Key Points
Congenital T-cell activation in patients with CTLA4 deficiency impairs transitional-to-FO B-cell maturation.
FO B-cell maturation can be rescued in CTLA4 deficiency by CTLA4-replacement therapy in vivo.
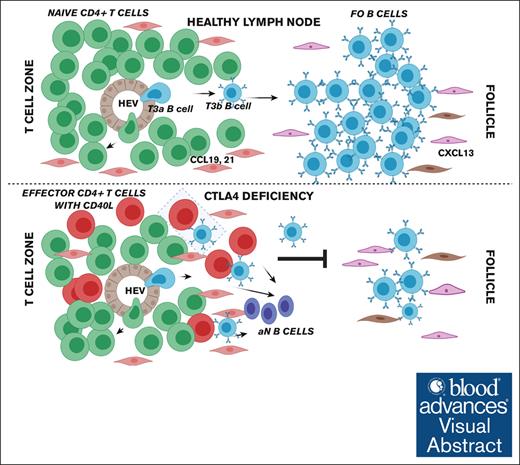
Visual Abstract
Patients with cytotoxic T-lymphocyte–associated protein 4 (CTLA4) deficiency exhibit profound humoral immune dysfunction, yet the basis for the B-cell defect is not known. We observed a marked reduction in transitional-to-follicular (FO) B-cell development in patients with CTLA4 deficiency, correlating with decreased CTLA4 function in regulatory T cells, increased CD40L levels in effector CD4+ T cells, and increased mammalian target of rapamycin complex 1 (mTORC1) signaling in transitional B cells (TrBs). Treatment of TrBs with CD40L was sufficient to induce mTORC1 signaling and inhibit FO B-cell maturation in vitro. Frequent cell-to-cell contacts between CD40L+ T cells and immunoglobulin D–positive CD27– B cells were observed in patient lymph nodes. FO B-cell maturation in patients with CTLA4 deficiency was partially rescued after CTLA4 replacement therapy in vivo. We conclude that functional regulatory T cells and the containment of excessive T-cell activation may be required for human TrBs to mature and attain metabolic quiescence at the FO B-cell stage.
Introduction
Using a cohort of patients with hyperactivating PIK3CD mutations causing activated PI3K-δ syndrome, we have shown that constitutive mammalian target of rapamycin complex 1 (mTORC1) signaling in human transitional B cells (TrBs) impairs development before the follicular (FO) B-cell stage.1 Whether extrinsic signals also influence transitional-to-FO B-cell development in humans is not known.
Cytotoxic T-lymphocyte–associated protein 4 (CTLA4) is a critical negative regulator of T-cell costimulation that is constitutively expressed on T regulatory cells (Tregs) and activated effector T cells. CTLA4 deficiency was first described in 2014 as an autosomal-dominant condition of congenital Treg dysfunction and constitutive T-cell hyperactivation with variable clinical penetrance.2-4 Although CTLA4 expression is restricted to T cells in humans, patients with CTLA4 deficiency clinically have both cellular and humoral immune dysfunction, including decreased circulating B cells, hypogammaglobulinemia, recurrent infections, and robust autoimmune pathology.4 There is no evidence for CTLA4 expression in nonmalignant human B-lineage cells, including B cells from patients with CTLA4 deficiency,2 and interestingly mice with the engineered homozygous deletion of Ctla4 have no reported attenuation of B-cell numbers or function.
The mechanism of T-cell–mediated autoimmune disease in CTLA4 deficiency stems logically from the role of CTLA4 in negatively regulating T-cell activation and maintaining peripheral T-cell tolerance.5,6 In contrast, the mechanism of B-cell dysfunction in CTLA4 deficiency remains poorly understood. Here, using a cohort of patients with CTLA4 deficiency, including at time points before and after therapy with CTLA4 immunoglobulin, we show that functional Tregs are required for the acquisition of metabolic quiescence at the mature FO B-cell stage.
Methods
Patients
This research was approved by the Mass General Brigham institutional review board. Patients with CTLA4 mutations by next-generation sequencing were included, and CTLA4 deficiency was validated using intracellular flow cytometry to assay total cellular CTLA4 protein levels and CTLA4 functional analysis by CD80 transendocytosis in CD4+FOXP3+ Tregs (Figure 2). Members of 1 family were either homozygous or heterozygous for a 3′ untranslated region (UTR) variant in CTLA4, and the functional relevance of this variant was validated, in part, by establishing that these individuals expressed extremely low levels (median fluorescence intensity [MFI] of <103) of total cellular CTLA4 (as established by intracellular flow cytometry for this protein in CD4+FOXP3+ Tregs; supplemental Figure 4). The exact numbers of patients with CTLA4 deficiency and healthy controls used per assay are provided in the figure legends 1-4 and 6 and supplemental Table 1.
Increased frequency of circulating TrBs and decreased frequency of circulating FO B cells in patients with CTLA4 deficiency. Flow cytometric analysis of peripheral blood B-cell populations in patients with CTLA4 deficiency (n = 11) and healthy controls (n = 16). (A) Representative plots from a healthy control (left) and a patient with CTLA4 deficiency (right, homozygous CTLA4 3′ UTR mutation carrier), shown at 5% of events with gating strategy outlined (full description of B-cell gating strategy in supplemental Figure 1A). (B) Quantification of major B-cell subsets in the peripheral blood, including naïve, marginal zone (MZ), SWM, and DN populations as subset from total CD19+ B cells by the markers IgD and CD27. (C) Quantification of naïve (IgD+CD27–) B-cell subsets in the peripheral blood, including T1/2, T3a, and T3b TrBs, resting mature FO B cells, aN B cells, and MZP B cells as further subsets by the indicated markers. For the CD45RB by CD21 and count by CD73 B-cell subset plots, final IgD+CD27– B-cell frequency was derived as a percentage of the indicated parent gate. Symbols represent unique individuals; bars represent means (± standard deviation [SD]) of all data. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001 or as listed by 2-tailed Mann-Whitney U test. MZP, MZ precursor.
Increased frequency of circulating TrBs and decreased frequency of circulating FO B cells in patients with CTLA4 deficiency. Flow cytometric analysis of peripheral blood B-cell populations in patients with CTLA4 deficiency (n = 11) and healthy controls (n = 16). (A) Representative plots from a healthy control (left) and a patient with CTLA4 deficiency (right, homozygous CTLA4 3′ UTR mutation carrier), shown at 5% of events with gating strategy outlined (full description of B-cell gating strategy in supplemental Figure 1A). (B) Quantification of major B-cell subsets in the peripheral blood, including naïve, marginal zone (MZ), SWM, and DN populations as subset from total CD19+ B cells by the markers IgD and CD27. (C) Quantification of naïve (IgD+CD27–) B-cell subsets in the peripheral blood, including T1/2, T3a, and T3b TrBs, resting mature FO B cells, aN B cells, and MZP B cells as further subsets by the indicated markers. For the CD45RB by CD21 and count by CD73 B-cell subset plots, final IgD+CD27– B-cell frequency was derived as a percentage of the indicated parent gate. Symbols represent unique individuals; bars represent means (± standard deviation [SD]) of all data. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001 or as listed by 2-tailed Mann-Whitney U test. MZP, MZ precursor.
