Key Points
The coronary calcium score calculated on pretreatment PET/CT identifies patients with lymphoma at risk for cardiac events after anthracycline therapy.
Coronary calcium score may identify a target lymphoma population that is poised to benefit from tailored cardioprotective strategies.
Visual Abstract
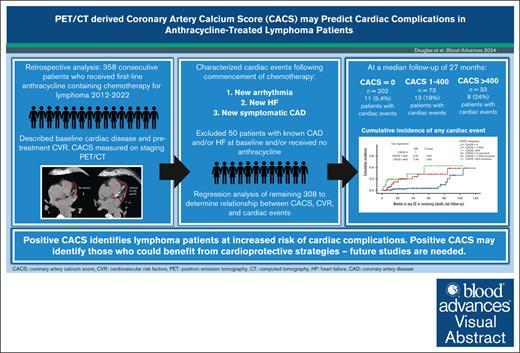
Anthracycline-mediated cardiotoxicity is a common concern after lymphoma therapy, particularly in patients with high cardiovascular risk (CVR). In noncancer populations, coronary artery calcium scoring (CACS) effectively identifies individuals who may benefit from aggressive CVR modification to lower the risk of cardiovascular events. Emerging evidence suggests that CACS can also predict cancer therapy-related cardiotoxicity, potentially identifying candidates for cardioprotective strategies. Our study aimed to evaluate whether CACS obtained from pretreatment positron emission tomography (PET)/computed tomography (CT) scans could stratify cardiac event risk in patients with lymphoma receiving anthracycline-based chemotherapy. We enrolled 358 consecutive patients with lymphoma treated between 2012 and 2022, calculating the CACS from their pretreatment PET/CT. We reviewed medical records to identify pre-existing cardiac conditions, CVR, and posttreatment cardiac events, including coronary events, heart failure (HF), and arrhythmias. Logistic and Cox regression models were used to assess associations between CVR, CACS categories (CACS = 0, CACS 1-400, CACS >400), and new cardiac events. At a median follow-up of 27 months (95% confidence interval [CI], 22.3-31.7) in patients without cardiac history, 10% experienced posttreatment cardiac events (HF, 14; arrhythmias, 9; coronary event, 1; combination, 8). Patients with a CACS >0 had more events (21 total, 20% vs 11 total, 5.4% for CACS = 0; P < .001). Elevated CACS was independently associated with HF (CACS 1-400: odds ratio [OR], 3.73; 95% CI, 1.21-11.43; P = .022; CACS >400: OR, 5.43; 95% CI, 1.47-20.03; P = .011) and any cardiac event (CACS 1-400: OR, 2.48; 95% CI, 1.02-6.04; P = .045; CACS >400: OR, 3.28; 95% CI, 0.91-10.68; P = .029). CACS may effectively stratify patients with lymphoma at risk of cardiac complications, thereby identifying a group poised to benefit from targeted preventive strategies.
Introduction
More than 50% of aggressive lymphomas are treated with anthracycline-containing chemotherapies. Five-year survival for patients with Hodgkin lymphoma and non-Hodgkin lymphoma exceeds 85% and 70%, respectively.1,2 However, there is a known significant cardiotoxicity risk associated with anthracyclines. Reducing the cancer therapy-related cardiotoxicity (CTRCT) burden in lymphoma survivorship is therefore a high priority.3,4 In Hodgkin lymphoma, survivors experience a fourfold to sixfold higher incidence of coronary artery disease (CAD) or heart failure (HF) than the general population. Mediastinal radiation and cumulative anthracycline dose independently increase risk.5 Cumulative incidence of cardiovascular disease in patients with non-Hodgkin lymphoma has been reported at 22% at 10 years, with a marked increase in HF compared with controls, in large population-based studies.3,6-8 There is a complex interplay of risks for CTRCT in patients with cancer. Anthracycline-mediated toxicity is attributed to numerous direct effects on cardiac myocytes.9 However, established cardiovascular risk (CVR) factors, including age, coronary disease, hypertension, dyslipidemia, diabetes, smoking, and obesity, are exceedingly common in the general community and have a significant influence on the development of CTRCT.6,10-12 Once established, anthracycline-related cardiomyopathy may be irreversible, and can significantly compromise the quality of life and overall survival of affected patients.13 Despite this, there are no lymphoma-specific guidelines recommending full risk assessment, intensive risk mitigation, or targeted monitoring for CVR in the longer term. In addition, comprehensive cardiac assessment and management at the time of lymphoma diagnosis are potentially heavy and not uniformly available for unselected populations. Identifying a target population to maximize the therapeutic yield of intensive CVR factor management is therefore desirable.
Coronary artery calcium scoring (CACS) measures calcium accumulation within the coronary arteries using cardiac computed tomography (CT). A positive CACS is a marker of coronary atherosclerosis, with increasing CACS correlating to higher cardiovascular event rates.14-16 CACS is used within the asymptomatic general population as a modality to reclassify CVR, where the indication for pharmacologic risk modification requires more clarity.17,18 CACS has not been validated specifically within cancer populations; however, evidence is emerging of its role in predicting CTRCT and other posttreatment cardiac events.19-22
Given the high risk of late cardiovascular events in patients with aggressive lymphoma, we aimed to evaluate the feasibility and value of CACS calculations using low-dose CT in patients with lymphoma undergoing routine staging whole-body position emission tomography (PET)/CT. We further aimed to describe the associations between baseline CVR, CACS, and cardiac disease rates in candidates for first-line anthracycline-containing lymphoma therapy.
