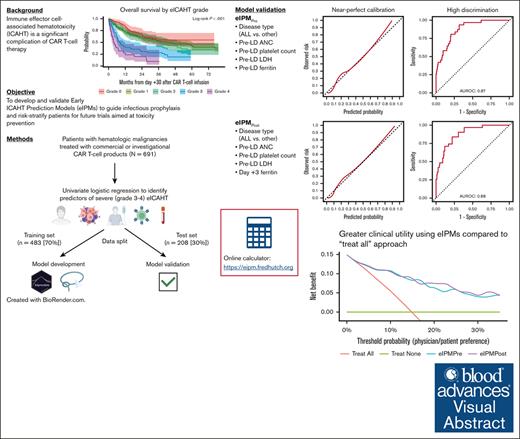
Key Points
Disease type, baseline cytopenia, lymphodepletion intensity, and systemic inflammation were strongly associated with severe eICAHT.
We validated 2 prediction models of grade 3 to 4 ICAHT with near-perfect calibration, high discrimination, and high net benefit.
Visual Abstract
Immune effector cell–associated hematotoxicity (ICAHT) is associated with morbidity and mortality after chimeric antigen receptor (CAR) T-cell therapy. To date, the factors associated with ICAHT are poorly characterized, and there is no validated predictive model of ICAHT as defined by current consensus criteria. Therefore, we performed comprehensive univariate analyses to identify factors associated with severe (grade 3-4) early ICAHT (eICAHT) in 691 patients who received commercial or investigational CAR T-cell therapy for hematologic malignancies. In univariate logistic regression, preinfusion factors associated with severe eICAHT included disease type (acute lymphoblastic leukemia), prelymphodepletion (pre-LD) blood counts including absolute neutrophil count (ANC), lactate dehydrogenase (LDH), and inflammatory (C-reactive protein [CRP], ferritin, and interleukin-6 [IL-6]) and coagulopathy biomarkers (D-dimer). Postinfusion laboratory markers associated with severe eICAHT included early and peak levels of inflammatory biomarkers (CRP, ferritin, and IL-6), coagulopathy biomarkers (D-dimer), peak cytokine release syndrome grade, and peak neurotoxicity grade. We trained (n = 483) and validated (n = 208) 2 eICAHT prediction models (eIPMs): eIPMPre including preinfusion factors only (disease type and pre-LD ANC, platelet count, LDH, and ferritin) and eIPMPost containing both preinfusion (disease type and pre-LD ANC, platelet count, and LDH) and early postinfusion (day +3 ferritin) factors. Both models generated calibrated predictions and high discrimination (area under the receiver operating characteristic curve in test set, 0.87 for eIPMPre and 0.88 for eIPMPost), with higher net benefit in decision curve analysis for eIPMPost. Individualized predictions of severe eICAHT can be generated from both eIPMs using our online tool (available at https://eipm.fredhutch.org).
Introduction
Chimeric antigen receptor (CAR) T-cell therapy has transformed the treatment landscape for B-cell and plasma cell malignancies. However, immune effector cell–associated hematotoxicity (ICAHT) has been increasingly recognized as a major adverse event across all target antigens and disease types.1-4 ICAHT is associated with high morbidity, transfusion dependency, growth factor requirement, and severe infections, with the latter representing the leading cause of nonrelapse mortality after CAR T-cell therapy.2,4-9 Recognizing that ICAHT was not adequately captured by the Common Terminology Criteria for Adverse Events criteria, the European Hematology Association (EHA) and the European Society for Blood and Marrow Transplantation (EBMT) recently created consensus criteria for grading ICAHT.8,10,11 The EHA/EBMT criteria define ICAHT based on the timing after CAR T-cell infusion (early, days 0-30; late, after day +30); early ICAHT (eICAHT) is additionally defined by the duration of neutropenia using thresholds of 500 cells per μL and 100 cells per μL (supplemental Table 1).11
An effective method to accurately identify patients at risk of severe eICAHT does not currently exist. Although our group and others have previously described factors associated with hematotoxicity after CAR T-cell therapy,4-6,8,12-14 a comprehensive, high-dimensional evaluation of clinical and laboratory predictors of eICAHT specifically assessed per the EHA/EBMT criteria has not been reported. In addition, although the CAR-HEMATOTOX model is commonly used to predict hematotoxicity after CAR T-cell therapy across disease types,5,8,9,13,14 it has a number of limitations, including the following: (1) it was developed specifically in patients with large B-cell lymphoma; (2) it predicts the duration of severe neutropenia (absolute neutrophil count [ANC] <500 cells per μL) ≥14 vs <14 days after CAR T-cell infusion, not severe ICAHT as defined per the consensus criteria, thus not capturing patients who might have grade 3-4 eICAHT due to ANC ≤100 cells per μL lasting ≥7 days; (3) it had relatively low sensitivity and specificity in the original validation cohorts (89% and 68%, respectively); (4) it does not generate continuous probabilities; and finally, (5) it does not include early postinfusion markers of inflammation, which are known to predict prolonged cytopenias.6,15 Thus, a predictive model of severe (grade 3-4) eICAHT that can be applied across all B-cell and plasma cell malignancies remains an unmet need to improve outcomes after CAR T-cell therapy.
To address these gaps, we performed comprehensive univariate analyses to identify preinfusion and postinfusion factors associated with severe eICAHT in a large cohort of 691 patients treated with CAR T-cell therapy at our institution. In addition, we independently validated 2 predictive models of severe eICAHT (eIPMPre and eIPMPost).
Methods
Study design
Adults who underwent their first infusion of CAR T-cell therapy for hematologic malignancies with commercial or investigational CD19, CD20, or B-cell maturation antigen (BCMA)-targeted CAR T-cell products at the Fred Hutchinson Cancer Center between 2013 and 28 April 2024 were included. All investigational products were autologous and had single-antigen targets. Laboratory values between day 0 (CAR T-cell infusion) and day +30 after CAR T-cell infusion were extracted from the electronic medical record. Day +3 laboratory values were recorded as the highest values between days +2 and +4. The data cutoff was 29 May 2024. This study was approved by the Fred Hutchinson Cancer Center Institutional Review Board.
Longitudinal ANC data
Longitudinal ANC values between day 0 and day +30 after CAR T-cell infusion were extracted from electronic medical records. Up to 7 consecutive daily missing ANC values were linearly interpolated.16 Patients who died before day +14 after CAR T-cell infusion, who had >7 consecutive days of missing ANC values, or who had a second CAR T-cell infusion within 30 days of the first CAR T-cell infusion were excluded from analysis.
Lymphodepletion type and intensity
Lymphodepletion (LD) regimens were categorized as cyclophosphamide and fludarabine (Cy/Flu) vs non-Cy/Flu. Cy/Flu regimens containing Cy 500 mg/m2 per day for 3 days, 60 mg/kg for 1 dose, or 30 mg/kg for 1 dose were categorized as high intensity. Cy/Flu regimens containing Cy 250 or 300 mg/m2 per day for 3 days or 1 g/m2 for 1 dose were categorized as low intensity.
Toxicity grading
The severity of cytokine release syndrome (CRS) was graded according to American Society of Transplantation and Cellular Therapy (ASTCT) criteria.17 The severity of neurotoxicity was graded according to the Common Terminology Criteria for Adverse Events version 4.0318 and ASTCT criteria17 in patients receiving CAR T-cell therapy before and after 2019, respectively. Immune effector cell–associated hemophagocytic lymphohistiocytosis-like syndrome (IEC-HS) was defined per the Hines et al criteria as meeting all the following criteria: prior active or resolved CRS, ferritin >2× the upper limit of normal, aspartate transaminase or alanine transaminase >5× upper limit of normal, and hypofibrinogenemia <150 mg/dL.19 The CAR-HEMATOTOX score was calculated using prelymphodepletion (pre-LD) values as previously described,5 with missing data imputed using bagged tree models. Grading of eICAHT per EHA/EBMT criteria was automated using the heatwaveR package in the R programming language as previously described.16
Statistical analyses
Median follow-up was estimated by predicting the censoring distribution (inverse Kaplan-Meier method). Overall survival (OS) was estimated by the Kaplan-Meier method; for analyses including eICAHT grade, which were assigned from ANC values between day 0 and +30 after CAR T-cell infusion, OS was landmarked at day +30. Associations between 58 clinically relevant patient, disease-related, and laboratory factors commonly obtained as standard of care and ICAHT outcomes were modeled using univariate and multivariable logistic regression.