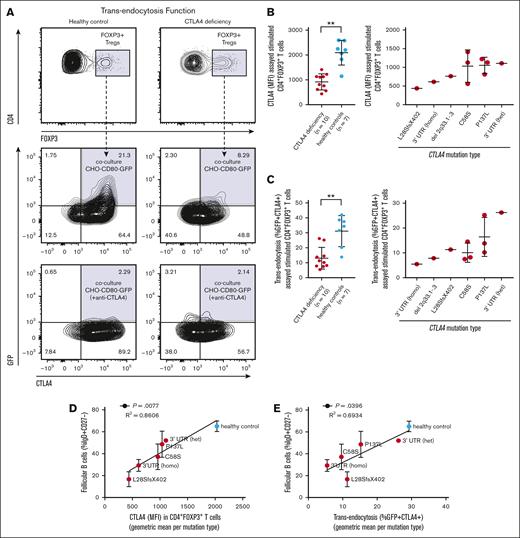
Reduced frequency of circulating FO B cells correlates with degree of congenital Treg dysfunction in CTLA4 deficiency. CHO-CD80-GFP cells were cocultured with activated T cells from the peripheral blood of patients with CTLA4 deficiency (n = 10) and healthy controls (n = 7). Gated CD4+FOXP3+ Tregs that had acquired GFP from CHO expressing CD80-GFP cells were scored positive for intact CTLA4-mediated transendocytosis. (A) Representative contour plots from a healthy control (left) and a patient with CTLA4 deficiency (right, heterozygous CTLA4 c.410C>T, p.P137L mutation carrier), shown at 2% of events with gating strategy outlined. Quantification of (B) total cellular CTLA4 levels by intracellular staining or (C) CTLA4 function by CD80-GFP transendocytosis as percent GFP+CTLA4+ in stimulated peripheral blood CD4+FOXP3+ Tregs from patients with CTLA4 deficiency compared with healthy controls (left) and compared by CTLA4 mutation type (right). Symbols represent unique individuals and bars represent means (± SD) of all data. ∗∗P < .01 by 2-tailed Mann-Whitney U test. (D-E) Correlations between FO B-cell frequency in the peripheral blood and severity of the underlying CTLA4 mutation type. Y-axes are FO B-cell frequency as percent IgD+CD27– B cells in the peripheral blood (data from Figure 1C, here shown as means ± standard error of the mean for all analyzed patients with the indicated CTLA4 mutation type or healthy control state, respectively). (D) X-axis is total cellular CTLA4 levels as MFI by intracellular staining in stimulated CD4+FOXP3+ Tregs (data from panel B, here consolidated as geometric mean per CTLA4 mutation type or healthy control state, respectively). (E) X-axis is CTLA4 function as CD80-GFP transendocytosis (percent GFP+CTLA4+) in stimulated CD4+FOXP3+ Tregs (data from panel C, here consolidated as geometric mean per CTLA4 mutation type or healthy control state, respectively). Simple linear regression with correlation coefficient (R2) and P value shown. Homo, homozygous; het, heterozygous.
Reduced frequency of circulating FO B cells correlates with degree of congenital Treg dysfunction in CTLA4 deficiency. CHO-CD80-GFP cells were cocultured with activated T cells from the peripheral blood of patients with CTLA4 deficiency (n = 10) and healthy controls (n = 7). Gated CD4+FOXP3+ Tregs that had acquired GFP from CHO expressing CD80-GFP cells were scored positive for intact CTLA4-mediated transendocytosis. (A) Representative contour plots from a healthy control (left) and a patient with CTLA4 deficiency (right, heterozygous CTLA4 c.410C>T, p.P137L mutation carrier), shown at 2% of events with gating strategy outlined. Quantification of (B) total cellular CTLA4 levels by intracellular staining or (C) CTLA4 function by CD80-GFP transendocytosis as percent GFP+CTLA4+ in stimulated peripheral blood CD4+FOXP3+ Tregs from patients with CTLA4 deficiency compared with healthy controls (left) and compared by CTLA4 mutation type (right). Symbols represent unique individuals and bars represent means (± SD) of all data. ∗∗P < .01 by 2-tailed Mann-Whitney U test. (D-E) Correlations between FO B-cell frequency in the peripheral blood and severity of the underlying CTLA4 mutation type. Y-axes are FO B-cell frequency as percent IgD+CD27– B cells in the peripheral blood (data from Figure 1C, here shown as means ± standard error of the mean for all analyzed patients with the indicated CTLA4 mutation type or healthy control state, respectively). (D) X-axis is total cellular CTLA4 levels as MFI by intracellular staining in stimulated CD4+FOXP3+ Tregs (data from panel B, here consolidated as geometric mean per CTLA4 mutation type or healthy control state, respectively). (E) X-axis is CTLA4 function as CD80-GFP transendocytosis (percent GFP+CTLA4+) in stimulated CD4+FOXP3+ Tregs (data from panel C, here consolidated as geometric mean per CTLA4 mutation type or healthy control state, respectively). Simple linear regression with correlation coefficient (R2) and P value shown. Homo, homozygous; het, heterozygous.
Flow cytometric analysis of peripheral blood B cells and T cells
A total of 5 million previously frozen peripheral blood mononuclear cells (PBMCs) were analyzed for B-cell or T-cell staining, respectively. The distinction between transitional and FO human B cells was accomplished by ABCB1 transporter efflux profiling, using MitoTracker Green (ThermoFisher Scientific),1,7,8 in combination with CD73 surface staining.1,9 B cells were run on a Symphony 5-laser BD fluorescence-activated cell sorter (5S Symphony) and sorted on a fluorescence-activated cell sorter Aria II (BD Biosciences). The primary B-cell panel used is listed in supplemental Table 2. For T-cell staining, a similar protocol was applied. Primary T-cell panels, both extracellular and intracellular, including CD40L, are listed in supplemental Tables 3 and 4, respectively, and additional details are provided in the supplemental Methods.
Analysis of total cellular CTLA4 levels and CD80 transendocytosis function in Tregs
Chinese hamster ovary (CHO) cells stably overexpressing CD80–green fluorescent protein (GFP) were kindly provided by the Sansom laboratory.10 Analyses of total cellular CTLA4 levels and CTLA4-mediated transendocytosis were performed by intracellular flow cytometry as previously described2 using the True-Nuclear transcription factor buffer set (BioLegend). After coculture, CD4+FOXP3+ Tregs that had acquired GFP from CHO expressing CD80-GFP cells were scored positive for intact CTLA4-mediated transendocytosis. Additional details are provided in the supplemental Methods.
In vitro B-cell differentiation and phospho–flow cytometry
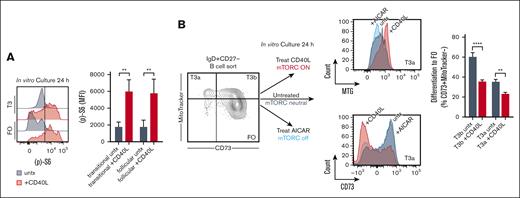
PBMCs isolated from fresh healthy donor human leukopacks were surface labeled, as previously described.1 In Figure 5A, amounts of phosphorylated (p)-S6 were analyzed at 24 hours in culture, in the absence or presence of CD40L treatment as indicated, using (p)-S6 Ser235/236 (monoclonal cupk43k, Thermo Fisher Scientific) and an intracellular phospho-staining protocol (supplemental Methods). In Figure 5B, sorted T3a and T3b B cells were cultured for 24 hours in the absence or presence of CD40L treatment, or 5-aminoimidazole-4-carboxamide ribonucleotide treatment to inhibit mTORC signaling as a control.1 T3a and T3b B cells were then assayed by extracellular flow cytometry for differentiation to the FO B-cell stage by the acquisition of surface CD73 and the loss of MitoTracker staining. Additional details are provided in the supplemental Methods.