Methods
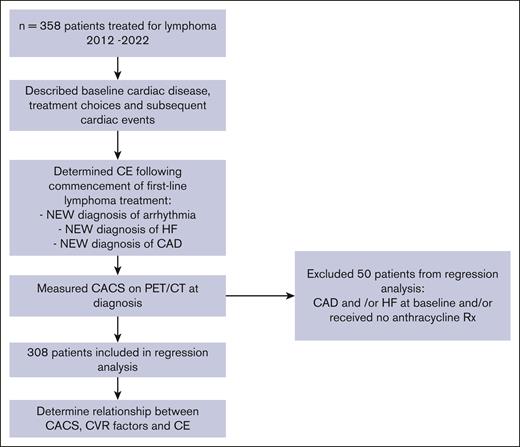
This single-center study identified consecutive patients from our institutional database with treatment-naïve aggressive lymphoma who required first-line anthracycline-containing combination chemotherapy between January 2012 and May 2022 (Figure 1). Eligible adult patients were required to have a staging PET/CT within 2 months of diagnosis and have received ≥1 cycle of chemotherapy. Clinical data were extracted from electronic patient records and included demographics, lymphoma type and treatment, cardiac disease history, and CVR factors as defined by Australian cardiovascular disease risk assessment guidelines: body mass index ≥25, estimated glomerular filtration rate <45 mL/min, hypercholesterolemia, smoking, hypertension, and diabetes.17 Family history and ethnicity were not routinely recorded and therefore were not collected. Details of any cardiac events during the follow-up period (from treatment commencement until last clinical review) were also collected and defined as: (1) new acute myocardial infarct and/or need for coronary intervention (“new CAD”), (2) new onset heart failure (“new HF”): included symptomatic inpatient or outpatient presentation and/or new echocardiogram findings of left ventricular dysfunction <50%, noting that no routine posttreatment cardiac function monitoring was performed, (3) new onset arrhythmia (“new arrhythmia”). Representations with known prior arrhythmias were not considered to be new cardiac events.
Low-dose (120 kVp, nominal current of 50 mA with a maximum of 80 mA), noncontrast CT performed in conjunction with fluorodeoxyglucose PET performed for the initial staging of lymphoma was used to calculate the coronary artery calcium score by qualified nuclear medicine physicians. The scores were universally cross-checked and approved by a single senior nuclear medicine physician to ensure quality and consistency of reporting. PET/CT was performed using Philips Ingenuity TF 128 (Philips Medical System, Cleveland, OH). The CT component was viewed using OsiriX MD (Switzerland) version 14.0, a medical imaging Digital Imaging Communications in Medicine viewer. The calcium scoring plugin in the OsiriX MD software allowed calcium score calculation, which is Agatston equivalent to a threshold of 130 Hounsfield units. Each vessel was individually tracked, and every focus of calcification was marked. A final CACS was generated, showing the individual calcium score of each vessel and the total CACS summed from the individual scores. This methodology differs from the CACS performed for population-based vascular risk assessment, in which the CT slice size was prospectively controlled and gating was applied. The slice sizes were 2 to 4 mm, and the CT scans were uniformly nongated. Therefore, the CACS values obtained in this study cannot be directly compared with those obtained from routine CACS assessments. CACS categories commonly used for vascular risk assessment include: 0, 1 to 100, 100 to 400, and >400.18 However, due to smaller numbers with CACS ≥1 in our data set, we divided CACS into 3 categories: 0 vs ≥1 agatston units (AU) (ie, any positivity), 1 to 400, and >400 AU.
Descriptive statistics were used to summarize data, including frequencies and proportions for discrete data and median and range for continuous data. The follow-up time was calculated using the reverse Kaplan-Meier method. χ2 or Fisher exact tests were used for comparison of categorical variables, with statistical significance set at P < .05. Univariable and multivariable logistical regression analyses were performed to identify the associations between CACS, baseline CVR factors, and the development of new heart failure or arrhythmias, using odds ratio (OR) and 95% confidence intervals (CI). The cumulative incidence of cardiac events was determined using the reverse Kaplan-Meier method, with Cox regression and hazard ratios (HR) (with corresponding 95% CI) to determine the impact of CACS on the cumulative incidence of cardiac events. Receiver operating characteristic analyses were used to determine the sensitivity and specificity of the pretreatment CACS for new cardiac events. Statistical analysis was performed using SPSS statistical software version 25 (IBM Corp, 2017). This study was approved by the institutional review board (LNR17/Austin/186).