Multivariable prediction models were developed in accordance with the Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD) statement.20 Model specification and variable selection were based on recommendations by Riley et al.21 Briefly, based on a prevalence of severe eICAHT of 16%, shrinkage factor of 0.9, Cox-Snell R squared statistic of 0.1, and multiplicative margin of error of 1.1, the minimum sample size required for new multivariable development with 6 parameters was estimated to be 510.21 Variables included in multivariable modeling were selected based on the strength of association with severe eICAHT in univariate logistic regression, subject-matter knowledge, and their availability as part of standard of care (eg, bone marrow involvement was not included because bone marrow biopsies are not routinely obtained in patients with large B-cell lymphoma). Before multivariable modeling, the training set was preprocessed as follows using the recipes package of the tidymodels ecosystem in R22: (1) missing data for continuous variables were imputed using bagged tree models (step_impute_bag() function) based on disease type (acute lymphoblastic leukemia [ALL] vs other), LD regimen (low-dose Cy/Flu vs other); receipt of bridging therapy; pre-LD and day +3 ANC; pre-LD and day +3 platelet count; pre-LD lactate dehydrogenase (LDH); pre-LD, day +0, and day +3 ferritin; day +3 C-reactive protein (CRP); and day +3 D-dimer; (2) continuous variables were normalized; and (3) categorical variables were converted to binary numeric terms.
The data set was randomly split into a training set consisting of 70% of observations (n = 483) and an independent validation set (referred to as “test set” throughout the manuscript) consisting of the remaining 30% of observations (n = 208), stratified by severe eICAHT to ensure equal proportions of severe ICAHT in each set (supplemental Table 2). Models were first trained on the training set and least absolute shrinkage and selection operator-regularized with bootstrap resampling and parameter tuning based on the best area under the receiver operating characteristic curve (AUROC). The penalty term chosen based on parameter tuning was 1 × 10–10 for the model including LD regimen and 0.00316 for the other models. The trained models were evaluated for calibration (agreement between predictions and observed frequencies; eg, in patients predicted to have a 10% risk of grade 3-4 ICAHT, 10% actually developed grade 3-4 ICAHT), discrimination (ability to correctly rank the risk of 2 randomly selected patients [1, perfect discrimination; 0.5, equivalent to a coin flip]; AUROC), and net benefit (proportion of true positives minus the weighted proportion of false positives based on the probability threshold; can be visualized across a range of probability thresholds via decision curve analysis23) in the test set. Sensitivity and specificity of the models were calculated on the test set based on an optimal cut point maximizing the Youden index.
All statistical analyses were performed using R software version 4.4.1 (R Core Team, Vienna, Austria) and the following packages: caret, cutpointr, dcurves, ggplot2, glmnet, gtsummary, heatwaveR, pmsampsize, probably, runway, tidymodels, and vip. A web application to generate predictions of severe eICAHT based on our multivariable models can be accessed at https://eipm.fredhutch.org/. A reproducible data supplement can be found at https://emilyliangmd.github.io/eipm/.
Results
Patient, disease, and treatment characteristics and long-term outcomes
A total of 691 patients were included (Table 1; supplemental Figure 1). The median age at CAR T-cell infusion was 61 years (interquartile range, 51-68). Sixty-three percent of patients (n = 437) were male, and 84% (n = 583) were White. Thirty-four percent (n = 234) had received prior hematopoietic cell transplantation (HCT). The most common disease types were aggressive non-Hodgkin lymphoma (NHL; n = 318 [46%]) and indolent NHL (mantle cell lymphoma [MCL], n = 37 [5%]; other indolent NHL, n = 117 [17%]). The most common CAR T-cell products were the investigational CD19-targeted CAR T-cell product JCAR014 (n = 193 [28%]), axicabtagene ciloleucel (n = 140 [20%]), and lisocabtagene maraleucel (n = 87 [13%]). Four hundred twenty-two patients (61%) received bridging therapy.
Patient, disease, and treatment characteristics
| Characteristic . | N = 691 . |
|---|---|
| Age | |
| Median (IQR) | 61 (51-68) |
| Range | 19-84 |
| Sex | |
| Male | 437 (63%) |
| Female | 254 (37%) |
| Race | |
| White | 583 (84%) |
| Asian | 58 (8%) |
| Black or African American | 18 (3%) |
| American Indian or Alaska Native | 13 (2%) |
| Multiple | 8 (1%) |
| Unknown | 7 (1%) |
| Native Hawaiian or other Pacific Islander | 4 (1%) |
| Ethnicity | |
| Not Hispanic or Latino | 629 (91%) |
| Hispanic or Latino | 52 (8%) |
| Unknown | 10 (1%) |
| Prior HCT | 234 (34%) |
| Disease | |
| Aggressive NH∗ | 318 (46%) |
| Multiple myeloma/plasma cell leukemia | 120 (17%) |
| Other indolent NHL† | 117 (17%) |
| ALL‡ | 99 (14%) |
| MCL | 37 (5%) |
| Bone marrow involvement by morphology, %§ | |
| Median (IQR) | 0 (0-30) |
| Range | 0-100 |
| Missing | 267 |
| Bone marrow involvement by flow cytometry, %§ | |
| Median (IQR) | 0 (0-19) |
| Range | 0-99 |
| Missing | 252 |
| Received bridging therapy|| | 422 (61%) |
| Aggressive NHL | 153 (48%) |
| Multiple myeloma/plasma cell leukemia | 108 (90%) |
| Other indolent NHL | 69 (59%) |
| ALL | 59 (60%) |
| MCL | 33 (89%) |
| LD regimen | |
| Low-intensity Cy/Flu | 403 (58%) |
| High-intensity Cy/Flu | 253 (37%) |
| Other¶ | 35 (5%) |
| Total CAR T-cell dose, × 106cells | |
| Median (IQR) | 170 (111-402) |
| Range | 5-43 692 |
| CAR T-cell product | |
| CD19 targeted | |
| JCAR014# | 193 (28%) |
| Axicabtagene ciloleucel | 140 (20%) |
| Lisocabtagene maraleucel | 87 (13%) |
| Brexucabtagene autoleucel | 47 (7%) |
| JCAR021# | 44 (6%) |
| Tisagenlecleucel | 15 (2%) |
| CD20 targeted | |
| Investigational product∗∗ | 45 (7%) |
| BCMA targeted | |
| Ciltacabtagene autoleucel | 52 (8%) |
| Investigational product# | 38 (5%) |
| Idecabtagene vicleucel | 30 (4%) |
| CAR T-cell product category | |
| Investigational CAR T-cell product with 4-1BB costimulatory domain†† | 320 (46%) |
| Commercial CD19-targeted CAR T-cell product with CD28 costimulatory domain | 187 (27%) |
| Commercial CD19-targeted CAR T-cell product with 4-1BB costimulatory domain | 102 (15%) |