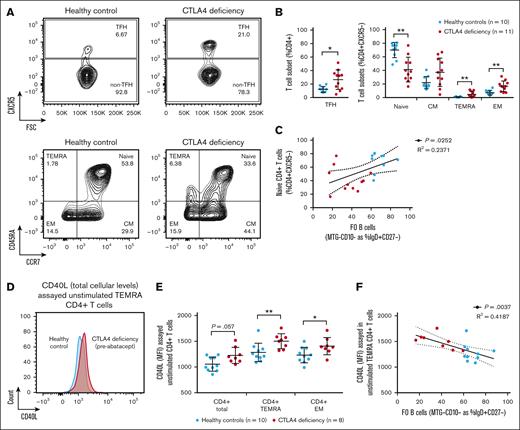
Increased CD40L levels in effector CD4+ T cells in CTLA4 deficiency correlate with a decrease in the frequency of circulating FO B cells. (A) Extracellular flow cytometric analysis of peripheral blood T-cell populations in patients with CTLA4 deficiency (n = 11) and healthy controls (n = 10). Representative contour plots from a healthy control (left) and 2 patients with CTLA4 deficiency (right, heterozygous CTLA4 c.410C>T, p.P137L mutation carriers) shown at 5% of events (full description of T-cell gating strategy in supplemental Figure 1B). (B) Quantification of the major CD4+ T-cell subsets defined by the extracellular markers CXCR5, CCR7, and CD45RA, including TFH, naïve, CM, TEMRA, and EM populations. Symbols represent unique individuals; bars represent means (± SD) of all data. ∗P < .05; ∗∗P < .01 by 2-tailed Mann-Whitney U test. (C) Correlation between FO B-cell frequency as percent IgD+CD27– B cells in the peripheral blood (data from Figure 1C; x-axis) and naïve CD4+ T-cell frequency as percent CD4+CXCR5– T cells in the peripheral blood (data from Panel B; y-axis). Simple linear regression with correlation coefficient (R2), P value, and 95% confidence interval (CI) shown. (D) Intracellular flow cytometric analysis of peripheral blood T-cell populations in patients with CTLA4 deficiency (n = 8) and healthy controls (n = 10). Representative overlaid histograms from a healthy control (blue) and a patient with CTLA4 deficiency (red, heterozygous CTLA4 c.173G>C p.C58S mutation carrier) shown. (E) Quantification of total cellular CD40L levels (MFI) by intracellular staining in the major CD4+ T-cell subsets described above in patients with CTLA4 deficiency and healthy controls. Symbols represent unique individuals; bars represent means (± SD) of all data. ∗P < .05; ∗∗P < .01 or as listed by 2-tailed Mann-Whitney U test. (F) Correlation between FO B-cell frequency as percent IgD+CD27– B cells in the peripheral blood (data from Figure 1C; x-axis) and total cellular CD40L levels as MFI in TEMRA CD4+ T cells (data from panel E; y-axis). Simple linear regression with correlation coefficient (R2), P value, and 95% CI shown. CM, central memory; EM, effector memory; TFH, T follicular helper.
Increased CD40L levels in effector CD4+ T cells in CTLA4 deficiency correlate with a decrease in the frequency of circulating FO B cells. (A) Extracellular flow cytometric analysis of peripheral blood T-cell populations in patients with CTLA4 deficiency (n = 11) and healthy controls (n = 10). Representative contour plots from a healthy control (left) and 2 patients with CTLA4 deficiency (right, heterozygous CTLA4 c.410C>T, p.P137L mutation carriers) shown at 5% of events (full description of T-cell gating strategy in supplemental Figure 1B). (B) Quantification of the major CD4+ T-cell subsets defined by the extracellular markers CXCR5, CCR7, and CD45RA, including TFH, naïve, CM, TEMRA, and EM populations. Symbols represent unique individuals; bars represent means (± SD) of all data. ∗P < .05; ∗∗P < .01 by 2-tailed Mann-Whitney U test. (C) Correlation between FO B-cell frequency as percent IgD+CD27– B cells in the peripheral blood (data from Figure 1C; x-axis) and naïve CD4+ T-cell frequency as percent CD4+CXCR5– T cells in the peripheral blood (data from Panel B; y-axis). Simple linear regression with correlation coefficient (R2), P value, and 95% confidence interval (CI) shown. (D) Intracellular flow cytometric analysis of peripheral blood T-cell populations in patients with CTLA4 deficiency (n = 8) and healthy controls (n = 10). Representative overlaid histograms from a healthy control (blue) and a patient with CTLA4 deficiency (red, heterozygous CTLA4 c.173G>C p.C58S mutation carrier) shown. (E) Quantification of total cellular CD40L levels (MFI) by intracellular staining in the major CD4+ T-cell subsets described above in patients with CTLA4 deficiency and healthy controls. Symbols represent unique individuals; bars represent means (± SD) of all data. ∗P < .05; ∗∗P < .01 or as listed by 2-tailed Mann-Whitney U test. (F) Correlation between FO B-cell frequency as percent IgD+CD27– B cells in the peripheral blood (data from Figure 1C; x-axis) and total cellular CD40L levels as MFI in TEMRA CD4+ T cells (data from panel E; y-axis). Simple linear regression with correlation coefficient (R2), P value, and 95% CI shown. CM, central memory; EM, effector memory; TFH, T follicular helper.
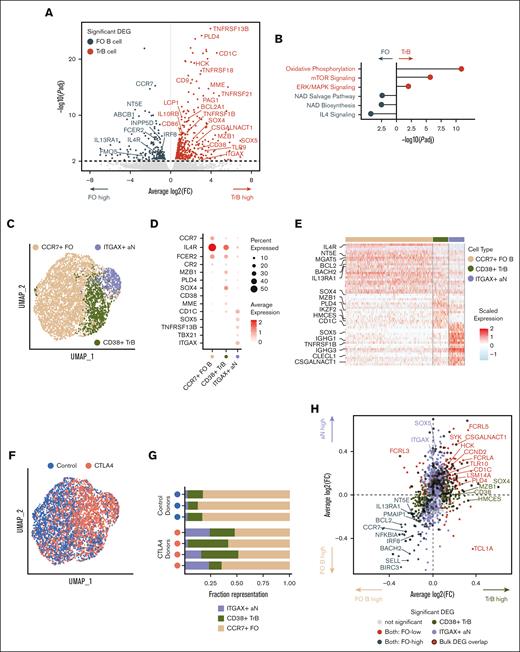
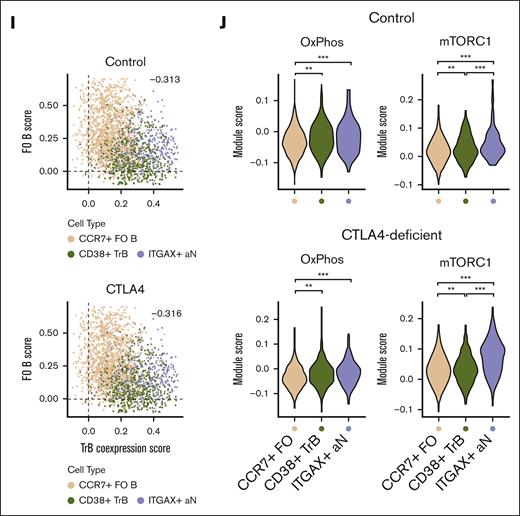
Increased mTORC1 signaling due to abundance of transitional and ABC-like aN B-cell clusters in patients with CTLA4 deficiency. (A) Volcano plot of shared differential gene expression analysis between bulk RNA-seq samples of flow-sorted resting FO and TrB populations (T1/2, T3; n = 3 healthy controls; significant differentially expressed genes defined by adjusted P value ≤ .01). (B) Adjusted P value of ingenuity pathway analysis pathway overlaps with significantly differentially expressed genes in TrB vs resting FO B cells. (C) Uniform manifold approximation and projection (UMAP) projection of total naïve (IgD+CD27–) B cells profiled by scRNA-seq from patients with CTLA4 deficiency (n = 4) and healthy controls (n = 3), annotated by naïve B-cell subtype. (D) Dot plot showing the marker gene expression of FO, TrB, and aN B-cell types by cell type annotation; color represents average normalized gene expression and size represents the percent cells with non-zero gene expression. (E) Heat map of scaled gene expression of top 30 differentially expressed genes between annotated cell types. (F) UMAP projection of total naïve (IgD+CD27–) B cells profiled by scRNA-seq, annotated by control or patients with CTLA4 deficiency. (G) Fraction representation of each annotated cell type within each donor scRNA-seq sample. (H) Average log2-fold change of significant differentially expressed genes (adjusted P value ≤ .05) between distinguishing CD38+ TrBs vs CCR7+ FO B cells plotted on the x-axis and average log2-fold change of significant differentially expressed genes between ITGAX+ ABC-like aN B cells vs CCR7+ FO B cells plotted on the y-axis, showing correlation in differential gene expression distinguishing both TrBs and ABC-like aN B cells from FO B cells. Differentially expressed genes that overlap with significant differentially expressed genes from the bulk RNA-seq analysis in panel A are outlined. (I) Single-cell module scores defined by TrB and ABC-like aN cells shared differentially expressed genes in both TrB and ABC-like aN cells plotted on the x-axis (TrB coexpression score), vs module scores defined by shared FO differentially expressed genes in FO cells on the y-axis (FO B score). Single cells from healthy controls are plotted separately from single cells from patients with CTLA4 deficiency to demonstrate consistent trends in cell type population scores. Pearson correlation rho values (−0.313 for healthy controls, −0.316 for patients with CTLA4 deficiency) were both significant (P < 2E−16). (J) Violin plots of module scores over msigDB hallmark oxidative phosphorylation and mTORC1 signaling pathways (see “Methods”) in healthy controls and patients with CTLA4 deficiency. Violin plots of single-cell module scores shown and evaluated for significance separately for healthy controls and patients with CTLA4 deficiency; ∗∗P < .001; ∗∗∗P < .0001. DEG, differentialy expressed gene; ERK, extracellular signal-regulated kinase; IL4, interleukin 4; msigDB, molecular signatures database; NAD, nicotinamide adenine dinucleotide.