Results
During the 10-year study period, 358 eligible patients were identified. The median follow-up was 40 months (95% CI, 31.1-48.9). Baseline demographics, treatment, and CVR characteristics are detailed in Table 1. Pre-existing cardiac disease was documented in 70 of 358 (19.6%) patients, including arrhythmia (n = 26; all atrial fibrillation), HF (n = 6), CAD (n = 18), and >1 diagnosis (n = 20). The patient analysis workflow is detailed in Figure 1.
Baseline characteristics and CVR factors
| Whole cohort . | CVR comparison according to CACS in 308-patient subset without prior CAD or HF . | ||||
|---|---|---|---|---|---|
| Characteristics . | n (%) . | CACS = 0 (N = 202) . | CACS 1-400 (N = 73) . | CACS >400 (N = 33) . | P value . |
| Total | 308 (100) | 202 | 73 | 33 | NA |
| Male sex | 199 (56) | 97 (48%) | 48 (66%) | 22 (67%) | .011 |
| Median age, y (range) | 66 (19-93) | 55 (19-93) | 69 (44-93) | 72 (46-89) | <.001 |
| No. of chemotherapy cycles, median (range) | 6 (1-6) | 6 (1-6) | 6 (1-6) | 6 (1-6) | .646 |
| Mediastinal radiotherapy exposure | 9 (3) | 8 (4%) | 1 (1%) | 0 (0%) | .301 |
| Modifiable CVR factors | |||||
| BMI ≥25 | 212 (59) | 102 (51%) | 54 (74%) | 19 (57.6%) | .002 |
| eGFR <45 | 33 (9) | 14 (7%) | 5 (7%) | 4 (12.1%) | .56 |
| Hypercholesterolaemia | 100 (28) | 28 (14%) | 26 (36%) | 16 (48.5%) | <.001 |
| Hypertension | 138 (39) | 48 (24%) | 33 (45%) | 20 (60.6%) | <0.001 |
| Diabetes | 64 (18) | 16 (8%) | 21 (42.9%) | 12 (36.4%) | <.001 |
| Smoking history | 130 (36) | 63 (31%) | 25 (23.6%) | 19 (57.6%) | .049 |
| Any cardiac risk factor | 239 (67) | 110 (55%) | 54 (28.1%) | 29 (90%) | <.001 |
| ≥2 cardiac risk factors | 135 (38) | 42 (21%) | 31 (32.3%) | 23 (70%) | <.001 |
| Median number cardiac RFs (range) | 1 (0-4) | 1 (0-4) | 1 (0-4) | 2 (0-4) | <.001 |
| Cardiac events on treatment | |||||
| New arrhythmia | 20 (6) | 7 (3.5%) | 3 (4%) | 6 (18%) | .002 |
| New heart failure | 26 (7) | 6 (3%) | 9 (12%) | 6 (18%) | <.001 |
| New CAD | 11 (3) | 1 (0.5%) | 2 (3%) | 1 (3%) | .222 |
| Any cardiac event | 44 (12) | 11 (5.4%) | 13 (18%) | 8 (24.2%) | <.001 |
| Months to first cardiac event, median (range) | 14.5 (1-104) | 72 (6-104) | 14 (6-84) | 5 (1-54) | .002 |
| Diagnosis | |||||
| DLBCL | 275 (77) | ||||
| Other high-grade DLBCL | 24 (7) | ||||
| PTCL | 2 (1) | ||||
| HL | 55 (15) | ||||
| Indolent lymphoma | 2 (1) | ||||
| Whole cohort . | CVR comparison according to CACS in 308-patient subset without prior CAD or HF . | ||||
|---|---|---|---|---|---|
| Characteristics . | n (%) . | CACS = 0 (N = 202) . | CACS 1-400 (N = 73) . | CACS >400 (N = 33) . | P value . |
| Total | 308 (100) | 202 | 73 | 33 | NA |
| Male sex | 199 (56) | 97 (48%) | 48 (66%) | 22 (67%) | .011 |
| Median age, y (range) | 66 (19-93) | 55 (19-93) | 69 (44-93) | 72 (46-89) | <.001 |
| No. of chemotherapy cycles, median (range) | 6 (1-6) | 6 (1-6) | 6 (1-6) | 6 (1-6) | .646 |
| Mediastinal radiotherapy exposure | 9 (3) | 8 (4%) | 1 (1%) | 0 (0%) | .301 |
| Modifiable CVR factors | |||||
| BMI ≥25 | 212 (59) | 102 (51%) | 54 (74%) | 19 (57.6%) | .002 |
| eGFR <45 | 33 (9) | 14 (7%) | 5 (7%) | 4 (12.1%) | .56 |
| Hypercholesterolaemia | 100 (28) | 28 (14%) | 26 (36%) | 16 (48.5%) | <.001 |
| Hypertension | 138 (39) | 48 (24%) | 33 (45%) | 20 (60.6%) | <0.001 |
| Diabetes | 64 (18) | 16 (8%) | 21 (42.9%) | 12 (36.4%) | <.001 |
| Smoking history | 130 (36) | 63 (31%) | 25 (23.6%) | 19 (57.6%) | .049 |
| Any cardiac risk factor | 239 (67) | 110 (55%) | 54 (28.1%) | 29 (90%) | <.001 |
| ≥2 cardiac risk factors | 135 (38) | 42 (21%) | 31 (32.3%) | 23 (70%) | <.001 |
| Median number cardiac RFs (range) | 1 (0-4) | 1 (0-4) | 1 (0-4) | 2 (0-4) | <.001 |
| Cardiac events on treatment | |||||
| New arrhythmia | 20 (6) | 7 (3.5%) | 3 (4%) | 6 (18%) | .002 |
| New heart failure | 26 (7) | 6 (3%) | 9 (12%) | 6 (18%) | <.001 |
| New CAD | 11 (3) | 1 (0.5%) | 2 (3%) | 1 (3%) | .222 |
| Any cardiac event | 44 (12) | 11 (5.4%) | 13 (18%) | 8 (24.2%) | <.001 |
| Months to first cardiac event, median (range) | 14.5 (1-104) | 72 (6-104) | 14 (6-84) | 5 (1-54) | .002 |
| Diagnosis | |||||
| DLBCL | 275 (77) | ||||
| Other high-grade DLBCL | 24 (7) | ||||
| PTCL | 2 (1) | ||||
| HL | 55 (15) | ||||
| Indolent lymphoma | 2 (1) | ||||
BMI, body mass index; DLBCL, diffuse large B-cell lymphoma; eGFR, estimated glomerular filtration rate; HL, Hodgkin lymphoma; PTCL, peripheral T-cell lymphoma; RFs, risk factors.
