an arbitrary time cutoff. Although it was anticipated that this could result in a “natural experiment,” we found that the majority of patients receiving CAR T-cell therapy during the liso-cel default period actually received axi-cel. This was likely due to limitations in apheresis slot availability, resulting in wait times for apheresis that were deemed too long by clinicians, prompting axi-cel use if collection could be arranged earlier. As such, patients with higher-risk disease requiring urgent treatment may have disproportionately received axi-cel. Additionally, on comparison of patient characteristics, the axi-cel cohort was significantly younger than the liso-cel cohort (62 vs 73 years). This difference may reflect greater physician comfort with liso-cel administration in older patients, given lower rates of toxicity reported in the liso-cel clinical trials than those with axi-cel.1,2 Thus, in this study and other real-world analyses of liso-cel and axi-cel, awareness of these potential biases in the choice of patient and therapy is needed.
Notably, outcomes in these 2 real-world cohorts compared favorably with the outcomes of phase 2 trials in patients receiving CAR T-cell therapy for the third line or later. In our real-world axi-cel cohort, the CRR was 72%, compared with 58% in ZUMA-1.1 In the liso-cel cohort, the CRR was 62%, compared with 53% in TRANSCEND NHL-001.2 Similar findings with regard to 12-month PFS and OS were also noted. Other real-world studies for axi-cel have shown comparable efficacy outcomes with clinical trial populations.9-13
Rates of CRS and ICANS were significantly higher in the axi-cel cohort, reflecting findings from clinical trials and real-world comparisons of axi-cel with tisagenlecleucel (tisa-cel).14 However, there was no significant difference found between rates of grade 3+ CRS or grade 3+ ICANS between patients receiving infusion with axi-cel and those with liso-cel. This may reflect more aggressive use of tocilizumab for lower-grade CRS, with 80% of the axi-cel cohort receiving tocilizumab, compared with 32% of those in the liso-cel cohort. The median onset of CRS in the axi-cel cohort was later than previously seen, and in the liso-cel cohort, it was earlier than previously seen, such that there was no real difference in the timing of this toxicity after the 2 products.
Further analysis demonstrated that elevated levels of baseline inflammatory markers, particularly CRP and IL-6 at day 0, were associated with decreased treatment efficacy, increased risk of any-grade ICANS, and prolonged cytopenias. These findings align with those of other recent studies, including the CAR-HEMATOTOX model, in which baseline inflammatory markers including CRP and ferritin were associated with toxicity and inferior PFS.15 Markers of tumor burden, including LDH at day 0 and tumor bulk >5 cm, were associated with a decreased likelihood of achieving CR, reflecting findings from other studies showing that high tumor burden at the time of CAR T-cell therapy is associated with poorer response to treatment.
We acknowledge the limitations of this study, including its single-center, retrospective nature. This study included CAR T-cell recipients and was not able to capture all patients considered for CAR T-cell therapy; as such, there may be bias via the exclusion of patients who were intended to receive CAR T cells but were ultimately not able to receive therapy. Additionally, 10 patients received nonconforming liso-cel products on an expanded access protocol, which is not available at all institutions. Low event rates may limit the power of the comparisons between cohorts in terms of efficacy and toxicity, resulting in differences that approach but do not reach statistical significance in this study. Additionally, given that our study reflects the real-world commercial use of CAR T-cell therapies in clinical practice, heterogeneity of patient management exists with regard to factors such as time point of pretreatment scan and patients lost to follow-up after returning to local health centers.
Although this analysis focused on liso-cel and axi-cel therapies, it should be noted that tisa-cel is another option as third-line or later therapy in R/R LBCL. Other studies have reported on relative outcomes with tisa-cel and axi-cel in R/R LBCL.14,16 During the time period of this study, however, tisa-cel was not routinely used in this context, so we did not include this regimen in the analysis.