Increased mTORC1 signaling due to abundance of transitional and ABC-like aN B-cell clusters in patients with CTLA4 deficiency. (A) Volcano plot of shared differential gene expression analysis between bulk RNA-seq samples of flow-sorted resting FO and TrB populations (T1/2, T3; n = 3 healthy controls; significant differentially expressed genes defined by adjusted P value ≤ .01). (B) Adjusted P value of ingenuity pathway analysis pathway overlaps with significantly differentially expressed genes in TrB vs resting FO B cells. (C) Uniform manifold approximation and projection (UMAP) projection of total naïve (IgD+CD27–) B cells profiled by scRNA-seq from patients with CTLA4 deficiency (n = 4) and healthy controls (n = 3), annotated by naïve B-cell subtype. (D) Dot plot showing the marker gene expression of FO, TrB, and aN B-cell types by cell type annotation; color represents average normalized gene expression and size represents the percent cells with non-zero gene expression. (E) Heat map of scaled gene expression of top 30 differentially expressed genes between annotated cell types. (F) UMAP projection of total naïve (IgD+CD27–) B cells profiled by scRNA-seq, annotated by control or patients with CTLA4 deficiency. (G) Fraction representation of each annotated cell type within each donor scRNA-seq sample. (H) Average log2-fold change of significant differentially expressed genes (adjusted P value ≤ .05) between distinguishing CD38+ TrBs vs CCR7+ FO B cells plotted on the x-axis and average log2-fold change of significant differentially expressed genes between ITGAX+ ABC-like aN B cells vs CCR7+ FO B cells plotted on the y-axis, showing correlation in differential gene expression distinguishing both TrBs and ABC-like aN B cells from FO B cells. Differentially expressed genes that overlap with significant differentially expressed genes from the bulk RNA-seq analysis in panel A are outlined. (I) Single-cell module scores defined by TrB and ABC-like aN cells shared differentially expressed genes in both TrB and ABC-like aN cells plotted on the x-axis (TrB coexpression score), vs module scores defined by shared FO differentially expressed genes in FO cells on the y-axis (FO B score). Single cells from healthy controls are plotted separately from single cells from patients with CTLA4 deficiency to demonstrate consistent trends in cell type population scores. Pearson correlation rho values (−0.313 for healthy controls, −0.316 for patients with CTLA4 deficiency) were both significant (P < 2E−16). (J) Violin plots of module scores over msigDB hallmark oxidative phosphorylation and mTORC1 signaling pathways (see “Methods”) in healthy controls and patients with CTLA4 deficiency. Violin plots of single-cell module scores shown and evaluated for significance separately for healthy controls and patients with CTLA4 deficiency; ∗∗P < .001; ∗∗∗P < .0001. DEG, differentialy expressed gene; ERK, extracellular signal-regulated kinase; IL4, interleukin 4; msigDB, molecular signatures database; NAD, nicotinamide adenine dinucleotide.
CD40L induces mTORC signaling and arrests FO B-cell maturation in vitro. (A) Levels of (p)-S6 by intracellular flow cytometry in transitional (T) and FO B-cell subsets, sorted and cultured for 24 hours in vitro with CD40L treatment (+CD40L) compared with without (untx) as indicated. Histograms are representative of 3 independent experiments. Quantified data, from 3 independent experiments, are means (± SD) of all data. ∗∗P < .01 by unpaired Student t test. (B) Differentiation by extracellular flow cytometry of T3a and T3b B cells, sorted and cultured for 24 hours in vitro with, compared with, without CD40L treatment or AICAR treatment to inhibit mTORC signaling as a control. Histograms are representative of 3 independent experiments, here showing differences in T3a B-cell levels of MitoTracker (MTG) and CD73 at 24 hours in culture by treatment condition as indicated. Differentiation to the FO B-cell stage was scored by the acquisition of the CD73+MTG− phenotype. Quantified data, from 2 independent experiments, are means (± SD) of all data. ∗∗P < .01; ∗∗∗∗P < .0001 by unpaired Student t test. AICAR, 5-aminoimidazole-4-carboxamide ribonucleotide; untx, untreated.
CD40L induces mTORC signaling and arrests FO B-cell maturation in vitro. (A) Levels of (p)-S6 by intracellular flow cytometry in transitional (T) and FO B-cell subsets, sorted and cultured for 24 hours in vitro with CD40L treatment (+CD40L) compared with without (untx) as indicated. Histograms are representative of 3 independent experiments. Quantified data, from 3 independent experiments, are means (± SD) of all data. ∗∗P < .01 by unpaired Student t test. (B) Differentiation by extracellular flow cytometry of T3a and T3b B cells, sorted and cultured for 24 hours in vitro with, compared with, without CD40L treatment or AICAR treatment to inhibit mTORC signaling as a control. Histograms are representative of 3 independent experiments, here showing differences in T3a B-cell levels of MitoTracker (MTG) and CD73 at 24 hours in culture by treatment condition as indicated. Differentiation to the FO B-cell stage was scored by the acquisition of the CD73+MTG− phenotype. Quantified data, from 2 independent experiments, are means (± SD) of all data. ∗∗P < .01; ∗∗∗∗P < .0001 by unpaired Student t test. AICAR, 5-aminoimidazole-4-carboxamide ribonucleotide; untx, untreated.