Values in bold indicate statistically significant P values <0.05.
Baseline CVR assessment and management
Overall, 239 patients (67%) had ≥1 documented modifiable CVR factor, and 135 (38%) had ≥2. The most common risk factors were elevated body mass index in 212 (59%), smoking in 130 (36%), and hypercholesterolemia in 100 (28%). No pre-emptive cardiology reviews were performed for patients to formally assess or optimize risk in the absence of established cardiac disease. Patients with cardiac disease, particularly HF, received lower cumulative doses of anthracycline (median 175 mg/m2 doxorubicin) than those without (median 300 mg/m2); however, dose reduction/omission was nonuniform. New cardiac events occurred in 29% of patients with pre-existing cardiac disease (Table 2) and were significantly increased compared with those without pre-existing cardiac disease (OR, 2.23; 95% CI, 1.1-4.5; P = .025). Those with a history of CAD had a particularly high rate of subsequent cardiac events (CE) (13/18, 72.2%). Among the patients with known pre-existing cardiac disease, 52 of 70 (74.3%) had a cardiologist actively involved in their care.
Cardiac events after commencement of lymphoma treatment
| . | N . | Total new events (%) . | New CAD event . | New HF . | New arrhythmia . |
|---|---|---|---|---|---|
| No cardiac history | 286 | 29 (10.2) | 4 | 18 | 16 |
| Prior cardiac history | 70 | 20 (29) | 7 | 7 | 3 |
| CAD | 18 | 13 (72.2) | 5 | 3 | 2 |
| HF | 6 | 1 (16.6) | 1 | 0 | 0 |
| Arrhythmia | 26 | 3 (11.5) | 0 | 3 | 0 |
| >1 cardiac disease | 20 | 3 (15) | 1 | 1 | 1 |
| . | N . | Total new events (%) . | New CAD event . | New HF . | New arrhythmia . |
|---|---|---|---|---|---|
| No cardiac history | 286 | 29 (10.2) | 4 | 18 | 16 |
| Prior cardiac history | 70 | 20 (29) | 7 | 7 | 3 |
| CAD | 18 | 13 (72.2) | 5 | 3 | 2 |
| HF | 6 | 1 (16.6) | 1 | 0 | 0 |
| Arrhythmia | 26 | 3 (11.5) | 0 | 3 | 0 |
| >1 cardiac disease | 20 | 3 (15) | 1 | 1 | 1 |
Baseline CACS and new cardiac events
Of the 308 patients with no prior history of HF or CAD who received anthracycline, 204 (66%) received ≥250 mg/m2 doxorubicin equivalent. The remaining 104 patients received a median dose of 150 mg/m2 (interquartile range, 75-225). The reasons for lower anthracycline doses were not uniformly available. One hundred and four (34%) patients had a baseline CACS of >0, whereas 202 (66%) had a baseline CACS of 0 (Table 1). Those with elevated CACS (1-400 or >400) were statistically more likely to be male (48% vs 66% [CACS 1-400] and 67% [CACS >400], P = .011), older (55 years vs 69 [CACS 1-400] and 72 [CACS >400], P < .001), and had higher numbers of modifiable CVR factors (21% had ≥2 factors vs 32% [CACS 1-400] and 70% [CACS >400], P < .001). Overall, at a median follow-up of 27 months (95% CI, 22.3-31.7) for this cohort with no cardiac history, 32 (10%) patients experienced at least 1 posttreatment cardiac event (new-onset HF, n = 14; new arrhythmia, n = 9; new CAD event, n = 1; combination of events, n = 8). Cardiac events were more common in those with elevated CACS than in those with CACS = 0 (n = 11, 5.4% vs n = 13, 18% [CACS 1-400] and n = 8, 24% [CACS >400], P < .001) Median time to first cardiac event was shorter in those with elevated CACS (72 vs 14 months [CACS 1-400] and 5 months [CACS >400]), P < 0.001).