| Commercial BCMA-targeted CAR T-cell product | 82 (12%) |
| Pre-LD ANC, ×103cells per μL | |
| Median (IQR) | 2.84 (1.79-4.29) |
| Range | 0.00-54.03 |
| Missing | 1 |
| Pre-LD Hb (g/dL) | |
| Median (IQR) | 10.70 (9.50-12.35) |
| Range | 7.00-17.10 |
| Pre-LD platelet count, × 103/μL | |
| Median (IQR) | 142 (89-202) |
| Range | 6-790 |
| Characteristic . | N = 691 . |
|---|---|
| Age | |
| Median (IQR) | 61 (51-68) |
| Range | 19-84 |
| Sex | |
| Male | 437 (63%) |
| Female | 254 (37%) |
| Race | |
| White | 583 (84%) |
| Asian | 58 (8%) |
| Black or African American | 18 (3%) |
| American Indian or Alaska Native | 13 (2%) |
| Multiple | 8 (1%) |
| Unknown | 7 (1%) |
| Native Hawaiian or other Pacific Islander | 4 (1%) |
| Ethnicity | |
| Not Hispanic or Latino | 629 (91%) |
| Hispanic or Latino | 52 (8%) |
| Unknown | 10 (1%) |
| Prior HCT | 234 (34%) |
| Disease | |
| Aggressive NH∗ | 318 (46%) |
| Multiple myeloma/plasma cell leukemia | 120 (17%) |
| Other indolent NHL† | 117 (17%) |
| ALL‡ | 99 (14%) |
| MCL | 37 (5%) |
| Bone marrow involvement by morphology, %§ | |
| Median (IQR) | 0 (0-30) |
| Range | 0-100 |
| Missing | 267 |
| Bone marrow involvement by flow cytometry, %§ | |
| Median (IQR) | 0 (0-19) |
| Range | 0-99 |
| Missing | 252 |
| Received bridging therapy|| | 422 (61%) |
| Aggressive NHL | 153 (48%) |
| Multiple myeloma/plasma cell leukemia | 108 (90%) |
| Other indolent NHL | 69 (59%) |
| ALL | 59 (60%) |
| MCL | 33 (89%) |
| LD regimen | |
| Low-intensity Cy/Flu | 403 (58%) |
| High-intensity Cy/Flu | 253 (37%) |
| Other¶ | 35 (5%) |
| Total CAR T-cell dose, × 106cells | |
| Median (IQR) | 170 (111-402) |
| Range | 5-43 692 |
| CAR T-cell product | |
| CD19 targeted | |
| JCAR014# | 193 (28%) |
| Axicabtagene ciloleucel | 140 (20%) |
| Lisocabtagene maraleucel | 87 (13%) |
| Brexucabtagene autoleucel | 47 (7%) |
| JCAR021# | 44 (6%) |
| Tisagenlecleucel | 15 (2%) |
| CD20 targeted | |
| Investigational product∗∗ | 45 (7%) |
| BCMA targeted | |
| Ciltacabtagene autoleucel | 52 (8%) |
| Investigational product# | 38 (5%) |
| Idecabtagene vicleucel | 30 (4%) |
| CAR T-cell product category | |
| Investigational CAR T-cell product with 4-1BB costimulatory domain†† | 320 (46%) |
| Commercial CD19-targeted CAR T-cell product with CD28 costimulatory domain | 187 (27%) |
| Commercial CD19-targeted CAR T-cell product with 4-1BB costimulatory domain | 102 (15%) |
| Commercial BCMA-targeted CAR T-cell product | 82 (12%) |
| Pre-LD ANC, ×103cells per μL | |
| Median (IQR) | 2.84 (1.79-4.29) |
| Range | 0.00-54.03 |
| Missing | 1 |
| Pre-LD Hb (g/dL) | |
| Median (IQR) | 10.70 (9.50-12.35) |
| Range | 7.00-17.10 |
| Pre-LD platelet count, × 103/μL | |
| Median (IQR) | 142 (89-202) |
| Range | 6-790 |
JCAR014, investigational CAR T-cell product containing 4-1BB costimulatory domain, murine CD19-targeted single-chain variable fragment (scFv), and infused as 1:1 ratio of CD4+:CD8+ CAR T cells; JCAR021, investigational CAR T-cell product containing 4-1BB costimulatory domain, fully human CD19-targeted scFv, and infused as 1:1 ratio of CD4+:CD8+ CAR T cells.
BCMA, B-cell maturation antigen; Hb, hemoglobin; IQR, interquartile range; LD, lymphodepletion.
Large B-cell lymphoma, Burkitt lymphoma, central nervous system lymphoma, pleiomorphic MCL.
Follicular lymphoma, chronic lymphocytic leukemia, marginal zone lymphoma, Waldenstrom macroglobulinemia, hairy cell leukemia.
Including 1 patient with lymphoid blast phase of chronic myeloid leukemia.
Performed within 30 days of CAR T-cell infusion; for patients with ALL, the Children’s Oncology Group (COG) result was used.
Any antineoplastic therapy administered after leukapheresis and before lymphodepletion, including corticosteroids, intrathecal chemotherapy, and radiation therapy; percentages of patients receiving bridging therapy within each disease type are calculated from the total number of patients with the respective disease type.
Other LD regimens included single-agent cyclophosphamide (n = 20), single-agent bendamustine (n = 6), cyclophosphamide/etoposide ± dexamethasone (n = 8), and single-agent fludarabine (n = 1).
Contained a 4-1BB costimulatory domain.
Contained a dual 4-1BB and CD28 costimulatory domain.
Including the 45 patients who received the investigational CD20-targeted CAR T-cell product containing both 4-1BB and CD28 costimulatory domains.
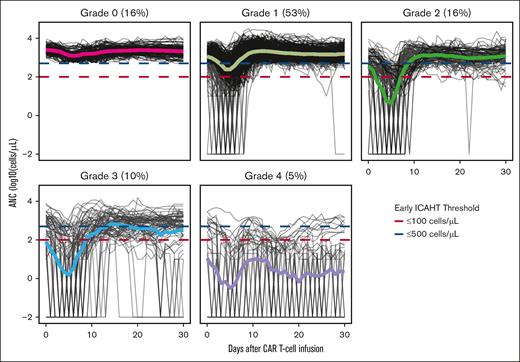
Most patients experienced CRS (n = 516 [75%]), including 35 (5%) who experienced grade ≥3 CRS and 33 (5%) who experienced IEC-HS. Two-hundred eighty patients (41%) experienced neurotoxicity, including 208 (30%) who experienced grade ≥2 neurotoxicity. Any grade eICAHT occurred in 579 patients (84%), including grades 1, 2, 3, and 4 in 366 (53%), 111 (16%), 67 (10%), and 36 (5%) patients, respectively (Figure 1). Between days 0 and 30 after CAR T-cell infusion, 584 (85%) received granulocyte colony-stimulating factors (G-CSFs), and 2 patients received thrombopoietin receptor agonists. Severe (grade 3-4) eICAHT occurred in 19 patients (12%) with indolent NHL, 35 (11%) with aggressive NHL, 36 (36%) with ALL, and 12 (10%) with multiple myeloma or plasma cell leukemia. The median follow-up was 32.9 months (interquartile range, 12.3-63.0). The median OS was 34.6 months (95% confidence interval [CI], 27.1-42.6); 3-year OS was 48.5% (95% CI, 44.1-53.2).
Longitudinal ANC data categorized by eICAHT grade. Solid lines represent means of each grade. Blue and red dashed lines represent ANC cutoffs for eICAHT (ANC ≤500 and ≤100 cells per μL, respectively).
Longitudinal ANC data categorized by eICAHT grade. Solid lines represent means of each grade. Blue and red dashed lines represent ANC cutoffs for eICAHT (ANC ≤500 and ≤100 cells per μL, respectively).
Predictors of severe eICAHT
We applied univariate logistic regression to evaluate the association between severe eICAHT and 58 variables, including patient and disease characteristics, preinfusion and postinfusion laboratory values, and CAR T-cell peak toxicity grades. Because MCL has been previously reported to be associated with severe eICAHT,8 this disease type was classified separately from other indolent NHL. The results of the univariate logistic regression models are shown in Table 2 and supplemental Figure 2, and the summary statistics for these variables are shown in supplemental Table 3.