Bulk RNA-seq analysis
Analyzed data were from existing RNA-sequencing (RNA-seq) libraries. RNA had been isolated from sorted transitional (T1/2 and T3a/b) and FO B cells from 3 healthy individuals using the RNeasy-Plus micro kit (Qiagen) with RNA-seq libraries prepared as previously described.11 Additional details are provided in the supplemental Methods.
scRNA-seq
We used the Seq-Well S3 platform for massively parallel single-cell RNA-seq (scRNA-seq) to capture transcriptomes of single cells on barcoded messenger RNA capture beads, as previously described.12 Approximately 15 000 immunoglobulin D–positive (IgD+)CD27– B cells sorted from the PBMCs of patients with CTLA4 deficiency (n = 4 individuals, including 1 patient before and after abatacept treatment) and healthy individuals (n = 3) were loaded onto individual arrays containing barcoded messenger RNA capture beads. Cell-lysis, RNA hybridization, reverse transcription, whole-transcriptome amplification, and library construction were performed for each sample array before sequencing using an Illumina 75 Cycle NextSeq500/550v2 kit, sample demultiplexing, and hg19 genome alignment. Dimensionality reduction, differential gene expression, and module scoring analysis for characterizing IgD+CD27– naïve B-cell subpopulations are described in detail in the supplemental Methods.
Multicolor immunofluorescence staining of tissues
Tissue samples were fixed in formalin, embedded in paraffin, and sectioned. Details of staining and imaging processes are provided in the supplemental Methods.
Statistical analyses
Data were analyzed using GraphPad Prism version 9.00. Student t and Mann-Whitney U tests were used to compare continuous variables between 2 groups. For >2 group comparisons, analysis of variance with post-hoc Tukey test was used. Wilcoxon signed-rank test was used for pair-wise comparisons. Correlation analyses and linear regression models were used to examine associations between study variables. P values are 2-sided, and a P value < .05 was considered significant.
Results
Increased frequencies of transitional and activated naïve B cells and decreased frequency of FO B cells in patients with CTLA4 deficiency
Patients with CTLA4 deficiency were longitudinally followed-up for immune deficiency, autoimmunity, and end-organ lymphoinfiltrative pathology (supplemental Table 1).2-4,13 We analyzed peripheral blood B cells by flow cytometry in all patients with CTLA4 deficiency (Figure 1) using the outlined B-cell panel (supplemental Table 2) and gating strategy (supplemental Figure 1A). Consistent with previous reports, we observed a lower frequency of circulating class-switched memory (SWM) (IgD–CD27+) B cells and a higher frequency of B cells in the naïve (IgD+CD27–) gate (Figure 1A-B).2,3 Dissecting the heterogeneous IgD+CD27– B-cell compartment in greater detail, we observed increased TrBs in patients with CTLA4 deficiency, including T1/2 (CD10+MitoTracker+), T3a (CD10–MitoTracker+CD45RB–CD73–), and T3b (CD10–MitoTracker+CD45RB–CD73+) B cells, as recently defined.1,9 In contrast, we found a significant reduction in circulating CD10–MitoTracker– FO B cells (Figure 1A,C), consistent with impaired B-cell maturation between the late transitional and FO B-cell stages. Although circulating FO B cells were reduced in patients with CTLA4 deficiency, we observed a higher frequency of circulating activated naïve (aN; CD21loCD11chi) B cells (Figure 1A,C).
In patients with CTLA4 deficiency, absolute numbers of B cells in the peripheral blood were quantified and, despite relative lymphopenia in a subset of patients, counts of circulating transitional and aN B cells increased whereas counts of FO B cells decreased in patients with disease compared with healthy controls (supplemental Figure 2). In the naïve (IgD+CD27–) B-cell gate, we observed higher absolute counts of circulating aN B cells (P = .01), a trend toward higher counts of circulating late TrBs (T3a, P = .10), and a trend toward lower counts of circulating FO B cells (FO, P = .15) in patients with CTLA4 deficiency than in healthy controls.
Circulating FO B-cell deficiency correlates with reduced CTLA4 levels and function in Tregs in patients with CTLA4 deficiency
Apart from the rare detection of CTLA4 in human B cells in CD5+ monoclonal malignancies,14,15CTLA4 is understood to be primarily expressed by T cells. In our studies, intracellular flow cytometry revealed extremely low abundance of total cellular CTLA4 protein levels in transitional and FO human B cells (MFI of <103 in all naïve [IgD+CD27–] B cells analyzed; supplemental Figure 3). We therefore hypothesized that the observed impairment in transitional-to-FO B-cell maturation in patients with CTLA4 deficiency was secondary to the underlying Treg dysfunction and resulting T-cell hyperactivation.
We tested this hypothesis through analysis of mutation-specific effects on Tregs in patients with CTLA4 deficiency compared with healthy controls. Given that heterozygous mutations in CTLA4 can elicit a dominant-negative effect, we additionally probed Treg CTLA4 function through CD80 transendocytosis.2 All unique deletion, missense, and noncoding region CTLA4 mutations caused decreased CTLA4 total cellular levels (Figure 2A-B) and/or the attenuation of CTLA4-mediated transendocytosis function (Figure 2A,C) in CD4+FOXP3+ Tregs from patients with CTLA4 deficiency compared with healthy controls. Members of 1 family harbored a 3′ UTR variant sequence in the CTLA4 gene. The likely functional relevance of this variant was supported by the observations of extremely low total cellular abundance of CTLA4 protein by intracellular staining in CD4+FOXP3+ Tregs (MFI of <103 in the homozygous carrier state and below the healthy control level in the heterozygous carrier state; supplemental Figure 4). Finally, loss of circulating FO B cells, as identified in Figure 1, correlated by linear regression with both lower total cellular CTLA4 levels (P = .0077, R2 = 0.86; Figure 2D) and lower CTLA4-mediated CD80 transendocytosis function (P = .0396, R2 = 0.69; Figure 2E) as assayed in CD4+FOXP3+ Tregs by CTLA4 mutation type or healthy control state, respectively. These data suggest that the loss of circulating FO B cells is directly proportional to the underlying magnitude of the Treg dysfunction that occurs in CTLA4 deficiency.
Circulating FO B-cell deficiency correlates with increased CD40L levels in CD4+ effector T cells in patients with CTLA4 deficiency
We investigated the T-cell compartment of patients with CTLA4 deficiency using intracellular and extracellular multiparameter flow cytometry (supplemental Tables 3 and 4; supplemental Figure 1B). As expected in CTLA4 deficiency,2,3 we observed a lower frequency of naïve CD4+ T cells in circulation and higher frequencies of T follicular helper (TFH), effector memory, and T effector memory CD45RA+ (TEMRA) CD4+ T cells in circulation (Figure 3A-B). The loss of circulating naïve CD4+ T cells correlated by linear regression with the loss of circulating FO B cells identified in Figure 1 (P = .0252, R2 = 0.24; Figure 3C). In patients with CTLA4 deficiency, absolute numbers of CD4+ T cells in the peripheral blood were quantified and, despite relative lymphopenia in a subset of patients, there were increased circulating counts of TEMRA CD4+ cells compared with healthy controls that trended toward significance (P = .07) and decreased circulating counts of naïve CD4+ T cells compared with healthy controls that met significance (P < .05; supplemental Figure 5).
By intracellular flow cytometry, effector CD4+ T cells from patients with CTLA4 deficiency compared with healthy controls had higher total cellular levels of CD40L, with the largest increase relative to healthy controls seen in TEMRA CD4+ T cells (Figure 3D-E). The increase in CD40L levels, in unstimulated TEMRA CD4+ T cells specifically, correlated by linear regression with the loss of circulating FO B cells with a stronger correlation coefficient (P = .0037, R2 = 0.42; Figure 3F). These data suggest that in a human state of CTLA4 deficiency, there is a relative increase in both the proportion of circulating effector CD4+ T cells and the levels of activating ligands within those effector cells, the latter of which correlates more strongly with the loss of FO B cells in the peripheral blood.