Two cardiac deaths occurred: 1 attributed to an ischemic event secondary to new CAD (CACS = 5039) and the other to HF and arrhythmia (CACS = 0).
Association between baseline CVR, CACS, and cardiac events
Univariable regression analyses are presented in Table 3. For the development of any new cardiac event, statistically significant associations were found for age >60 years, hypertension, any CVR factor, ≥2 CVR factors, CACS 1 to 400, and CACS >400. For the development of new arrhythmia, associations were found for age >60 years, hypercholesterolemia, hypertension, ≥2 CVR factors, and CACS >400. For the development of new heart failure, statistically significant associations included age >60 years, hypertension, ≥2 CVR factors, CACS 1 to 400, and CACS >400. Analysis of the CACS vascular risk categories of 0, 1 to 400, and >400 AU identified a relationship between higher CACS values and cardiac events. For any event, the OR for CACS 1 to 400 was 3.66 (95% CI, 1.97-9.24; P = .003), and the OR for CACS >400 was 5.50 (95% CI, 2.02-14.9; P = .001). New coronary events were rare within this analysis (4 events only); thus, analysis was not performed.
Univariable regression analysis: probability of developing cardiac events
| Covariate . | Any cardiac event . | New arrhythmia . | New heart failure . | |||
|---|---|---|---|---|---|---|
| OR (95% CI) . | P value . | OR (95% CI) . | P value . | OR (95% CI) . | P value . | |
| Male sex | 1.69 (0.78-3.63) | .182 | 1.20 (0.44-3.28) | .726 | 1.73 (0.69-4.43) | .248 |
| Age (per 10-y increase) | 1.78 (1.32-2.39) | <.001 | 2.06 (1.31-3.24) | .002 | 1.50 (1.09-2.04) | .011 |
| Age >60 y | 6.37 (2.18-18.6) | .001 | 12.36 (1.61-94.8) | .016 | 3.49 (1.15-10.6) | .028 |
| Total anthracycline dose | 0.99 (0.99-1.01) | .592 | 1.001 (0.995-1.01) | .595 | 0.998 (0.99-1.00) | .576 |
| Modifiable CVR factors | ||||||
| BMI ≥25 | 2.93 (1.23-7.00) | .016 | 3.37 (0.94-12.1) | .062 | 1.91 (0.72-5.06) | .195 |
| eGFR <45 | 1.25 (0.35-4.45) | .729 | _ | .998 | 2.09 (0.57-7.67) | .267 |
| Hypercholesterolaemia | 2.22 (1.03-4.81) | .042 | 3.65 (1.32-10.1) | .013 | 1.36 (0.51-3.66) | .539 |
| Hypertension | 4.57 (2.11-9.92) | <.001 | 6.67 (2.09-21.2) | .001 | 3.56 (1.43-8.90) | .007 |
| Diabetes | 1.55 (0.63-3.82) | .338 | 2.54 (0.84-7.67) | .098 | 1.28 (0.41-3.99) | .667 |
| Smoking history | 0.70 (0.32-1.56) | .702 | 0.41 (0.12-1.47) | .173 | 0.91 (0.36-2.30) | .839 |
| No. of cardiac risk factors | ||||||
| No risk factors | 1 | 1 | 1 | |||
| 1 risk factor | 3.61 (1.13-11.6) | .031 | 2.82 (0.53-14.8) | .222 | 3.12 (0.80-12.1) | .101 |
| ≥2 risk factors | 5.24 (1.69-16.30) | .004 | 5.47 (1.15-25.9) | .033 | 4.06 (1.08-15.2) | .038 |
| Coronary artery calcium score (positive vs negative) | ||||||
| CACS = 0 (n = 202) | 1 | 1 | 1 | |||
| CACS ≥1 (n = 106) | 4.27 (1.97-9.24) | <.001 | 2.60 (0.94-7.19) | .066 | 5.48 (2.06-14.6) | .001 |
| Coronary calcium score (categorized) | ||||||
| CACS = 0 (n = 202) | 1 | 1 | 1 | |||
| CACS 1-400 (n = 73) | 3.66 (1.56-8.60) | .003 | 0.74 (0.21-2.67) | .65 | 2.69 (1.08-6.67) | .033 |
| CACS >400 (n = 33) | 5.50 (2.02-14.9) | .001 | 5.82 (1.96-17.26) | .001 | 3.79 (1.36-10.59) | .011 |
| Covariate . | Any cardiac event . | New arrhythmia . | New heart failure . | |||
|---|---|---|---|---|---|---|
| OR (95% CI) . | P value . | OR (95% CI) . | P value . | OR (95% CI) . | P value . | |
| Male sex | 1.69 (0.78-3.63) | .182 | 1.20 (0.44-3.28) | .726 | 1.73 (0.69-4.43) | .248 |
| Age (per 10-y increase) | 1.78 (1.32-2.39) | <.001 | 2.06 (1.31-3.24) | .002 | 1.50 (1.09-2.04) | .011 |
| Age >60 y | 6.37 (2.18-18.6) | .001 | 12.36 (1.61-94.8) | .016 | 3.49 (1.15-10.6) | .028 |