Univariate logistic regression model evaluations of predictors of grade 3 to 4 eICAHT
| Characteristic . | N . | Event, n . | OR . | 95% CI . | P value . |
|---|---|---|---|---|---|
| Age | 691 | 102 | 0.97 | 0.96-0.98 | <.001 |
| Sex | 691 | 102 | |||
| Female | — | — | |||
| Male | 0.84 | 0.55-1.30 | .44 | ||
| Race/ethnicity | 691 | 102 | |||
| White | — | — | |||
| Non-Hispanic Black or African American | 1.19 | 0.27-3.73 | .79 | ||
| Other | 0.69 | 0.32-1.31 | .29 | ||
| Prior HCT | 691 | 102 | |||
| No | — | — | |||
| Yes | 1.25 | 0.80-1.92 | .31 | ||
| Disease | 691 | 102 | |||
| Aggressive NHL | — | — | |||
| Other indolent NHL | 1.19 | 0.61-2.23 | .60 | ||
| ALL | 4.62 | 2.70-7.95 | <.001 | ||
| MCL | 0.98 | 0.28-2.65 | .97 | ||
| MM/PCL | 0.90 | 0.43-1.75 | .76 | ||
| Bone marrow involvement by morphology, log10, % | 424 | 65 | 1.43 | 1.22-1.68 | <.001 |
| Bone marrow involvement by flow cytometry, log10, % | 439 | 69 | 1.52 | 1.29-1.81 | <.001 |
| Received bridging therapy | 691 | 102 | |||
| No | — | — | |||
| Yes | 2.05 | 1.29-3.35 | .003 | ||
| Lymphodepletion regimen | 691 | 102 | |||
| Low-intensity Cy/Flu | — | — | |||
| High-intensity Cy/Flu | 1.45 | 0.93-2.25 | .10 | ||
| Other | 2.44 | 1.03-5.35 | .031 | ||
| Lymphodepletion regimen, low-dose Cy/Flu vs other | 691 | 102 | |||
| Low-intensity Cy/Flu | — | — | |||
| Other | 1.56 | 1.02-2.38 | .040 | ||
| Total CAR T-cell dose, log10, × 106 cells | 691 | 102 | 0.42 | 0.29-0.60 | <.001 |
| CAR target | 691 | 102 | |||
| BCMA | — | — | |||
| CD19 | 1.78 | 0.98-3.54 | .076 | ||
| CD20 | 0.64 | 0.14-2.15 | .51 | ||
| Costimulatory domain | 691 | 102 | |||
| 4-1BB | — | — | |||
| CD28 | 1.02 | 0.63-1.62 | .93 | ||
| Dual 4-1BB and CD28 | 0.40 | 0.09-1.13 | .13 | ||
| Peak CRS grade | 691 | 102 | 2.01 | 1.60-2.56 | <.001 |
| Peak neurotoxicity grade | 691 | 102 | 1.51 | 1.29-1.76 | <.001 |
| IEC-HS | 689 | 102 | |||
| No | — | — | |||
| Yes | 3.12 | 1.41-6.51 | .003 | ||
| Pre-LD ANC, log10, × 103 cells per μL | 690 | 102 | 0.15 | 0.08-0.24 | <.001 |
| D +3 ANC, log10, × 103 cells per μL | 691 | 102 | 0.53 | 0.47-0.61 | <.001 |
| Pre-LD Hb, g/dL | 691 | 102 | 0.61 | 0.52-0.70 | <.001 |
| Pre-LD platelet count, log10, × 103/μL | 691 | 102 | 0.06 | 0.03-0.11 | <.001 |
| D +3 platelet count, log10, × 103/μL | 691 | 102 | 0.06 | 0.03-0.11 | <.001 |
| Pre-LD LDH, log10, U/L | 677 | 101 | 9.95 | 4.72-21.5 | <.001 |
| Pre-LD CRP, log10, mg/L | 439 | 58 | 3.44 | 2.24-5.43 | <.001 |
| D +0 CRP, log10, mg/L | 644 | 85 | 3.24 | 2.21-4.84 | <.001 |
| D +3 CRP, log10, mg/L | 643 | 91 | 3.54 | 2.30-5.65 | <.001 |
| D +5 CRP, log10, mg/L | 444 | 81 | 3.36 | 2.10-5.57 | <.001 |
| D +7 CRP, log10, mg/L | 549 | 77 | 3.73 | 2.36-6.08 | <.001 |
| Peak CRP, log10, mg/L | 683 | 98 | 16.7 | 7.91-38.4 | <.001 |
| Pre-LD ferritin, log10, ng/mL | 486 | 69 | 5.57 | 3.42-9.51 | <.001 |
| D +0 ferritin, log10, ng/mL | 658 | 90 | 9.87 | 5.82-17.5 | <.001 |
| D +3 ferritin, log10, ng/mL | 649 | 94 | 9.24 | 5.65-15.8 | <.001 |
| D +5 ferritin, log10, ng/mL | 445 | 80 | 6.74 | 4.17-11.5 | <.001 |
| D +7 ferritin, log10, ng/mL | 555 | 81 | 5.49 | 3.65-8.57 | <.001 |
| Peak ferritin, log10, ng/mL | 691 | 102 | 6.90 | 4.80-10.2 | <.001 |
| Pre-LD IL-6, log10, pg/mL | 130 | 14 | 4.13 | 1.58-11.4 | .004 |
| D +0 IL-6, log10, pg/mL | 336 | 41 | 2.95 | 1.68-5.24 | <.001 |
| D +3 IL-6, log10, pg/mL | 476 | 71 | 1.44 | 1.11-1.86 | .005 |
| D +5 IL-6, log10, pg/mL | 356 | 60 | 1.56 | 1.18-2.07 | .002 |
| D +7 IL-6, log10, pg/mL | 413 | 59 | 2.13 | 1.53-2.99 | <.001 |
| Peak IL-6, log10, pg/mL | 551 | 79 | 2.60 | 2.00-3.43 | <.001 |
| Pre-LD fibrinogen, log10, mg/dL | 378 | 60 | 3.87 | 0.53-29.4 | .19 |
| D +0 fibrinogen, log10, mg/dL | 606 | 79 | 7.19 | 1.13-46.4 | .037 |
| D +3 fibrinogen, log10, mg/dL | 613 | 85 | 3.85 | 0.66-23.2 | .14 |
| D +5 fibrinogen, log10, mg/dL | 404 | 69 | 0.80 | 0.16-4.14 | .79 |
| D +7 fibrinogen, log10, mg/dL | 508 | 70 | 0.68 | 0.17-2.78 | .58 |
| Nadir fibrinogen, log10, mg/dL | 661 | 94 | 0.07 | 0.03-0.18 | <.001 |
| Pre-LD D-dimer, log10, mg/L FEU | 341 | 57 | 2.97 | 1.50-5.92 | .002 |
| D +0 D-dimer, log10, mg/L FEU | 557 | 72 | 4.85 | 2.65-9.00 | <.001 |
| D +3 D-dimer, log10, mg/L FEU | 561 | 79 | 4.20 | 2.41-7.41 | <.001 |
| D +5 D-dimer, log10, mg/L FEU | 373 | 63 | 4.01 | 2.21-7.44 | <.001 |
| D +7 D-dimer, log10, mg/L FEU | 455 | 60 | 3.58 | 2.09-6.19 | <.001 |
| Peak D-dimer, log10, mg/L FEU | 651 | 92 | 4.69 | 3.00-7.49 | <.001 |
| Pre-LD PTT, log10, s | 455 | 71 | 5.04 | 0.18-114 | .32 |
| Day +0 PTT, log10, s | 502 | 69 | 7.63 | 0.43-111 | .14 |
| Day +3 PTT, log10, s | 494 | 74 | 7.22 | 1.05-45.2 | .038 |
| Day +5 PTT, log10, s | 298 | 55 | 4.70 | 0.78-26.0 | .079 |
| Day +7 PTT, log10, s | 402 | 59 | 4.81 | 0.67-30.0 | .10 |
| Peak PTT, log10, s | 606 | 91 | 9.09 | 2.90-27.9 | <.001 |
| Characteristic . | N . | Event, n . | OR . | 95% CI . | P value . |
|---|---|---|---|---|---|
| Age | 691 | 102 | 0.97 | 0.96-0.98 | <.001 |
| Sex | 691 | 102 | |||
| Female | — | — | |||
| Male | 0.84 | 0.55-1.30 | .44 | ||
| Race/ethnicity | 691 | 102 | |||
| White | — | — | |||
| Non-Hispanic Black or African American | 1.19 | 0.27-3.73 | .79 | ||
| Other | 0.69 | 0.32-1.31 | .29 | ||
| Prior HCT | 691 | 102 | |||
| No | — | — | |||
| Yes | 1.25 | 0.80-1.92 | .31 | ||
| Disease | 691 | 102 | |||