Increased mTORC1 signaling defines aberrant B-cell populations in patients with CTLA4 deficiency
We performed both bulk and single-cell transcriptomics to obtain, in part, a window into the metabolic state of TrBs in CTLA4 deficiency. Increased mTORC1 signaling is a transcriptional hallmark of TrBs. A differential gene expression analysis comparing flow-sorted circulating transitional and resting FO B cells from a previously published bulk RNA-seq data set revealed upregulation of activation markers in these TrBs, including CD38, BCL2A1, and CD86, and decreased expression of canonical FO B-cell marker genes (eg, CCR7, FCER2, and IL13RA1).1 FO B cells were additionally defined by increased expression of NT5E, encoding for CD73, and ABCB1 (Figure 4A). Pathway enrichment analysis over these differentially expressed genes revealed enrichment for nicotinamide adenine dinucleotide signaling and biosynthesis pathways in FO B cells, in contrast to strong enrichment for mTORC1 signaling and oxidative phosphorylation pathways in TrBs (Figure 4B). These data confirm that maturation stage–restricted metabolic dependencies define transcriptional signatures separating transitional and FO B cells.1
To resolve the dominant TrB populations in CTLA4 deficiency and underlying signaling patterns, we performed scRNA-seq on total naïve (IgD+CD27–) B cells from 4 patients with CTLA4 deficiency and 3 healthy controls. Along with CCR7+ FO B cells, clustering analysis resolved a subpopulation of TrBs, which most closely resemble the T3 TrB stage given increased expression of SOX4, CD38, and HMCES, in addition to sparse detection of the T1/2 marker MME. We additionally characterized a subpopulation of ITGAX+TBX21+ B cells. Although CD11chiTbet+ cells have sometimes been generically referred to as age-associated B cells (ABCs) (Figure 4C-E),16,17 there are at least 3 distinct categories of ITGAX+TBX21+ B cells in humans, including aN B cells (IgD+CD27–),18 double-negative 2 (DN2) B cells (IgD–CD27–),19 and a CD27+TBX21+ITGAX+ population with high somatic hypermutation, suggesting germinal center derivation.20 Compared with healthy controls, decreases in the relative proportions of FO B cells in patients with CTLA4 deficiency were accompanied by increased proportions of both TrBs and ABC-like aN B cells (Figure 4F-G), further corroborating our flow cytometry findings (Figure 1).
Next, we asked whether the ABC-like aN B-cell population in CTLA4 deficiency exhibits transcriptional similarities to TrB populations, possibly providing clues to their origins in this disease. Interestingly, many genes differentially expressed by ABC-like aN B cells compared with CCR7+ FO B cells were also differentially expressed by TrBs, including PLD4, MZB1, and CD1C.21,22 We curated a signature of shared differentially expressed genes in TrBs and ABC-like aN B cells compared with FO B cells (Figure 4H). Many of these genes were also differentially expressed in bulk-sorted TrBs compared with resting FO B cells from healthy controls (Figure 4A,H). Scoring all cells by this signature similarly segregated both TrBs and ABC-like aN B cells from their FO counterparts in both healthy controls and patients with CTLA4 deficiency (Figure 4I), suggesting that ABC-like aN B cells may share core TrB identity gene expression with the canonical TrB population in CTLA4 deficiency.
Given the relative accumulation of both TrBs and ABC-like aN B cells in CTLA4 deficiency, we explored whether the ABC-like aN B cells used the same underlying metabolic signatures as TrBs. By module scoring B-cell subpopulations, we found increased transcriptomic signatures of oxidative phosphorylation in both TrBs and ABC-like aN B cells compared with FO B cells. We additionally observed elevated mTORC1 signaling in TrBs compared with FO B cells, and further increased mTORC1 signaling in ABC-like aN B cells compared with both TrB and FO B cells. These metabolic trends between these 3 subpopulations were consistent in both healthy controls and B cells from patients with CTLA4 deficiency, suggesting that mTORC1 signaling strongly defines ABC-like aN B cells (Figure 4J). Collectively, these findings implicate a potential TrB origin for ABC-like aN B cells and nominate mTORC1 signaling as both an inhibitory pathway for normal FO B-cell maturation and permissive for ABC-like aN B-cell maturation in the context of increased CD4+ T-cell activation.
CD40L induces mTORC1 signaling and inhibits transitional-to-FO B-cell maturation in vitro
Using an in vitro B-cell differentiation model, we previously showed that transitional-to-FO B-cell maturation is enhanced with adenosine 5′-monophosphate-activated protein kinase stimulation and mTORC1 inhibition.2 We have used this model system (Figure 5) to further probe the direct effects of CD40L on TrB signaling and developmental potential. We observed increased (p)-S6 levels in CD40L-treated TrB (Figure 5A), suggesting that CD40L can induce mTORC1 signaling in human TrBs. We also observed decreased generation of FO B cells in TrB cultures treated for 24 hours with CD40L compared with those left untreated (Figure 5B). Together with previously published data, these results suggest that mTORC signaling critically regulates human transitional-to-FO B-cell development. Although inhibition of mTORC augments transitional-to-FO B-cell maturation,2 induction of mTORC by CD40L, as quantified by (p)-S6 levels, limits transitional-to-FO B-cell maturation potential in vitro.
To determine whether CD40L+ T cells interact in vivo with cells that correspond to cells in the naïve B-cell gate (that includes TrBs) in patients with common variable immunodeficiency caused by congenital syndromes of T-cell activation (activated PI3K-δ syndrome and CTLA4 deficiency), we examined lymph nodes using immunofluorescence microscopy. Cell-to-cell contacts between CD40L+ T cells and IgD+CD27– B cells occurred prominently in the T-cell zones and more frequently in patients with congenital T-cell activation as compared with secondary lymphoid organs of healthy controls (supplemental Figure 6). Finally, we identified that late TrBs (T3a B cells) acquire surface CCR7 (supplemental Figure 7) and therefore can potentially recirculate to secondary lymphoid organs at this stage of maturation. Together, these data suggest that activated T cells may directly interact with IgD+CD27– B cells, as early as the late TrB stage, in human lymphoid organs.
FO B-cell maturation is restored with CTLA4 immunoglobulin treatment in patients with CTLA4 deficiency
In a subset of our CTLA4 deficiency cohort, onset of autoimmune and lymphoinfiltrative disease necessitated initiation of abatacept (CTLA4 immunoglobulin) therapy. Robust clinical response to abatacept has been demonstrated in CTLA4 deficiency4,23 and phenotypically similar disorders (homozygous lipopolysaccharide-responsive and beige-like anchor protein and guanine nucleotide exchange factor deficiencies).24-26 Clinical response, dose, and duration of treatment are detailed in supplemental Table 1. In patients treated for at least 3 months, we observed a robust decrease in circulating early transitional (T1/2) and T3a B cells and a corresponding increase in FO B cells in circulation (Figure 6A).