This study is the first, to our knowledge, to compare the efficacy and toxicities of axi-cel and liso-cel for the treatment of R/R LBCL in the commercial setting. These real-world data in patients with ≥2 prior lines of therapy showed similar outcomes with both products, compared with their respective pivotal phase 2 studies, further supporting the generalizability of these trials.1,2 Efficacy outcomes including response rates, PFS, and OS were not significantly different on direct comparison of the axi-cel and liso-cel cohorts, but when accounting for differences in patient and disease characteristics, an inferior ORR and PFS were observed in the liso-cel cohort. Conversely, the overall toxicity profiles, particularly with CRS and ICANS, appeared more favorable for liso-cel than axi-cel. The “brain-to-vein” time,17 measured as the time from the consideration of CAR T-cell therapy for a patient to the time of CAR T-cell infusion, was significantly longer for liso-cel than axi-cel, even for patients treated at a later time point when manufacturing slot availability had improved for liso-cel. Given these findings, the choice of product should be individualized; in addition to efficacy, other factors including risk factors for toxicity, the manufacturing time of CAR T cells, and medical costs, are relevant. Future evaluations with larger, multicenter real-world cohorts are needed to further evaluate the possible difference in efficacy between axi-cel and liso-cel and to provide additional insights into the toxicities of these CAR T-cell products.
Acknowledgments
D.A.Q. has received training support and funding from the Lymphoma Research Foundation.
C.A.J. is a scholar in Clinical Research of the Leukemia & Lymphoma Society.
Authorship
Contribution: A.L., D.M., and C.A.J. conceived and designed the study. A.L., D.A.Q., D.M., and C.A.J. interpreted findings; A.L., D.A.Q., and C.A.J. wrote the manuscript; R.A.R. performed biostatistical analysis; D.M., C.D., J.D.C., and A.S. performed chart review and data collection; P.A., J.L.C., D.C.F., E.D.J., A.I.K., A.S.L., R.W.M., E.M.P., and C.A.J. provided care for patients included in the study; and all authors provided important feedback and reviewed the final manuscript.
Conflict-of-interest disclosure: D.Q. reports advisory board fees from Genmab and ADC Therapeutics. P.A. reports consulting roles for Merck, Bristol Myers Squibb (BMS), Pfizer, Affimed, Adaptive, Infinity, ADC Therapeutics, Celgene, MorphoSys, Daiichi Sankyo, Miltenyi, Tessa, Genmab, C4, Enterome, Regeneron, Epizyme, AstraZeneca, Genentech/Roche, Xencor, Foresight, and ATB Therapeutics; research funding from Kite, Merck, BMS, Affimed, Adaptive, Tensha, Otsuka, Sigma Tau, Genentech/Roche, IGM Biosciences, and AstraZeneca; and honoraria from Merck and BMS. J.L.C. reports consulting fees from ADC Therapeutics, Seagen, Kite, Incite/MorphoSys, and Regeneron, and research funding from Genentech/Roche, Merck, AbbVie, and Bayer. E.D.J. reports consulting fees from Syros and Takeda, and research funding from Acerta, Janssen, Novartis, and Pharmacyclics. A.S.L. reports advisory board fees from Kite and Seagen; consulting fees from Research to Practice, Genmab, Adaptive Biotechnologies, BMS, AbbVie, Intellia, and Epizyme; and research funding from BMS, Merck, Genentech/Roche, and Genmab. C.A.J. served as a consultant for Kite/Gilead, Novartis, BMS/Celgene, Abintus Bio, ImmPACT Bio, Caribou Bio, MorphoSys, ADC Therapeutics, AbbVie, AstraZeneca, Ipsen, Sana, Synthekine, Daiichi Sankyo, and Janssen, and has received research funding from Kite/Gilead and Pfizer. The remaining authors declare no competing financial interests.
Correspondence: David A. Qualls, Dana-Farber Cancer Institute, 450 Brookline Ave, Dana 200C5, Boston, MA 02215; email: david_qualls@dfci.harvard.edu.
References
Author notes
A.L. and D.A.Q. contributed equally to this study and are joint first authors.
Original deidentified data are available on request from the corresponding author, David A. Qualls (david_qualls@dfci.harvard.edu).
The full-text version of this article contains a data supplement.