| Aggressive NHL | — | — | |||
| Other indolent NHL | 1.19 | 0.61-2.23 | .60 | ||
| ALL | 4.62 | 2.70-7.95 | <.001 | ||
| MCL | 0.98 | 0.28-2.65 | .97 | ||
| MM/PCL | 0.90 | 0.43-1.75 | .76 | ||
| Bone marrow involvement by morphology, log10, % | 424 | 65 | 1.43 | 1.22-1.68 | <.001 |
| Bone marrow involvement by flow cytometry, log10, % | 439 | 69 | 1.52 | 1.29-1.81 | <.001 |
| Received bridging therapy | 691 | 102 | |||
| No | — | — | |||
| Yes | 2.05 | 1.29-3.35 | .003 | ||
| Lymphodepletion regimen | 691 | 102 | |||
| Low-intensity Cy/Flu | — | — | |||
| High-intensity Cy/Flu | 1.45 | 0.93-2.25 | .10 | ||
| Other | 2.44 | 1.03-5.35 | .031 | ||
| Lymphodepletion regimen, low-dose Cy/Flu vs other | 691 | 102 | |||
| Low-intensity Cy/Flu | — | — | |||
| Other | 1.56 | 1.02-2.38 | .040 | ||
| Total CAR T-cell dose, log10, × 106 cells | 691 | 102 | 0.42 | 0.29-0.60 | <.001 |
| CAR target | 691 | 102 | |||
| BCMA | — | — | |||
| CD19 | 1.78 | 0.98-3.54 | .076 | ||
| CD20 | 0.64 | 0.14-2.15 | .51 | ||
| Costimulatory domain | 691 | 102 | |||
| 4-1BB | — | — | |||
| CD28 | 1.02 | 0.63-1.62 | .93 | ||
| Dual 4-1BB and CD28 | 0.40 | 0.09-1.13 | .13 | ||
| Peak CRS grade | 691 | 102 | 2.01 | 1.60-2.56 | <.001 |
| Peak neurotoxicity grade | 691 | 102 | 1.51 | 1.29-1.76 | <.001 |
| IEC-HS | 689 | 102 | |||
| No | — | — | |||
| Yes | 3.12 | 1.41-6.51 | .003 | ||
| Pre-LD ANC, log10, × 103 cells per μL | 690 | 102 | 0.15 | 0.08-0.24 | <.001 |
| D +3 ANC, log10, × 103 cells per μL | 691 | 102 | 0.53 | 0.47-0.61 | <.001 |
| Pre-LD Hb, g/dL | 691 | 102 | 0.61 | 0.52-0.70 | <.001 |
| Pre-LD platelet count, log10, × 103/μL | 691 | 102 | 0.06 | 0.03-0.11 | <.001 |
| D +3 platelet count, log10, × 103/μL | 691 | 102 | 0.06 | 0.03-0.11 | <.001 |
| Pre-LD LDH, log10, U/L | 677 | 101 | 9.95 | 4.72-21.5 | <.001 |
| Pre-LD CRP, log10, mg/L | 439 | 58 | 3.44 | 2.24-5.43 | <.001 |
| D +0 CRP, log10, mg/L | 644 | 85 | 3.24 | 2.21-4.84 | <.001 |
| D +3 CRP, log10, mg/L | 643 | 91 | 3.54 | 2.30-5.65 | <.001 |
| D +5 CRP, log10, mg/L | 444 | 81 | 3.36 | 2.10-5.57 | <.001 |
| D +7 CRP, log10, mg/L | 549 | 77 | 3.73 | 2.36-6.08 | <.001 |
| Peak CRP, log10, mg/L | 683 | 98 | 16.7 | 7.91-38.4 | <.001 |
| Pre-LD ferritin, log10, ng/mL | 486 | 69 | 5.57 | 3.42-9.51 | <.001 |
| D +0 ferritin, log10, ng/mL | 658 | 90 | 9.87 | 5.82-17.5 | <.001 |
| D +3 ferritin, log10, ng/mL | 649 | 94 | 9.24 | 5.65-15.8 | <.001 |
| D +5 ferritin, log10, ng/mL | 445 | 80 | 6.74 | 4.17-11.5 | <.001 |
| D +7 ferritin, log10, ng/mL | 555 | 81 | 5.49 | 3.65-8.57 | <.001 |
| Peak ferritin, log10, ng/mL | 691 | 102 | 6.90 | 4.80-10.2 | <.001 |
| Pre-LD IL-6, log10, pg/mL | 130 | 14 | 4.13 | 1.58-11.4 | .004 |
| D +0 IL-6, log10, pg/mL | 336 | 41 | 2.95 | 1.68-5.24 | <.001 |
| D +3 IL-6, log10, pg/mL | 476 | 71 | 1.44 | 1.11-1.86 | .005 |
| D +5 IL-6, log10, pg/mL | 356 | 60 | 1.56 | 1.18-2.07 | .002 |
| D +7 IL-6, log10, pg/mL | 413 | 59 | 2.13 | 1.53-2.99 | <.001 |
| Peak IL-6, log10, pg/mL | 551 | 79 | 2.60 | 2.00-3.43 | <.001 |
| Pre-LD fibrinogen, log10, mg/dL | 378 | 60 | 3.87 | 0.53-29.4 | .19 |
| D +0 fibrinogen, log10, mg/dL | 606 | 79 | 7.19 | 1.13-46.4 | .037 |
| D +3 fibrinogen, log10, mg/dL | 613 | 85 | 3.85 | 0.66-23.2 | .14 |
| D +5 fibrinogen, log10, mg/dL | 404 | 69 | 0.80 | 0.16-4.14 | .79 |
| D +7 fibrinogen, log10, mg/dL | 508 | 70 | 0.68 | 0.17-2.78 | .58 |
| Nadir fibrinogen, log10, mg/dL | 661 | 94 | 0.07 | 0.03-0.18 | <.001 |
| Pre-LD D-dimer, log10, mg/L FEU | 341 | 57 | 2.97 | 1.50-5.92 | .002 |
| D +0 D-dimer, log10, mg/L FEU | 557 | 72 | 4.85 | 2.65-9.00 | <.001 |
| D +3 D-dimer, log10, mg/L FEU | 561 | 79 | 4.20 | 2.41-7.41 | <.001 |
| D +5 D-dimer, log10, mg/L FEU | 373 | 63 | 4.01 | 2.21-7.44 | <.001 |
| D +7 D-dimer, log10, mg/L FEU | 455 | 60 | 3.58 | 2.09-6.19 | <.001 |
| Peak D-dimer, log10, mg/L FEU | 651 | 92 | 4.69 | 3.00-7.49 | <.001 |
| Pre-LD PTT, log10, s | 455 | 71 | 5.04 | 0.18-114 | .32 |
| Day +0 PTT, log10, s | 502 | 69 | 7.63 | 0.43-111 | .14 |
| Day +3 PTT, log10, s | 494 | 74 | 7.22 | 1.05-45.2 | .038 |
| Day +5 PTT, log10, s | 298 | 55 | 4.70 | 0.78-26.0 | .079 |
| Day +7 PTT, log10, s | 402 | 59 | 4.81 | 0.67-30.0 | .10 |
| Peak PTT, log10, s | 606 | 91 | 9.09 | 2.90-27.9 | <.001 |
BCMA, B-cell maturation antigen; FEU, fibrinogen equivalent units; Hb, hemoglobin; LD, lymphodepletion; MM/PCL, multiple myeloma/plasma cell leukemia; PTT, partial thromboplastin time.
Boldface indicates P values < 0.05.
Baseline patient factors associated with severe eICAHT included ALL (reference, aggressive NHL, odds ratio [OR], 4.62; 95% CI, 2.70-7.95; P < .001) and greater bone marrow disease involvement (by morphology, OR, 1.43 per log10 increase in percentage; 95% CI, 1.22-1.68; P < .001; by flow cytometry, OR, 1.52 per log10 increase in percentage; 95% CI, 1.29-1.81; P < .001). Older age was associated with lower odds of severe eICAHT (OR, 0.97 per year increase; 95% CI, 0.96-0.98). Receipt of bridging therapy was associated with increased odds of severe eICAHT (OR, 2.05; 95% CI, 1.29-3.35; P = .003). Low-intensity Cy/Flu LD was associated with decreased odds of severe eICAHT (OR, 0.64; 95% CI, 0.42-0.98; P = .040). Higher CAR T-cell dose was associated with decreased odds of severe eICAHT (OR, 0.42 per log10 × 106 cell increase; 95% CI, 0.29-0.60; P < .001).