| Total anthracycline dose | 0.99 (0.99-1.01) | .592 | 1.001 (0.995-1.01) | .595 | 0.998 (0.99-1.00) | .576 |
| Modifiable CVR factors | ||||||
| BMI ≥25 | 2.93 (1.23-7.00) | .016 | 3.37 (0.94-12.1) | .062 | 1.91 (0.72-5.06) | .195 |
| eGFR <45 | 1.25 (0.35-4.45) | .729 | _ | .998 | 2.09 (0.57-7.67) | .267 |
| Hypercholesterolaemia | 2.22 (1.03-4.81) | .042 | 3.65 (1.32-10.1) | .013 | 1.36 (0.51-3.66) | .539 |
| Hypertension | 4.57 (2.11-9.92) | <.001 | 6.67 (2.09-21.2) | .001 | 3.56 (1.43-8.90) | .007 |
| Diabetes | 1.55 (0.63-3.82) | .338 | 2.54 (0.84-7.67) | .098 | 1.28 (0.41-3.99) | .667 |
| Smoking history | 0.70 (0.32-1.56) | .702 | 0.41 (0.12-1.47) | .173 | 0.91 (0.36-2.30) | .839 |
| No. of cardiac risk factors | ||||||
| No risk factors | 1 | 1 | 1 | |||
| 1 risk factor | 3.61 (1.13-11.6) | .031 | 2.82 (0.53-14.8) | .222 | 3.12 (0.80-12.1) | .101 |
| ≥2 risk factors | 5.24 (1.69-16.30) | .004 | 5.47 (1.15-25.9) | .033 | 4.06 (1.08-15.2) | .038 |
| Coronary artery calcium score (positive vs negative) | ||||||
| CACS = 0 (n = 202) | 1 | 1 | 1 | |||
| CACS ≥1 (n = 106) | 4.27 (1.97-9.24) | <.001 | 2.60 (0.94-7.19) | .066 | 5.48 (2.06-14.6) | .001 |
| Coronary calcium score (categorized) | ||||||
| CACS = 0 (n = 202) | 1 | 1 | 1 | |||
| CACS 1-400 (n = 73) | 3.66 (1.56-8.60) | .003 | 0.74 (0.21-2.67) | .65 | 2.69 (1.08-6.67) | .033 |
| CACS >400 (n = 33) | 5.50 (2.02-14.9) | .001 | 5.82 (1.96-17.26) | .001 | 3.79 (1.36-10.59) | .011 |
Values in bold indicate statistically significant P values <0.05.
Multivariable binomial regression analysis (Table 4), with age >60 years, >2 CVR risk factors, and CACS categories included in the regression model, identified that CACS 1 to 400 and CACS >400 were both independent risk factors for HF and any cardiac event, but were not specifically associated with arrhythmia. Again, for HF and any cardiac event, the relationship was stronger for CACS >400. For any cardiac event, OR for CACS 1 to 400 was 2.48 (95% CI, 1.02-6.04; P = .045) whereas OR for CACS >400 was 3.28 (95% CI, 1.13-9.53; P = .029). For HF, OR for CACS 1 to 400 was 3.72 (95% CI, 1.21-11.43; P = .022) whereas OR for CACS >400 was 5.43 (95% CI, 1.47-20.03; P = .011). We tested for multicollinearity using variance inflation factor analysis, which was <5 for all covariates, suggesting no significant collinearity within the multivariable analysis.
Multivariable regression analysis for probability of cardiac events
| Covariate . | All cardiac events . | New heart failure . | Arrhythmia . | |||
|---|---|---|---|---|---|---|
| OR (95% CI) . | P value . | OR (95% CI) . | P value . | OR (95% CI) . | P value . | |
| CACS 0 | 1 | 1 | 1 | |||
| CACS 1-400 | 2.48 (1.02-6.04) | .045 | 3.72 (1.21-11.43) | .022 | 0.70 (0.17-2.85) | .615 |
| CACS >400 | 3.28 (1.13-9.53) | .029 | 5.43 (1.47-20.03) | .011 | 3.12 (0.91-10.68) | .07 |
| ≥2 cardiac risk factors | 1.12 (0.50-2.52) | .789 | 0.98 (0.36-2.64) | .969 | 1.24 (0.41-3.73) | .70 |
| Age >60 y | 4.22 (1.34-13.30) | .014 | 2.11 (0. 62-7.18) | .233 | 9.93 (1.20-82.47) | .233 |
| Covariate . | All cardiac events . | New heart failure . | Arrhythmia . | |||
|---|---|---|---|---|---|---|
| OR (95% CI) . | P value . | OR (95% CI) . | P value . | OR (95% CI) . | P value . | |
| CACS 0 | 1 | 1 | 1 | |||
| CACS 1-400 | 2.48 (1.02-6.04) | .045 | 3.72 (1.21-11.43) | .022 | 0.70 (0.17-2.85) | .615 |
| CACS >400 | 3.28 (1.13-9.53) | .029 | 5.43 (1.47-20.03) | .011 | 3.12 (0.91-10.68) | .07 |
| ≥2 cardiac risk factors | 1.12 (0.50-2.52) | .789 | 0.98 (0.36-2.64) | .969 | 1.24 (0.41-3.73) | .70 |
| Age >60 y | 4.22 (1.34-13.30) | .014 | 2.11 (0. 62-7.18) | .233 | 9.93 (1.20-82.47) | .233 |
Values in bold indicate statistically significant P values <0.05.