Treatment with abatacept (CTLA4 immunoglobulin) restores FO B-cell maturation coincident with decreased CD40L levels on effector T cells in CTLA4 deficiency. (A-B) Flow cytometric analysis of peripheral blood B-cell populations in patients with CTLA4 deficiency before (n = 11) and after abatacept (n = 8) therapy (6 patients captured at paired intervals before/after abatacept therapy) and healthy controls (n = 16). Representative contour plots and histograms from a healthy control (left) and the same patient with CTLA4 deficiency (middle and right) before/after abatacept therapy (homozygous CTLA4 3′ UTR mutation carrier in panel A, and heterozygous CTLA4 c.410C>T, p.P137L mutation carrier in panel B) shown at 10% and 5% of events, respectively. Quantitation of B-cell subset frequency as percent IgD+CD27− B cells in the peripheral blood. Symbols represent unique individuals; bars represent means (± SD) of all data; dotted lines represent paired pretreatment to posttreatment time points. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001, or as listed by analysis of variance (ANOVA) for multiple comparisons or by Wilcoxon matched-pairs signed-rank test, as indicated. (C) scRNA-seq of total IgD+CD27− B cells (from heterozygous CTLA4 c.410C>T, p.P137L mutation carrier) before and after abatacept therapy. UMAP projection of cells is shown by cell type clustering and state of abatacept treatment. Fraction representation of each clustered scRNA-seq cell type. (D) Flow cytometric analysis of peripheral blood T-cell populations in patients with CTLA4 deficiency before (n = 8) and after abatacept (n = 6) therapy (4 patients captured at paired intervals before/after abatacept therapy) and healthy controls (n = 10). Representative overlaid histograms from the same patient with CTLA4 deficiency before/after abatacept therapy (heterozygous CTLA4 c.173G>C p.C58S mutation carrier). Quantitation of total cellular CD40L by intracellular staining in CD4+ T-cell subsets. Symbols represent unique individuals; bars represent means (± SD) of all data; dotted lines represent paired before to after treated unique patients. ∗P < .05 or as listed by ANOVA for multiple comparisons or by Wilcoxon matched-pairs signed-rank test, as indicated. (E) Correlation between FO B-cell frequency as percent IgD+CD27− B cells in the peripheral blood (data from panel A; x-axis) and total cellular CD40L levels as MFI in TEMRA CD4+ T cells (data from panel D; y-axis). Data are from untreated patients with CTLA4 deficiency, treated patients with CTLA4 deficiency, and healthy controls, as indicated. Simple linear regression with correlation coefficient (R2), P value, and 95% CI shown. ns, not significant.
Treatment with abatacept (CTLA4 immunoglobulin) restores FO B-cell maturation coincident with decreased CD40L levels on effector T cells in CTLA4 deficiency. (A-B) Flow cytometric analysis of peripheral blood B-cell populations in patients with CTLA4 deficiency before (n = 11) and after abatacept (n = 8) therapy (6 patients captured at paired intervals before/after abatacept therapy) and healthy controls (n = 16). Representative contour plots and histograms from a healthy control (left) and the same patient with CTLA4 deficiency (middle and right) before/after abatacept therapy (homozygous CTLA4 3′ UTR mutation carrier in panel A, and heterozygous CTLA4 c.410C>T, p.P137L mutation carrier in panel B) shown at 10% and 5% of events, respectively. Quantitation of B-cell subset frequency as percent IgD+CD27− B cells in the peripheral blood. Symbols represent unique individuals; bars represent means (± SD) of all data; dotted lines represent paired pretreatment to posttreatment time points. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001, or as listed by analysis of variance (ANOVA) for multiple comparisons or by Wilcoxon matched-pairs signed-rank test, as indicated. (C) scRNA-seq of total IgD+CD27− B cells (from heterozygous CTLA4 c.410C>T, p.P137L mutation carrier) before and after abatacept therapy. UMAP projection of cells is shown by cell type clustering and state of abatacept treatment. Fraction representation of each clustered scRNA-seq cell type. (D) Flow cytometric analysis of peripheral blood T-cell populations in patients with CTLA4 deficiency before (n = 8) and after abatacept (n = 6) therapy (4 patients captured at paired intervals before/after abatacept therapy) and healthy controls (n = 10). Representative overlaid histograms from the same patient with CTLA4 deficiency before/after abatacept therapy (heterozygous CTLA4 c.173G>C p.C58S mutation carrier). Quantitation of total cellular CD40L by intracellular staining in CD4+ T-cell subsets. Symbols represent unique individuals; bars represent means (± SD) of all data; dotted lines represent paired before to after treated unique patients. ∗P < .05 or as listed by ANOVA for multiple comparisons or by Wilcoxon matched-pairs signed-rank test, as indicated. (E) Correlation between FO B-cell frequency as percent IgD+CD27− B cells in the peripheral blood (data from panel A; x-axis) and total cellular CD40L levels as MFI in TEMRA CD4+ T cells (data from panel D; y-axis). Data are from untreated patients with CTLA4 deficiency, treated patients with CTLA4 deficiency, and healthy controls, as indicated. Simple linear regression with correlation coefficient (R2), P value, and 95% CI shown. ns, not significant.
Coincident with restoration of the appropriate balance of FO and TrB in the blood, we observed a decrease in activated B cells after abatacept therapy in patients with CTLA4 deficiency, specifically with a marked reduction in the frequency of circulating CD21loCD11chi B cells (Figure 6B). Accounting for high interpatient variability, we performed scRNA-seq on 1 treated patient and compared pretherapy with posttherapy naïve (IgD+CD27–) B-cell transcriptomes. Using unbiased uniform manifold approximation and projection clustering, a distinct pretreatment cluster of ABC-like aN B cells emerged that resolved after treatment with abatacept (Figure 6C). Additionally, the abatacept-mediated change in B-cell differentiation was coincident with resolution of the high CD40L levels previously observed on TEMRA CD4+ T cells to those seen in healthy controls (Figure 6D). In contrast to the robust changes in the B-cell compartment, total subsets of effector memory and TEMRA CD4+ T cells remained significantly unchanged before to after treatment with abatacept (supplemental Figure 8). Finally, correlation by linear regression remained significant between CD40L levels, in unstimulated TEMRA CD4+ T cells specifically, and circulating frequencies of FO B cells, including now patients with CTLA4 deficiency in pre–abatacept-treated and post–abatacept-treated states (P = .0206, R2 = 0.22; Figure 6E). Together, these data suggest baseline extrafollicular T-dependent B-cell activation in CTLA4 deficiency and restoration of normal FO B-cell differentiation after ≥3 months of abatacept therapy.
Discussion
Patients with CTLA4 deficiency have well described humoral immune dysfunction, characterized by lymphopenia, reduced class-SWM B cells, and expanded activated CD21lo B cells.2-4 Potential underlying mechanisms for this B-cell dysregulation include increased B-cell apoptosis,27,28 B-cell trafficking to end-organs, and/or impaired B-cell activation and differentiation. Here, we demonstrated an increased frequency of circulating late TrBs with a corresponding loss of FO B cells in patients with CTLA4 deficiency. Because these late TrBs are MitoTracker+CD10–, these data explain the lack of previous descriptions of impaired transitional-to-FO B-cell development, because previous studies only used CD10, CD21, and CD38 surface levels to phenotype naïve B cells in patients with CTLA4 deficiency.3 Our data support a model of dysregulated B-cell differentiation, specifically between the late transitional and FO B-cell stages. In physiologic states of CTLA4 deficiency, congenital or iatrogenic, dysregulated, and hyperactive CD4+ T cells likely activate less mature TrBs, driving extrafollicular anti–self-responses. A large portion of the repertoire is thus diverted, B cells are less capable of generating germinal center responses, and immunodeficiency occurs as a consequence.
Although circulating FO B cells were proportionately reduced in patients with CTLA4 deficiency, we observed higher frequencies of circulating aN B cells. These data align with the description of extrafollicular T-dependent B-cell activation in other strongly immune-activating conditions, including systemic autoimmunity,29 viral infection and immunization,30,31 and in patients with partial RAG deficiency.16 scRNA-seq supported a cluster of ITGAX+TBX21+ B cells in patients with CTLA4 deficiency, most consistent with ABC-like aN B cells. These ABC-like aN B cells also expressed a subset of genes that were shared with TrBs. Although the frequencies of class-SWM B cells were significantly attenuated in CTLA4 deficiency, the frequencies of IgD–CD27– DN B cells were not diminished. These findings also suggest a bias toward an extrafollicular rather than a germinal center response. A limitation of this work was the lack of characterization of DN B-cell subsets (DN1-4) and the absence of scRNA-seq analysis in B-cell compartments outside the IgD+CD27– “naïve” B-cell gate. These subpopulations will be characterized in future studies.