Pre-LD laboratory factors associated with grade 3 to 4 ICAHT included lower ANC (OR, 6.67 per log10 × 103 cells per μL decrease; 95% CI, 4.17-12.5; P < .001) and higher LDH (OR, 9.95 per log10U/L increase; 95% CI, 4.72-21.5; P < .001), CRP (OR, 3.44 per log10 mg/L increase; 95% CI, 2.24-5.43; P < .001), ferritin (OR, 5.57 per log10 ng/mL increase; 95% CI, 3.42-9.51; P < .001), interleukin-6 (IL-6; OR, 4.13 per log10 pg/mL increase; 95% CI, 1.58-11.4; P = .004), and D-dimer (OR, 2.97 per log10 mg/L fibrinogen equivalent unit [FEU] increase; 95% CI, 1.50-5.92; P = .001).
Early postinfusion laboratory factors most strongly associated with grade 3 to 4 ICAHT included higher day +3 CRP (OR, 3.54 per log10 mg/L; 95% CI, 2.30-5.65; P < .001), day +3 ferritin (OR, 9.24 per log10mg/L; 95% CI, 5.65-15.8; P < .001), and day +3 D-dimer (OR, 4.20 per log10 mg/L FEU increase; 95% CI, 2.41-7.41; P < .001). Peak CRS grade (OR, 2.01 per grade increase; 95% CI, 1.60-2.56; P < .001), peak neurotoxicity grade (OR, 1.51 per grade increase; 95% CI, 1.29-1.76; P < .001), and occurrence of IEC-HS (OR, 3.12; 95% CI, 1.41-6.51; P = .003) were also associated with grade 3 to 4 ICAHT.
Predictive models of severe eICAHT
We first assessed the classification performance of the CAR-HEMATOTOX low- vs high-risk categories to predict severe eICAHT in the test set; its sensitivity, specificity, and AUROC in predicting grade 3 to 4 ICAHT were 97%, 44%, and 0.70, respectively (Table 3; supplemental Figure 3). Because the CAR-HEMATOX does not generate probabilities, calibration could not be assessed.
Sensitivity, specificity, AUROC, and Brier scores of the CAR-HEMATOTOX score, eIPMPre, and eIPMPost in the test set
| . | CAR-HEMATOTOX . | eIPMPre . | eIPMPost . |
|---|---|---|---|
| Sensitivity | 97% | 94% | 84% |
| Specificity | 44% | 68% | 78% |
| AUROC | 0.70 | 0.87 | 0.88 |
| Brier score | N/A | 0.087 | 0.085 |
| . | CAR-HEMATOTOX . | eIPMPre . | eIPMPost . |
|---|---|---|---|
| Sensitivity | 97% | 94% | 84% |
| Specificity | 44% | 68% | 78% |
| AUROC | 0.70 | 0.87 | 0.88 |
| Brier score | N/A | 0.087 | 0.085 |
The Brier score (mean of squared differences between predicted and observed events) cannot be computed for the CAR-HEMATOTOX score because it does not generate predicted probabilities. N/A, not applicable.
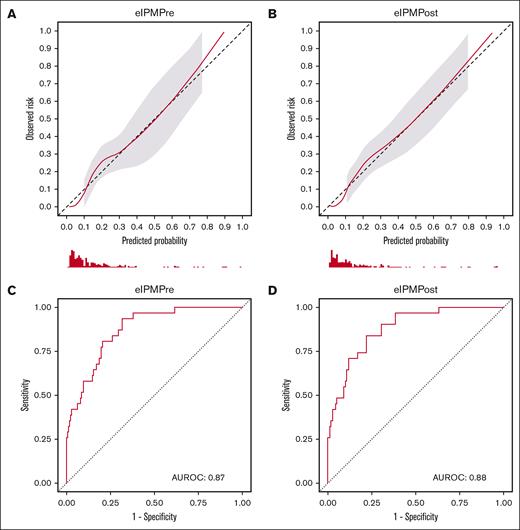
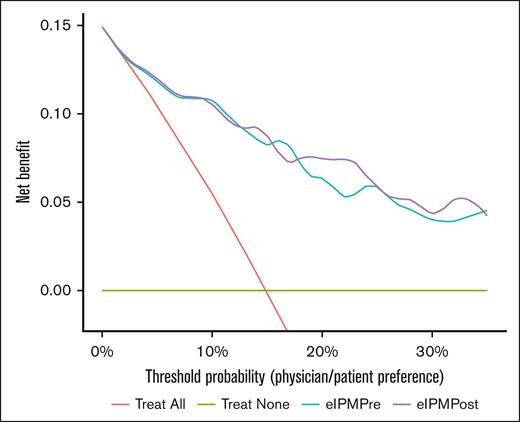
Because the CAR-HEMATOTOX score was developed before the development of ICAHT criteria, we developed and validated new models predictive of severe eICAHT specifically, using modern modeling techniques based on the TRIPOD statement20 and the tidymodels framework.22 We trained a multivariable least absolute shrinkage and selection operator-regularized logistic regression model including disease type (ALL vs other), pre-LD ANC, platelet count, LDH, and ferritin (eIPMPre; supplemental Table 4). When applied to the test set, eIPMPre showed near-perfect calibration (Figure 2A; supplemental Figure 4A) and demonstrated high discrimination with an AUROC of 0.87 (Table 3; Figure 2C). Sensitivity and specificity of the model based on an optimal cut point using the Youden index were 94% and 68%, respectively (Table 3). In decision curve analysis, eIPMPre exhibited higher net benefit than a “treat all” approach across all probability thresholds starting at 3% (Figure 3). Neither the addition of receipt of bridging therapy nor LD regimen (low-dose Cy/Flu vs other) significantly improved the model (supplemental Table 5; supplemental Figures 5-7). The threshold-performance plot for eIPMPre is shown in supplemental Figure 8A.
Calibration and receiver operating characteristic (ROC) curves of eIPMPre and eIPMPost as assessed on the test set. (A-B) Calibration curves of eIPMPre (A) and eIPMPost (B). (C-D) ROC curves of eIPMPre (C) and eIPMPost (D).
Calibration and receiver operating characteristic (ROC) curves of eIPMPre and eIPMPost as assessed on the test set. (A-B) Calibration curves of eIPMPre (A) and eIPMPost (B). (C-D) ROC curves of eIPMPre (C) and eIPMPost (D).
Decision curves of “treat all,” “treat none,” eIPMPre, and eIPMPost approaches to grade 3 to 4 ICAHT. By visualizing the net benefit (proportion of true positives, “weighted” proportion of false positives vs false negatives) of various strategies across a range of probability thresholds, decision curves enable comparison of the clinical utility (net benefit) of these approaches. Across all probability thresholds, a clinical approach based on either eIPM confers greater clinical net benefit than a “treat all” or “treat none” approach. At higher probability thresholds >20% (ie, in situations when the costs of false positives are higher than false negatives), a clinical approach based on eIPMPost confers greater clinical net benefit than that based on eIPMPre.
Decision curves of “treat all,” “treat none,” eIPMPre, and eIPMPost approaches to grade 3 to 4 ICAHT. By visualizing the net benefit (proportion of true positives, “weighted” proportion of false positives vs false negatives) of various strategies across a range of probability thresholds, decision curves enable comparison of the clinical utility (net benefit) of these approaches. Across all probability thresholds, a clinical approach based on either eIPM confers greater clinical net benefit than a “treat all” or “treat none” approach. At higher probability thresholds >20% (ie, in situations when the costs of false positives are higher than false negatives), a clinical approach based on eIPMPost confers greater clinical net benefit than that based on eIPMPre.
We next hypothesized that the inclusion of postinfusion inflammatory markers, which reflect in vivo CAR T-cell–induced inflammation that can drive hematotoxicity,13,24 could improve eIPMPre. We specifically assessed early postinfusion markers because their timing can enable clinical intervention. We thus modified eIPMPre by replacing pre-LD ferritin with day +3 ferritin (eIPMPost; supplemental Table 4). When applied to our test set, eIPMPost showed comparable calibration (Figure 2B; supplemental Figure 4A) and higher discrimination than eIPMPre (AUROC, 0.88; Table 3; Figure 2D). The Youden-based sensitivity and specificity of eIPMPost were 84% and 78%, respectively (Table 3). Decision curve analysis showed higher net benefit of eIPMPost-based intervention than eIPMPre at probability thresholds >20% (Figure 3). Neither the addition of receipt of bridging therapy, day +3 CRP, or day +3 D-dimer nor the substitution of day +3 ANC and platelet count for the pre-LD values significantly improved model performance (supplemental Table 5; supplemental Figures 5-7). The threshold-performance plot for eIPMPost is shown in supplemental Figure 8B.