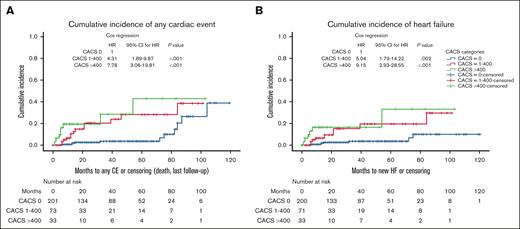
The cumulative incidence of cardiac events is shown in Figure 2. Time-dependent Cox regression, censored for death/last follow-up identified a statistically significant association between CACS categories and any cardiac event (for CACS 1-400: HR, 4.31; P < .001; 95% CI, 1.89-9.87; and for CACS >400: HR, 7.78; P < .001; 95% CI, 3.06-19.81), and HF (for CACS 1-400: HR, 5.04; P = .002; 95% CI, 1.79-14.22; and for CACS >400: HR, 9.15; P < 0.001; 95% CI, 2.93-28.55).
Cumulative incidence and COX regression for cardiac events according to CACS (censored at death/last follow-up). HF, heart failure; CE, cardiac event.
Cumulative incidence and COX regression for cardiac events according to CACS (censored at death/last follow-up). HF, heart failure; CE, cardiac event.
Receiver operating characteristic analyses with varying CACS cutoffs are presented in supplemental Figure 1, demonstrating modest sensitivity and specificity of CACS for HF (area under the curve, 0.698; 95% CI, 0.58-0.81), and also for any cardiac event (area under the curve, 0.671; 95% CI, 0.57-0.77).
Discussion
To our knowledge, this is the first large-scale study to assess the frequency and implications of CACS quantification from staging PET/CT in the context of baseline cardiac risk factor profiling in patients with lymphoma undergoing anthracycline chemotherapy. We identified that pre-existing cardiac disease and elevated CVR in candidates for anthracycline-based chemotherapy are common (19.6% and 67% of patients, respectively), and no uniform approach to formal cardiologic assessment and management exists. Patients with pre-existing cardiac disease are not unexpectedly at high risk of future events, particularly those with known CAD (new cardiac event rate, 72.2%), and new postchemotherapy cardiac events occurred early. Furthermore, CACS quantification using baseline staging PET/CT in patients with lymphoma is feasible. Importantly, in those with no cardiac history, elevated CACS was identified in 34%. In patients with CACS 1 to 400, this was associated with a >3-fold risk of new postchemotherapy cardiac events and a >2-fold risk of HF. In those with a CACS >400, there was a >5-fold risk of new postchemotherapy cardiac events and >3-fold risk of HF. The rates of new cardiac events, arrhythmia, and CAD are in keeping with recent large analyses: 5-year rates of grade 3 to 4 cardiovascular disease and HF were reported at 2.34% and 7.9% respectively in a pan-cancer systematic review,8 and a dedicated cyclophsophamide, doxorubicin, vincristine and prednisolone chemotherapy-specific population-based UK study with 5-year rates of arrhythmia at 1.3% to 5.7%, CAD at 0.8% to 2.0%, and HF of 0.6% to 4.6%.6
CACS is not widely used for CTRCT risk stratification in dedicated anthracycline-treated patient cohorts with lymphoma; however, data are emerging on the utility of CACS in identifying high cardiac risk patients within other cancer populations. Most studies are consistent with our results, showing a strong correlation between the CACS quantification and cardiac risk, including HF. A recent analysis of automated CACS on routine staging CT in patients with diffuse large B-cell lymphoma identified that at a median of 69 months, 24.66% of patients developed cancer therapy-related cardiac dysfunction, and 12.6% of patients developed major cardiac events. Risk of cancer therapy-related cardiac dysfunction and major cardiac events directly correlated with absolute CACS.19 Patients with solid cancers undergoing radiotherapy have similarly elevated cardiac risks associated with pretreatment CACS assessment.20-22 Although population-based CACS assessment has not traditionally been used for HF prediction, elevated CACS has been demonstrated in large studies to correlate with HF events, independently of symptomatic CAD.23 Coronary calcium denotes atherosclerotic change, which in addition to overt CAD, contributes to hypoperfusion, endothelial dysfunction, and symptomatic HF.24,25 Incorporating CACS to baseline lymphoma workup can potentially inform targeted risk factor management by identifying patients at increased risk of CTRCT, who benefit from primary prevention strategies such as statin therapy, which is used in asymptomatic noncancer patients with CACS >100.26-31 Atorvastatin prophylaxis was evaluated in patients with lymphoma receiving anthracyclines in the Statins to Prevent the Cardiotoxicity of Anthracyclines trial.32 However, the heterogeneous population, lack of CVR stratification and CACS assessment have limited applicability to routine care.