Similar to patients with PI3K-δ gain-of-function mutation,1 patients with CTLA4 deficiency demonstrated a transcriptomic signature of increased mTORC1 activation in naïve (IgD+CD27–) B cells. Specifically, through scRNA-seq we observed increased transcriptomic signatures of oxidative phosphorylation and elevated mTORC1 signaling in transitional and ABC-like aN B cells compared with FO B cells. Moreover, CD40L induced (p)-S6 signaling in TrBs and impaired in vitro generation of FO B cells. These data support 1 model whereby TrBs are developmentally impaired in patients with CTLA4 deficiency through the interaction “in trans” with CD40L on activated effector CD4+ T cells. We accept that enhanced conversion of naïve FO B cells into short-lived aN B cells could also potentially contribute to a relative decrease in circulating FO B cells and that an inflammatory milieu beyond CD40L may have contributed, in a yet to be fully explained manner, to the survival and expansion of TrBs. Although here we suggest lymphoid organs to be 1 site of potential T–B-cell interaction, patients with CTLA4 deficiency have prominent infiltration of nonlymphoid organs (eg, lungs) with expanded T- and B-cell populations, which could also drive pathologic T–B-cell interactions.2-4 Anatomical differences in B-cell niches between mice and human may further contribute to the different species-specific phenotypes of mutations that influence T–B-cell collaboration.32 Finally, although possible that inherited CTLA4 deficiency could cause direct TrB dysfunction that is corrected by CTLA4 immunoglobulin replacement therapy, we failed to demonstrate discernable intracellular RNA levels of CTLA4 in naïve (IgD+CD27–) B cells, including transitional and FO B-cell subsets, and intracellular protein levels were not reliably detectable in T3/FO B cells by flow cytometry (supplemental Figure 3).
In summary, we observed maturational impairment of transitional-to-FO B cells that correlated linearly with lower CTLA4 levels and function in FOXP3+ Tregs and higher CD40L levels in TEMRA CD4+ T cells. Direct cell-to-cell contacts between CD40L+ activated T cells and IgD+CD27– B cells were visualized in the T-cell zones of patient lymph nodes. CTLA4 immunoglobulin corrected the B-cell phenotype, with a decrease in TrBs and ITGAX+ ABC-like aN B cells after therapy with abatacept by scRNA-seq. These findings indirectly suggest that functional Tregs are required for the maturation of metabolically quiescent mature FO B cells, which can be rescued using CTLA4 immunoglobulin therapy.
Acknowledgments
The authors thank Bodo Grimbacher and David Sansom for kindly providing the CHO cells stably overexpressing CD80-GFP,10 and the tissue processing core at the Ragon Institute for the processing and biobanking of the samples from patients with CTLA4 deficiency.
Funding was provided by the American Society of Hematology Research Training award for fellows, National Institutes of Health (NIH) grants 5T32HL007574-38, K23AI163350 (S.B.), U19 AI110495, P30-AI 060354 (M.G.), and T32-HL116275, Sala Elbaum Pediatric Research Scholars Program (K.H.), Ragon Institute (S.P.); and the American Academy of Allergy, Asthma & Immunology faculty development award and Bristol Myers Squibb (J.R.F.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Authorship
Contribution: J.R.F., H.A.-C., and S.P. contributed to conceptualization; J.R.F., S.B., E.W.-C., J.E.W., and A.K.S. were involved in patient sample contribution; J.R.F., K.H., H.A.-C., A.B., C.T., M.L.R., G.Y., V.M., R.C., K.P., N.K., and M.G. contributed to methodology; K.H., J.R.F., and S.P. were involved in funding acquisition; A.K.S., J.R.F., and S.P. were responsible for supervision; J.R.F., K.H., and H.A.-C. wrote the original draft of the manuscript; and all authors were involved in reviewing and editing of the manuscript.
Conflict-of-interest disclosure: J.R.F. was supported by an investigator-initiated research grant from Bristol Myers Squibb. A.K.S. is a founder and serves on the scientific advisory board of Honeycomb Biotechnologies, which is developing Seq-Well arrays for commercial use. S.P. serves on the scientific advisory boards of Abpro Inc, Paratus, and BE Biopharma Inc. H.A.-C. was supported by investigator-initiated research grants from Pfizer, AstraZeneca, Fresenius Kabi, and Boehringer Ingelheim. The remaining authors declare no competing financial interests.
The current affiliation for K.H. is Division of Pediatric Hematology-Oncology, Department of Pediatrics, Hassenfeld Children's Hospital at New York University Langone Health, New York University Grossman School of Medicine, New York, NY.
The current affiliation for J.R.F. is Clinical Immunodeficiency Program of Beth Israel Lahey Health, Division of Allergy and Immunology, Lahey Hospital & Medical Center, Burlington, MA.
Correspondence: Jocelyn R. Farmer, Clinical Immunodeficiency Program of Beth Israel Lahey Health, Division of Allergy and Immunology, Lahey Hospital & Medical Center, 31 Mall Rd, Burlington, MA 01805; email: jocelyn.farmer@lahey.org; and Shiv Pillai, Ragon Institute of Mass General, Massachusetts Institute of Technology, and Harvard, 600 Main St, Cambridge, MA 02139; email: spillai@mgh.harvard.edu.
References
Author notes
H.A.-C., K.H., J.R.F., and S.P. contributed equally to this work.
The single-cell RNA-sequencing data reported in this publication have been deposited in the National Center for Biotechnology Information’s Gene Expression Omnibus database (accession number GSE188449), and on the Broad Institute Single Cell Portal (accession number SCP1648).
All data needed to evaluate the conclusions in this article are present in the main article or the supplemental Materials.
The full-text version of this article contains a data supplement.

![Increased frequency of circulating TrBs and decreased frequency of circulating FO B cells in patients with CTLA4 deficiency. Flow cytometric analysis of peripheral blood B-cell populations in patients with CTLA4 deficiency (n = 11) and healthy controls (n = 16). (A) Representative plots from a healthy control (left) and a patient with CTLA4 deficiency (right, homozygous CTLA4 3′ UTR mutation carrier), shown at 5% of events with gating strategy outlined (full description of B-cell gating strategy in supplemental Figure 1A). (B) Quantification of major B-cell subsets in the peripheral blood, including naïve, marginal zone (MZ), SWM, and DN populations as subset from total CD19+ B cells by the markers IgD and CD27. (C) Quantification of naïve (IgD+CD27–) B-cell subsets in the peripheral blood, including T1/2, T3a, and T3b TrBs, resting mature FO B cells, aN B cells, and MZP B cells as further subsets by the indicated markers. For the CD45RB by CD21 and count by CD73 B-cell subset plots, final IgD+CD27– B-cell frequency was derived as a percentage of the indicated parent gate. Symbols represent unique individuals; bars represent means (± standard deviation [SD]) of all data. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001 or as listed by 2-tailed Mann-Whitney U test. MZP, MZ precursor.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/3/10.1182_bloodadvances.2024013267/4/m_blooda_adv-2024-013267-gr1.jpeg?Expires=1767701113&Signature=VHHzQWKN~ZEcJNyGbPNoTn30vGMS5fWqeI85m~b6K37gtuhorV61IBYw5mswnBPw1J2GNtpjsSrSzGBB3KbHaB2iISluvaHPcnhcQYs0x~BjB5L3uhud64JjTv5TTdA40Y1Lw2VJjKV~2SRY9JweNMolkBtEkJHm4aPe4PZxdAx7iJRDBK~kSS~QSsFdYP6Fy7R5UAXSP4N7mE14yYTSoISYn-sa-zkabsk~nLWlOB1dVHkL0YsYJoKZVo7SVgD5RooOToOqhf3mhmi8EVp-~9-atqKhDvk0Yes5ssqgL7V1PsCt7X1ZDEehKNd1d54EFPqUKfui-AN7i9NCe6ld9A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)