In the test set, the probabilities of severe eICAHT predicted by both eIPMs were strongly associated with the duration of neutropenia (supplemental Figure 9) and OS (supplemental Table 6). The performance metrics for eIPMPre and eIPMPost were comparable when evaluated in test subsets by ALL vs other disease types; although extreme probabilities remained relatively calibrated in the ALL subgroup, we observed underestimation of the actual risk in the 20% to 30% predicted range (supplemental Table 7; supplemental Figure 10).
Discussion
ICAHT is a major cause of morbidity and mortality after CAR T-cell therapy. Although there is a variety of interventions for ICAHT, including growth factors, infectious prophylaxis, and autologous stem cell boost, their practical use is limited by an inability to accurately predict ICAHT. To address this unmet need, we used a data set of 691 patients undergoing CAR T-cell therapy to generate and robustly validate 2 new models to predict severe (grade 3-4) eICAHT per EHA/EBMT criteria: eIPMPre and eIPMPost.
We performed comprehensive univariate analyses, identifying key factors associated with severe eICAHT. Unexpectedly, univariate modeling showed that higher CAR T-cell doses were associated with lower odds of severe eICAHT. This observation may reflect the administration of lower CAR T-cell doses in patients with ALL specifically than other disease types; patients with ALL with high disease burden treated with our investigational products JCAR014 and JCAR021 received lower doses of CAR T cells to mitigate the risk of severe CRS and ICANS, as described previously.25,26 Preinfusion disease burden as measured by LDH, preinfusion and postinfusion inflammatory markers (CRP, ferritin, and IL-6), and the severity of CRS/neurotoxicity were strongly predictive of grade 3 to 4 eICAHT. This is consistent with prior studies that have implicated systemic inflammation in driving hematotoxicity.2,4-6,8,14 Most of these prior studies, including 1 from our group that demonstrated a strong association between peak CRS/neurotoxicity grade and hematotoxicity, focused on patients receiving commercial or investigational CD19-targeted CAR T-cell products,5,6,8,12 with a smaller number of patients receiving commercial BCMA-targeted CAR T-cell products.4,8,14 In comparing our findings with those reported by Rejeski et al in their analysis of eICAHT in 549 patients with large B-cell lymphoma, MCL, and multiple myeloma undergoing CAR T-cell therapy,8 we similarly found that baseline cytopenias predicted severe eICAHT. A distinguishing feature of our work is the inclusion of patients with indolent NHL and ALL and investigational CAR T-cell products. We were able to confirm associations between systemic inflammation, including CRS/neurotoxicity severity, across a range of products and beyond the CD19 target. We also found that that patients with ALL were at significantly higher risk of developing severe eICAHT, corroborating the association between ALL and hematotoxicity previously described in a meta-analysis by Xia et al.12 In contrast to Rejeski et al,8 we could not confirm an association between MCL and severe eICAHT, although our cohort included only 37 patients with MCL. Furthermore, we found a strong association between severe eICAHT and markers of coagulopathy, including D-dimer levels. CAR T-cell–mediated inflammation, particularly CRS/neurotoxicity and associated endothelial damage, are known to be associated with coagulopathy.24,27-30 Our findings suggest that endothelial damage might also play a role in the pathophysiology of ICAHT.
In addition, we specifically evaluated the ability of the CAR-HEMATOTOX score, which is frequently used to predict hematotoxicity after CAR T-cell therapy,5,8,9,13,14 to predict severe eICAHT. Although the sensitivity of the CAR-HEMATOTOX score (high vs low) was high at 97%, it had very low specificity (44%; ie, prone to false-positive errors) and AUROC (0.70; low/intermediate discrimination). These results were expected because the CAR-HEMATOTOX score had low specificity in the initial validation cohort for the end point of duration of severe neutropenia lasting ≥14 vs <14 days after CAR T-cell therapy,5 not severe eICAHT specifically.
Given the absence of an available model to accurately predict severe eICAHT across disease types, we robustly validated 2 new multivariable models in an independent test set. We found that both eIPMPre (consisting of only pre-LD factors) and eIPMPost (consisting of pre-LD factors and day +3 ferritin) were near-perfectly calibrated in the test set, with eIPMPost having higher discrimination and net benefit at higher probability thresholds. Both models demonstrated greater net benefit than a “treat all” approach across all probability thresholds.
It is important to note that specifying a universal probability threshold based on the eIPMs is not possible or advised, because the acceptable risk depends on the clinical context.31 Instead, to translate the probabilities of severe eICAHT predicted by the eIPMs into clinical practice, providers should inform patients of their risk of severe eICAHT, and decisions should be formulated based on the specific clinical context and physician/patient preferences, including the potential consequences of false positives and false negatives. Decision curve analysis can help assess the clinical utility of predictive models, with the probability threshold on the x-axis representing the level of risk acceptable for a certain intervention or preference (ie, physician/patient preference). For example, administration of G-CSF or the addition of antifungal prophylaxis are generally considered to be low-risk interventions (ie, low cost of a false-positive result), whereas the potential harm of a severe infection is high (ie, high cost of a false-negative result). Thus, a relevant range of probability thresholds for these interventions would be 1% to 10% on the x-axis, (1:99-1:9 on the odds scale). As demonstrated in Figure 3, across most of the 1% to 10% probability threshold range, decisions based on the probabilities of severe eICAHT predicted by the eIPMs demonstrate greater net benefit than a “treat all” approach (ie, administering G-CSF or antifungal prophylaxis for all patients).
To our knowledge, these are the first models predicting severe hematotoxicity as defined specifically by grade 3 to 4 eICAHT. Predictions from the eIPMs can risk stratify patients and guide future studies of possible interventions such as modifying the intensity of LD, offering autologous stem cell boost if cryopreserved stem cells are available, using subsequent allogeneic HCT, and/or using additional infectious prophylaxis (particularly antifungal).7,32,33 Although institutions may have specific guidelines for administering prophylactic or early G-CSF, the eIPMs can inform current clinical practice by guiding providers to add antifungal prophylaxis or plan ahead for subsequent cellular therapies. For example, in a patient with multiple myeloma who has a high probability of severe eICAHT by the eIPMs and has cryopreserved stem cells at another institution, providers could plan ahead for possible autologous stem cell boost by transferring the stem cells to the current institution. Similarly, in a patient with ALL who has a high probability of severe eICAHT, providers could begin the donor search for a consolidative allogeneic HCT.
Strengths of our work include modeling severe eICAHT specifically, training and validation on one of the largest published cohorts of patients treated with CAR T-cell therapies, and transparent reporting in line with TRIPOD recommendations, using modern modeling techniques and allowing for maximum reproducibility. Transparent reporting is especially critical to allow for further external validation and potentially optimize our models. In addition, our models retain the continuous nature of numeric variables instead of dichotomizing them, thus providing greater statistical power and accounting for greater variability.34 Limitations of our model include the incorporation of investigational CAR T-cell products, which may affect generalizability. Because our models were generated with single-center data, validation of our models with an independent external data set will be important, especially given the increasing adoption of CAR T-cell therapy into earlier lines across the field as well as the use of approved products into new indications. Although our models are effective in predicting severe eICAHT, whether it can also predict the consequences of transfusion dependence and infections should be further evaluated. Lastly, late ICAHT (cytopenias beyond day +30) also significantly affects infection risk, transfusion requirements, patient outcomes, and quality of life. Additional studies to predict late ICAHT will be crucial to improve our understanding of this late toxicity and how to effectively mitigate it.
In conclusion, we identified disease type, pre-LD cytopenias, preinfusion and postinfusion inflammation, and lymphodepletion intensity as predictors of grade 3 to 4 eICAHT. We validated 2 predictive models (eIPMPre and eIPMPost) consisting of pre-LD and/or early postinfusion factors to predict grade 3 to 4 eICAHT. Online calculators are available to practitioners to easily generate personalized predictions (https://eipm.fredhutch.org/).