The benefit of using an existing baseline test to measure the CACS is significant for lymphomas. Patients with newly diagnosed lymphoma often require rapid diagnosis, staging, and treatment with complex chemotherapies, and lymphoma specialists lack specific cardiologic expertise and uniform access to specialized cardiac appraisals. In our study, CACS was conveniently performed on the baseline low-dose CT scan, sparing patients from additional radiation exposure and further tests, and it was found to be an objective marker robustly associated with cardiac events, particularly HF. Although patients with elevated CACS expectedly had higher CVR, no multicollinearity was identified in our analysis, and CACS remained significant in our multivariable analysis. Considering the recent development of comprehensive cardio-oncology guidelines,33 and that our patients developed cardiac events early during follow-up, maximizing the pretreatment evaluation period by using opportunistic PET/CT measurement of CACS as a guide to prioritize rapid referral to cardio-oncology services may improve patient care.
Our study has several limitations, particularly arising from the retrospective nature of clinical data collection. It may have been valuable to compare the clinical utility of formalized Framingham risk scores to CACS as predictors of cardiac events; however, Framingham components such as cholesterol level, ethnicity, and family history were not uniformly available. Additionally, it is likely that pre-existing cardiac disease, CVR factors, and cardiac events were underreported, and there was no routine postlymphoma cardiac surveillance, meaning that we were unable to report asymptomatic events, recently included in the definition of CTRCT as per the European Society of Cardiology.33 Whether patients received cardiac risk-modifying pharmacotherapy could not be reliably determined; yet importantly, we demonstrated an association between CACS and cardiac outcomes despite the possible use of cardioprotective medications. In addition, although 66% of the participants received >250 mg/m2 doxorubicin-equivalent anthracycline, some patients received less, which may have lowered the cardiac event rates and contributed to an underrepresentation of cardiac risk. Potentially, with such limitations leading to event underreporting, cardiac risk and correlations with CACS may indeed be stronger than suggested.
Despite the strong correlation with CAD in other studies, we could not demonstrate an association between elevated CACS and CAD events because of the low event number and relatively short follow-up period. In addition, despite the previous associations between mediastinal radiotherapy and cardiac events, this was not replicated in our data set. This is likely because there were few patients (n = 9, 3%) who received mediastinal radiotherapy, mean heart doses could not be elicited (and were likely variable), and the follow-up period was too short to determine the impact. Whether the location of coronary artery calcification impacts outcomes was not examined because of the low power with small event numbers; however, this variable may be worthy of investigation in larger studies.
We are now recruiting patients with lymphoma for a prospective observational study of CACS, involving systematic CVR assessment and posttreatment cardiac monitoring, which will allow further investigation of CACS utility in predicting cardiac outcomes (HREC/79155/Austin-2021).
Conclusion
With the increasing need to focus on improving long-term survivorship in patients with lymphoma, combined with the high cardiac event rates described in modern populations, there is a clear need for simplified objective approaches to identify patients at risk for preventable cardiac events. Our study demonstrates that CACS may be a valuable addition to routine cardiac assessment and may provide a modality to determine the group of patients most likely to benefit from tailored preventive approaches. Critically, early referral to cardio-oncology services may be enabled, to allow more effective risk mitigation and monitoring, which is important because of the early appearance of cardiac complications seen in our study. Larger prospective validation studies confirming the relationship between baseline CACS and cardiac outcomes, to progress risk stratification, and to identify the benefits of potential cardioprotective strategies are needed.
Acknowledgments
E.A.H. is supported by a National Health and Medical Research Council investigator grant. A.C.M. is supported by the National Heart Foundation Post-Doctoral Scholarship.
Authorship
Contribution: G.D., S.-T.L., A.C.M., and E.A.H. designed the study and oversaw the conduct, analysis, and manuscript preparation; E.S.S. and S.-T.L. performed coronary artery calcium scoring on staging positron emission tomography/computed tomography; N.W. collected clinical data; Z.L. performed statistical analysis; G.H. and G.C. assisted with manuscript preparation; and all authors approved the final manuscript.
Conflict-of-interest disclosure: E.A.H. has received research funding (paid to institution) from Roche, Bristol Myers Squibb, Merck KgA, AstraZeneca, TG Therapeutics, and Merck; consultancy or advisory fees (paid to institution) from Roche, Merck Sharpe & Dohme, AstraZeneca, Gilead, Antengene, Novartis, Regeneron, Janssen, Specialised Therapeutics, and Sobi; and travel expenses from AstraZeneca. G.D. has received honoraria from Orchard (Jazz) Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Genevieve Douglas, Department of Clinical Haematology, Austin Health, 145 Studley Rd, Heidelberg 3084, Australia; email: genevieve.douglas2@austin.org.au.
References
Author notes
A.C.M. and E.A.H. contributed equally to this work as senior authors.
Data are not publicly available and data queries or protocols are available upon request from the corresponding author, Genevieve Douglas (genevieve.douglas2@austin.org.au).
The full-text version of this article contains a data supplement.