Acknowledgments
The authors thank Monica Gerber from the Fred Hutch Data Science Lab for her assistance in developing the online early immune effector cell–associated hematotoxicity prediction model calculators.
This work was supported by the National Institutes of Health (NIH) National Heart, Lung, and Blood Institute (5T32HL007093 [E.C.L.]), the NIH National Cancer Institute (5T32CA951539 [J.J.H.]; P30 CA15704 [J.G.]), Fundación Española de Hematología y Hemoterapia (V.O.-M.), and Swim Across America (J.G.).
Authorship
Contribution: E.C.L. conceived of and designed the study, collected data, analyzed and interpreted data, and wrote the manuscript; J.G. conceived of and designed the study, analyzed and interpreted data, reviewed and edited the manuscript, and provided critical oversight; E.C.L., J.J.H., A.J.P., V.O.-M., and A.A. collected data; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: E.C.L. reports consultancy fees from Glass Health. V.O.-M. reports honoraria from Kite Pharma, Celgene, a Bristol Myers Squibb (BMS) company, and Janssen; travel grants from Kite Pharma, Celgene, a BMS company, Janssen, Roche, and Takeda; and consultancy fees from Celgene, a BMS company, Janssen, Pfizer, Novartis, and Miltenyi. N.W. reports membership on advisory committee for Mustang Bio. J.A.H. reports consultancy fees from AlloVir, Moderna, Deverra, Century Therapeutics, CSL Behring, GeoVax, Grifols, Karius, and Takeda; and research funding from AlloVir, Deverra, GeoVax, Takeda, and Merck. R.B. reports consultancy fees from BMS, Adaptive Biotech, GSK, Karyopharm, Legend Biotech, Janssen, Genentech, SparkCures, Sanofi, and Caribou; and research funding from Pfizer, Pack Health, and Novartis. A.J.C. reports consultancy fees from BMS and Adaptive, and research funding from Adaptive Biotechnologies, Harpoon, Nektar, BMS, Janssen, Sanofi, and AbbVie. D.G. reports research funding from Cellectar Biosciences, SpringWorks Therapeutics, Sanofi, Janssen Biotech, Seattle Genetics, and Juno Therapeutics, a BMS company; consultancy fees from Celgene, a BMS company, Ensoma, Janssen Biotech, and Seattle Genetics; membership on an entity's board of directors or advisory committees for GlaxoSmithKline; and patents and royalties in Juno Therapeutics, a BMS company. A.K.G. is a current holder of stock options in a privately held company, Compliment Corporation; reports consultancy fees from Incyte, Kite, MorphoSys/Incyte, ADCT, Acrotech, Merck, Karyopharm, Servier, BeiGene, Cellectar, Janssen, SeaGen, Epizyme, I-Mab Bio, Gilead, Genentech, Lilly, Caribou, and Fresenius Kabi; and reports research funding from Merck, I-Mab Bio, IgM Bio, Takeda, Gilead, AstraZeneca, Agios, Janssen, BMS, SeaGen, Teva, and Genmab. C.P. reports consultancy fees from BeiGene, Seattle Genetics, and Acrotech; and research funding from Incyte. M.S. reports consultancy fees from Fate Therapeutics, Genmab, MorphoSys/Incyte, Eli Lilly, BMS, Genentech, Kite, a Gilead company, AbbVie, Mustang Bio, ADC Therapeutics, BeiGene, AstraZeneca, Pharmacyclics, Janssen, MEI Pharma, and Regeneron; and research funding from Genmab, Vincerx, MorphoSys/Incyte, BMS, Genentech, AbbVie, Mustang Bio, BeiGene, AstraZeneca, Pharmacyclics, and TG Therapeutics. A.V.H. reports honoraria from Novartis, BMS, and Nektar Therapeutics; and research funding from BMS, Nektar Therapeutics, and Juno Therapeutics, a BMS company. B.G.T. reports consultancy fees from Mustang Bio and Proteios Technology; reports research funding from Mustang Bio and Juno Therapeutics, a BMS company; reports patents and royalties from Mustang Bio; and is a current holder of stock options in a privately held company, Proteios Technology. E.L.K. reports research funding from Juno Therapeutics, a BMS company. L.I. is a current equity holder in a publicly traded company, Mustang Bio. A.G.C. reports research funding from Juno Therapeutics. R.D.C. reports consultancy fees and honoraria from Amgen, Pfizer, Jazz, and Kite, a Gilead company; reports honoraria from Amgen, Autolus, Pfizer, Jazz, and Kite/Gilead; reports research funding from Amgen, Merck, Incyte, Pfizer, Servier, Vanda Pharmaceuticals, Kite/Gilead; reports membership on an entity's board of directors or advisory committees for Autolus and PeproMene Bio; and his spouse was employed by and owned stock in Seagen within the last 24 months. F.M. reports research funding from ExCellThera Inc. C.J.T. reports research funding from Juno Therapeutics, a BMS company, Nektar Therapeutics, and Nanostring; scientific advisory board fees from Caribou Biosciences, T-CURX, Myeloid Therapeutics, ArsenalBio, Cargo Therapeutics, Celgene, a BMS company, Differentia Bio, eGlint, and Advesya; data and safety monitoring board member fees from Kyverna; ad hoc advisory role/consulting (last 12 months) fees from Prescient Therapeutics, Century Therapeutics, IGM Biosciences, AbbVie, Boxer Capital, and Novartis; stock options in Eureka Therapeutics, Caribou Biosciences, Myeloid Therapeutics, ArsenalBio, Cargo Therapeutics, and eGlint; and the right to receive payment as an inventor on patents (intellectual property) related to CAR T-cell therapy. D.G.M. reports consultancy fees from A2 Biotherapeutics, Juno Therapeutics, Janssen, Legend Biotech, Mustang Bio, Novartis, Incyte, Gilead Sciences, Kite, a Gilead Sciences company, Pharmacyclics, Umoja, Celgene, a BMS company, Genentech, MorphoSys, BMS, Amgen, Navan Technologies, and BioLineRx; is a current holder of stock options in a privately-held companies, A2 Biotherapeutics and Navan Technologies; reports honoraria from A2 Biotherapeutics, Juno Therapeutics, Janssen, Legend Biotech, Mustang Bio, Novartis, Incyte, Gilead Sciences, Kite, a Gilead Sciences company, Pharmacyclics, Umoja, Celgene, a BMS company, Genentech, MorphoSys, BMS, Amgen, and Navan Technologies; reports membership of the scientific advisory board for A2 Biotherapeutics, Navan Technologies, and Chimeric Therapeutics; reports patents and royalties from Juno Therapeutics regarding the rights to royalties from Fred Hutch for patents licensed to Juno Therapeutics, a BMS company; reports research funding from Juno Therapeutics, a BMS company, Legend Biotech, Kite, a Gilead Sciences company, Celgene, a BMS company, and BMS; reports membership in CAR T steering committee in Lyell Immunopharma; reports membership in a scientific review committee of the Research Scholars Program in Hematologic Malignancies with Gilead Sciences; reports membership on an entity's board of directors or advisory committees in and participation on data safety monitory boards of Celgene, a BMS company, and BioLineRx; reports chair and membership of the lymphoma steering committee in Genentech; reports membership of the JCAR017 EAP-001 safety review committee, CLL strategic council, and the JCAR017-BCM-03 scientific steering committee under BMS; reports membership in clinical advisory board, CD19/CD20 bispecific CAR T-Cell Therapy Program in ImmPACT Bio; and membership in clinical advisory board for Interius. J.G. reports consultancy fees from Kite Pharma, MorphoSys, Legend Biotech, Janssen, and Sobi; honoraria from Kite Pharma, Legend Biotech, Janssen, and Sobi; research funding from MorphoSys, Angiocrine Bioscience, Celgene, a BMS company, Juno Therapeutics, a BMS company, and Sobi; and independent data review committee fees from Century Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Jordan Gauthier, Clinical Research Division, Fred Hutchinson Cancer Center, 1100 Fairview Ave N, Mail Stop D3-100, Seattle, WA 98109; email: jgauthier@fredhutch.org.
References
Author notes
A deidentified data set and a Docker application programming interface for external model validation are available on request from the corresponding author, Jordan Gauthier (jgauthier@fredhutch.org).
The full-text version of this article contains a data supplement.